Abstract
The Escherichia coli l-rhamnose-responsive transcription activators RhaS and RhaR both consist of two domains, a C-terminal DNA-binding domain and an N-terminal dimerization domain. Both function as dimers and only activate transcription in the presence of l-rhamnose. Here, we examined the ability of the DNA-binding domains of RhaS (RhaS-CTD) and RhaR (RhaR-CTD) to bind to DNA and activate transcription. RhaS-CTD and RhaR-CTD were both shown by DNase I footprinting to be capable of binding specifically to the appropriate DNA sites. In vivo as well as in vitro transcription assays showed that RhaS-CTD could activate transcription to high levels, whereas RhaR-CTD was capable of only very low levels of transcription activation. As expected, RhaS-CTD did not require the presence of l-rhamnose to activate transcription. The upstream half-site at rhaBAD and the downstream half-site at rhaT were found to be the strongest of the known RhaS half-sites, and a new putative RhaS half-site with comparable strength to known sites was identified. Given that cyclic AMP receptor protein (CRP), the second activator required for full rhaBAD expression, cannot activate rhaBAD expression in a ΔrhaS strain, it was of interest to test whether CRP could activate transcription in combination with RhaS-CTD. We found that RhaS-CTD allowed significant activation by CRP, both in vivo and in vitro, although full-length RhaS allowed somewhat greater CRP activation. We conclude that RhaS-CTD contains all of the determinants necessary for transcription activation by RhaS.
The RhaS protein functions to activate transcription of two of the operons in the Escherichia coli l-rhamnose regulon in response to the availability of l-rhamnose (11, 40). The two operons rhaBAD and rhaT encode the l-rhamnose catabolic enzymes (l-rhamnulokinase, l-rhamnose isomerase, and l- rhamnulose-1-phosphate aldolase) (2, 25) and an l-rhamnose-proton symporter that is responsible for transporting l-rhamnose into the cell (35), respectively. RhaS is encoded in an operon that also encodes a second l-rhamnose-responsive transcription activator, RhaR (37). RhaR activates transcription of the operon that encodes the two activator proteins, rhaSR (37, 38). All three operons in the l-rhamnose regulon also require a second activator protein, cyclic AMP (cAMP) receptor protein (CRP), for full transcription activation (11, 16, 40).
RhaS and RhaR are both members of the AraC/XylS family of transcription activators. This very large family of transcription activators is defined by sequence similarity in a 100-amino-acid region (10, 13). In all studied AraC/XylS family members, this 100-amino-acid region functions as a DNA-binding domain and is referred to here as the AraC/XylS family domain. Most family members contain one or more domains in addition to the AraC/XylS family domain, but a few family members consist only of this single domain, such as MarA and SoxS (13). AraC and XylS, the namesakes of the family, are examples of two-domain family members in which the nonfamily domain functions in both effector binding and dimerization (7, 19, 33).
Detailed molecular structures have been determined for the DNA-binding domains of two AraC/XylS family members, MarA and Rob (20, 28). MarA and Rob share particularly high sequence similarity, 51%, and the structures of their DNA-binding domains (the only domain of MarA) are nearly identical (with a root mean square deviation of 0.9 Å) (20). The DNA-binding domain of AraC/XylS family members contains two helix-turn-helix (HTH) DNA-binding motifs that contact consecutive major grooves of the DNA (20, 28). As a consequence, the binding site for each monomer (referred to as a half-site for dimers) is at least 17 bp long (13). In addition to DNA binding, the AraC/XylS family domain of a number of family members has been shown to be involved in transcription activation, making contacts with the C-terminal domain of the alpha subunit (α-CTD) of RNA polymerase (RNAP), the σ70 subunit of RNAP, or both (reviewed in reference 21).
Although membership in the AraC family is defined by sequence similarity within a single domain, RhaS and RhaR share amino acid sequence identity with each other, as well as with AraC, over their entire lengths. All three proteins are therefore predicted to have similar three-dimensional structures for both of their domains. The RhaS and RhaR N-terminal domains (NTDs) function in both ligand binding and dimerization (A. Kolin and S. Egan, unpublished results), while the CTDs are responsible for both DNA binding and direct contact with RNAP to activate transcription (4, 5, 42). We have previously identified several amino acid-base pair contacts that are involved in DNA binding by RhaS at rhaBAD (4). We have also identified two residues in RhaS and one in RhaR that are required to contact the σ70 subunit of RNAP to activate transcription and further have identified the residues in σ70 that each of these activator residues contacts (5, 42). Interestingly, the RhaS and RhaR residues involved in these contacts with σ70 are all located in one of the HTH motifs of the proteins.
Among AraC/XylS family proteins that consist of more than one domain, it is interesting that there have been a variety of findings regarding whether the DNA-binding domain alone is sufficient to activate transcription (7, 18, 19, 24, 26, 36). One example is the Pseudomonas putida activator XylS (XylS-ΔN209). When overexpressed to sufficiently high levels, XylS-ΔN209 can activate transcription of the TOL plasmid Pm promoter to the same high level as full-length XylS (19). Interestingly, at this high level of expression, full-length XylS becomes independent of its effector, activating to the same high levels in the absence and the presence of ligand. Another example is the DNA-binding domain of AraC. This domain alone could activate transcription of araBAD up to 15% as well as full-length AraC when alone or to the same level as full-length AraC when fused to an unrelated dimerization domain (7, 36). In contrast, the DNA-binding domain of the MelR protein (MelR173) is unable to activate transcription either at the wild-type target promoter (pmelAB) or at promoters where the promoter-proximal MelR half-site is improved (18; S. Busby, personal communication).
In the present study, we tested the C-terminal AraC/XylS family domains of RhaS and RhaR for their ability to bind DNA and activate transcription in the absence of their NTDs. We found that, while RhaS-CTD was able to activate transcription to high levels, RhaR-CTD could only activate to very low levels. DNase I footprinting indicated that both purified RhaS-CTD and RhaR-CTD were able to bind to DNA at their respective binding sites. Comparison of all of the RhaS half-sites showed that rhaI1 was the strongest site and that RhaS-CTD and full-length RhaS had similar profiles for binding to the different half-sites. Finally, we demonstrated the ability of RhaS-CTD to activate transcription in vitro and further that CRP was capable of significant in vitro activation in combination with RhaS-CTD.
MATERIALS AND METHODS
Culture media and conditions.
E. coli cultures for β-galactosidase assays were grown in MOPS (3-[N-morpholino]propanesulfonic acid)-buffered minimal medium using the protocol developed by Neidhardt et al. (4, 23). Tryptone broth (TB: 0.8% tryptone, 0.5% NaCl [pH 7.0]) was used to grow cultures in preparation for phage infection or transduction. CaCl2 was added (final concentration, 5 mM) to tryptone broth cultures used for bacteriophage P1 infection or transduction and maltose was added (final concentration 0.2%) to tryptone broth cultures used for bacteriophage λ infection or transduction. Tryptone-yeast extract (TY) liquid medium (0.8% tryptone, 0.5% yeast extract, and 0.5% NaCl [pH 7.0]) was used to grow cells for most other experiments. All cultures were grown at 37°C. Antibiotics were used as indicated at the following concentrations: ampicillin, 200 μg/ml; chloramphenicol, 25 μg/ml; kanamycin, 25 μg/ml; and tetracycline, 20 μg/ml.
General methods, strains, and plasmids.
Standard methods were used for restriction endonuclease digestion and ligation. Oligonucleotides synthesized for this study were synthesized by MWG-Biotech (High Point, NC). A list of oligonucleotides used in this study is available at http://www.molecularbiosciences.ku.edu/faculty/egan.html. The Expand high-fidelity PCR system (Roche; Indianapolis, IN) was used to amplify DNA fragments for cloning as well as to generate template DNA for sequencing of genes that were recombined onto the chromosome. DNA sequencing was performed at the Molecular Research Core Facility at Idaho State University. The DNA sequence of both strands was determined for the entire cloned region of all cloned, mutagenized, and recombined DNA fragments.
Table 1 contains the list of strains and plasmids used in this study. All strains used in β-galactosidase assays were derived from ECL116 (1). All lacZ fusions used in this study were translational fusions, with the exception of Φ(rhaT-lacZ)Δ84, which was a transcriptional fusion. The lacZ fusions are named such that “Φ” stands for fusion and the upstream endpoint of each fusion relative to the transcription start site (for example, −84, but without the minus sign) is given after the “Δ.” The lacZ translational fusions were initially constructed on the plasmid pRS414, while the transcriptional fusion was constructed on pRS415 (32). The lacZ fusions used in all experiments, except those in Fig. 5, were then recombined onto the genome of bacteriophage λ and integrated into the bacterial chromosome as lysogens (32). Single-copy λ lysogens were identified using β-galactosidase assays and then confirmed using the Ter test (15). β-Galactosidase assays were performed using the Miller method, as previously described (4, 22). Specific activities were averaged from at least three independent assays, with two replicates in each assay. In all β-galactosidase assays, error was less than 20% of the average values.
TABLE 1.
Strains and plasmids used in this study
| E. coli strain or plasmid | Genotype | Source or reference |
|---|---|---|
| Strains | ||
| BL21(DE3) | F−ompT gal dcm lon hsdSB λDE3 | Novagen |
| ECL116 | F− ΔlacU169 endA hsdR thi | 1 |
| SME1048 | ECL116 recA::cat | Laboratory collection |
| SME1051 | ECL116 ΔrhaSR::kan | Laboratory collection |
| SME2986 | ECL116 λΦ(rhaB-lacZ)Δ84 ΔrhaSR::kan recA::cat | This study |
| SME2999 | ECL116 λΦ(rhaS-lacZ)Δ85 ΔrhaSR::kan | This study |
| SME3000 | ECL116 λΦ(rhaB-lacZ)Δ84 ΔrhaSR::kan | This study |
| SME3066 | ECL116 ΔrhaRSBAD zih-35::Tn10 | This study |
| SME3072 | ECL116 λΦ(rhaB-lacZ)Δ66 ΔrhaSR::kan | This study |
| SME3089 | ECL116 λΦ(rhaT-lacZ)Δ84 ΔrhaSR::kan | This study |
| SME3114 | ECL116 λΦ(rhaB-lacZ)Δ110 ΔrhaSR::kan recA::cat | This study |
| Plasmids | ||
| pBluescript II SK | AprlacZα | Stratagene |
| pET15b | AprlacI (ColE1 origin from pBR322) | Novagen |
| pHG165 | lacZα rop Apr (ColE1 origin from pBR322) | 34 |
| pRS414 | lac′ZYA | 32 |
| pSE101 | pTZ 18R Apr ′rhaTrhaSRrhaBA′ | Laboratory collection |
| pSE227 | pET15b rhaR196-312 (encodes N-terminal His6-tagged RhaR residues 196 through 312) | This study |
| pSE230 | pET15b rhaS163-278 (encodes N-terminal His6-tagged RhaS residues 163 through 278) | This study |
| pSE250 | pUC18 rhaSRrhaT′ wild type | This study |
| pSE262 | pHG165 + pSRcon promoter | This study |
| pSE265 | pSE262 rhaS | This study |
| pSE268 | pSE262 rhaS163-278 (encodes RhaS-CTD) | This study |
| pSE271 | pSU18 rhaS163-278 (encodes His6-tagged RhaS-CTD from lac promoter) | This study |
| pSE272 | pSU18 rhaR196-312 (encodes His6-tagged RhaR-CTD from lac promoter) | This study |
| pSE273 | pSU18 rhaS | This study |
| pSE274 | pSU18 rhaS163-278 (encodes RhaR-CTD) | This study |
| pSE276 | pRS414 Φ(rhaB-lacZ)Δ66 | This study |
| pSE283 | pTS134 with rhaBAD Δ110 promoter replacing rhaSR promoter | This study |
| pSU18 | lacZα Cmr (P15A ori) | 3 |
| pTS134 | pBluescript II SK rhaSR promoter | 44 |
| pUC18 | AprlacZα | 47 |
FIG. 5.
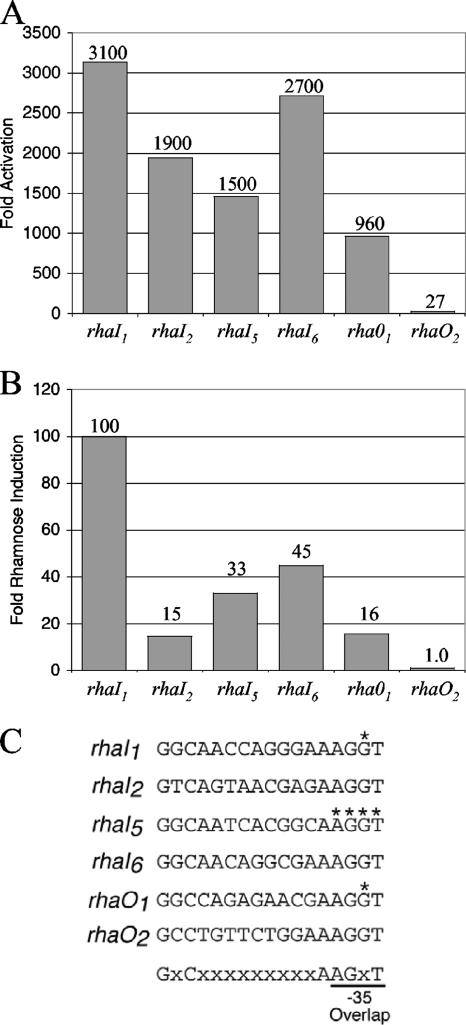
In vivo transcription activation by His6-RhaS-CTD or RhaS. The indicated RhaS half-sites, in the context of Φ(rhaB-lacZ)Δ66 on multicopy plasmids (pSE276 and derivatives), were assayed for β-galactosidase activity. Cells were grown in TY medium. (A) Activation by His6-RhaS-CTD, expressed from pSE271 in SME1051 [Δ(rhaSR)]. Activation (fold) was determined by dividing the activity obtained with RhaS-CTD by the activity of pSU18 alone (vector only) at each promoter. The activity of pSU18 alone ranged from 0.39 to 0.99 Miller units. (B) Transcription activation by RhaS expressed from the chromosome in SME1048 (wild-type rhaSR). Rhamnose induction (fold) was determined by dividing the activity of each fusion in the presence of rhamnose by the activity of the same fusion in the absence of rhamnose. The activities in the absence of rhamnose ranged from 0.21 to 0.29 Miller units. (C) The DNA sequences of the half-sites used in these experiments are shown. The asterisks indicate positions at which base pairs were changed from the wild-type half-site sequences (see Fig. 1) so that the DNA sequence of the overlapping −35 element was not changed. The bottom line indicates the sequence and position of the 6 important bp in the RhaS binding site that were used to identify rhaO1 and rhaO2.
Construction of plasmids for overexpression and purification of His6-RhaS-CTD and His6-RhaR-CTD proteins.
The N-terminal His6-tagged versions of RhaR-CTD and RhaS-CTD were expressed from pSE227 and pSE230, respectively. The RhaR- and RhaS-coding regions of pSE227 and pSE230 were amplified by PCR using pSE101 as the template and the following oligonucleotides: 2345 and 2346 for rhaR and 2349 and 2350 for rhaS. The PCR-amplified DNA was then ligated to pET15b using the NdeI and BamHI restriction sites such that the vector-encoded N-terminal His6 tag was fused in frame with the RhaS- and RhaR-coding regions.
Overexpression and purification of His6-RhaS-CTD and His6-RhaR-CTD.
All protein overexpression was performed in strain BL21(DE3) (Novagen). The cells were grown to an A600 of approximately 1.0, induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and incubated for an additional 3 h. After harvesting the cells, the cell pellets were resuspended in chromatography binding buffer (5 mM imidazole, 0.5 M sodium chloride, 20 mM Tris-HCl [pH 7.9]) and sonicated. After sonication, the lysate was centrifuged to separate soluble proteins from insoluble proteins. At this level of overexpression, the vast majority of the proteins were present in the insoluble, pellet fraction. Therefore, the pellet fractions from the sonication lysates were resuspended in chromatography binding buffer containing 6 M urea and rocked overnight at 4°C to solubilize the His6-RhaS-CTD and His6-RhaR-CTD proteins. The next day, the urea-containing suspensions were centrifuged to remove the remaining insoluble protein and the supernatant fractions were loaded onto immobilized metal affinity chromatography columns made with Ni+-charged Chelex 20 resin (Sigma) that had been pre-equilibrated with binding buffer containing 6 M urea. The columns were washed with five volumes of binding buffer (containing 6 M urea) and then with 3 volumes of wash buffer (60 mM imidazole, 0.5 M sodium chloride, 20 mM Tris-HCl [pH 7.9]) containing 6 M urea. In order to allow refolding of the protein on the column, the columns were washed with three volumes of wash buffer without urea and the His6-tagged proteins were then eluted with 3 volumes of elution buffer (0.5 M imidazole, 0.5 M sodium chloride, 20 mM Tris-HCl [pH 7.9]). CRP with no added His6 tag was also purified by immobilized metal affinity chromatography, using the procedure previously described (43).
Construction of RhaS, RhaS-CTD, and RhaR-CTD expression plasmids for in vivo experiments.
In order to test the ability of His6-RhaS-CTD and His6-RhaR-CTD to activate transcription in vivo, the respective genes were subcloned from pSE230 and pSE227 into pSU18 to make pSE271 and pSE272, respectively (using primers 2453 and 2454 in both cases). The subcloning involved digesting the PCR products with EcoRI and HindIII and then ligating them to similarly digested pSU18. In the resulting constructs, the lac promoter of pSU18 drives transcription and the ribosome binding site from pET15b (which was subcloned along with the open reading frame from pSE230 and pSE227) drives translation. A non-His6-tagged version of RhaS-CTD (pSE274) in pSU18 and an equivalent version of full-length RhaS (pSE273) were also constructed by PCR amplification from pSE101 and addition of a primer-encoded Shine-Dalgarno sequence that was equivalent to the Shine-Dalgarno sequence in pET15b. The upstream primers were 2571 for RhaS and 2574 for RhaS-CTD, and the downstream primer was 2542 in both cases.
Plasmid pSE262 was constructed by adding a constitutive promoter to pHG165. The inserted promoter in pSE262 (pSRcon) is the core rhaSR promoter, except the −35 hexamer was changed so that it matches the consensus −35 hexamer sequence (therefore, the −35 sequence is TTGACA, and the −10 sequence is TACTAT). Also, pSE262 has a Shine-Dalgarno sequence (GAAGGA) followed immediately by a BamHI site. Placement of a translational start codon immediately downstream of the BamHI site provides the correct spacing relative to the Shine-Dalgarno sequence for ribosome recognition. The rhaS gene and the gene encoding RhaS-CTD were amplified by PCR from pSE250 with primers 2731 or 2732 and 2542 and ligated to pSE262 to make pSE265 and pSE268, respectively.
Western blots to compare in vivo expression of His6-RhaS-CTD and His6-RhaR-CTD.
To compare His6-RhaS-CTD expression to His6-RhaR-CTD expression in vivo, cultures of the strains used in β-galactosidase assays (Table 2) were grown to mid-log phase in TB with chloramphenicol. Cells were sedimented and resuspended in TB to identical cell densities (using A600). Five hundred microliters of each sample was sonicated and separated into soluble and insoluble fractions by centrifugation. The insoluble pellets were resuspended in 500 μl TB. The total protein concentration in each sample was determined by Bradford (6) protein assay (Bio-Rad, Hercules, CA). Soluble fractions were standardized to identical protein concentrations, as were insoluble fractions, with insoluble fractions generally containing approximately 10-fold less protein than soluble fractions (the same ratio found before the minor adjustments to standardize protein concentrations). The samples were then analyzed using Western blots. Equal amounts of protein from the standardized His6-RhaS-CTD- and His6-RhaR-CTD-containing cell fractions were loaded onto two 15% sodium dodecyl sulfate-polyacrylamide gels, electrophoresed, and blotted onto a nitrocellulose membrane using standard procedures. We also loaded known amounts of either purified His6-RhaS-CTD or His6-RhaR-CTD on the gels to allow for quantification of His6-RhaS-CTD or His6-RhaR-CTD present in the soluble and insoluble fractions of each sample. We added total lysate from the vector-only sample (collected before fractionation) to the samples with purified protein to prevent differences in antibody-antigen binding due to the lack of other proteins in the sample. The primary antibodies (anti-RhaS and anti-RhaR) were custom-made polyclonal rabbit antibodies from Cocalico Biologicals (Reamstown, PA). Anti-RhaS antibody was preadsorbed against a lysate of strain SME3066 (ΔrhaRSBAD) in order to remove rabbit antibodies to other E. coli proteins. This preadsorption step was not necessary for anti-RhaR antibody. The Alexa Fluor 680-labeled secondary antibody (anti-rabbit) was obtained from Molecular Probes (Eugene, OR). The blots were imaged using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE).
TABLE 2.
Transcription activation by His6-RhaS-CTD and His6-RhaR-CTD
| Promoter fusiona | β-Galactosidase sp act (Miller units)b
|
His6-RhaS-CTD or His6-RhaR-CTD activation (fold)c | |
|---|---|---|---|
| Vector only | His6-RhaS-CTD or His6-RhaR-CTD | ||
| Φ(rhaB-lacZ)Δ84 | 0.021 | 21 (His6-RhaS-CTD) | 1,000 (His6-RhaS-CTD) |
| Φ(rhaT-lacZ)Δ84d | 0.29 | 51 (His6-RhaS-CTD) | 180 (His6-RhaS-CTD) |
| Φ(rhaS-lacZ)Δ85 | 0.41 | 0.82 (His6-RhaR-CTD) | 2.0 (His6-RhaR-CTD) |
Each strain carried a single-copy lacZ fusion integrated into the chromosome as a λ lysogen and also Δ(rhaSR).
β-Galactosidase activity was determined as described in Materials and Methods. The vector-only sample was pSU18. His6-RhaS-CTD was expressed from pSE271, and His6-RhaR-CTD was expressed from pSE272.
Activation values (fold) were calculated by dividing the activity in the presence of His6-RhaS-CTD or His6-RhaR-CTD by the activity in the presence of vector only.
Φ(rhaT-lacZ)Δ84 is a transcriptional fusion. All other lacZ fusions in this study were translational fusions.
DNase I footprinting.
The template DNAs for DNase I footprinting were generated by PCR using the following templates and primers: rhaBAD was amplified from pSE101 using primers 2371 and 2410, rhaT was amplified from ECL116 cells using primers 2096 (32P-labeled) and 2097 for one strand and primers 2655 (32P-labeled) and 2656 for the other strand, and rhaSR was amplified from pSE101 using primers 2371 and 2409. DNase I footprinting was performed as previously described (44). Gels were imaged by autoradiography. In addition to the results shown, similar results were obtained when the other DNA strand was labeled. All DNase I footprinting experiments were carried out at least twice.
Construction of rhaI half-site fusions on plasmids.
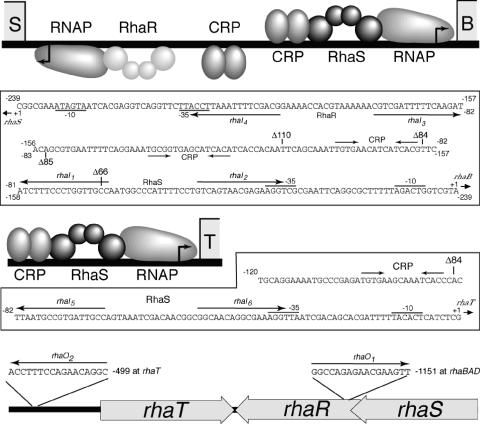
The half-site fusions used to compare the strengths of various RhaS DNA half-sites were constructed in the context of a Φ(rhaB-lacZ)Δ66 fusion (Fig. 1) in pRS414. At the wild-type rhaBAD promoter, the rhaI2 half-site overlaps the −35 hexamer of RNAP by 4 bp. The other RhaS half-sites were placed in the position of rhaI2; however, the DNA sequence of the 4-bp overlap with the −35 hexamer was not changed (Fig. 5C). (Constructs in which the −35 sequence was changed to match that of the rhaI1, rhaI5, and rhaO1 half-site resulted in extremely low expression [unpublished results].) The DNA sequence surrounding each half-site was identical in every case. The wild-type Φ(rhaB-lacZ)Δ66 fusion (pSE276) was created by PCR with oligonucleotides 2414 and 744, using pSE101 as the template. The other half-site fusions were constructed by PCR using an oligonucleotide-encoded half-site in the upstream primer (2413, 2441, 2442, 2445, and 2446) and oligonucleotide 744 downstream, and the resulting plasmids were named pSE276 rhaIX (where “X” represents the half-site number).
FIG. 1.
RhaS and RhaR binding sites within the l-rhamnose regulon. For each promoter (rhaSR, rhaBAD, and rhaT), there is a schematic showing the known proteins and binding sites and below each schematic is the DNA sequence of that promoter region. Within the rhaSR-rhaBAD intergenic region, labels for the rhaSR promoter are below the DNA sequence and labels for the rhaBAD promoter are above the DNA sequence. The upstream endpoints of the lacZ fusions used in this study (in bp upstream from the transcription start site) are indicated with Δ. The RhaS and RhaR binding sites are shown as half-sites and labeled with the half-site number (rhaIx). The orientation of each half-site is indicated with an arrow. RhaS (dark gray) and RhaR (light gray) are depicted as dimers, with each monomer consisting of two domains, a C-terminal DNA-binding domain and an N-terminal dimerization domain, depicted as spheres. The bottom figure shows the approximate positions of the rhaO half-sites (which are outside of the promoter regions) within the l-rhamnose region.
In vitro transcription assays.
Single-round in vitro transcription assays were carried out with core RNAP and σ70 purified as described previously (48, 49). Reconstitution of σ70 with core RNAP was carried out by mixing 4 μg core RNAP and 0.7 μg σ70 (1:1 molar ratio) in 100 μl of RNAP storage buffer (50 mM Tris-HCl [pH 8.0], 50% glycerol, 0.1 mM NaEDTA, 0.1 mM dithiothreitol, 50 mM NaCl), incubating the mixture at 25°C for 10 min, and then storing it at −20°C. To prepare the transcription reaction, His6-RhaS-CTD and/or CRP was incubated with rhaBAD promoter template DNA (PCR amplified with oligonucleotides 744 and 2654) in IVT reaction buffer (final concentrations in reaction mixture, 20 mM Tris-HCl [pH 7.9], 50 mM KCl, 4 mM MgCl2, 1 mM dithiothreitol, 0.1 mM KEDTA, 0.1 mg/ml bovine serum albumin, 50 mM l-rhamnose, 0.2 mM cAMP) at 37°C for 10 min. RNAP was then added (10 nM final concentration), and the reaction mixture was incubated for 5 min at 37°C. An initiation mixture was added (final concentrations in reaction mixture, 0.2 mM each ATP, CTP, and GTP; 0.02 mM UTP; 100 mg/ml heparin; 0.2 μCi [α-32P]UTP [3,000 Ci/mmol]). The reaction mixture was next incubated at 37°C for 10 min, and then the reaction was stopped by addition of 0.25 volume of stop solution (7 M urea, 0.1 M KEDTA, 0.4% sodium dodecyl sulfate, 20 mM Tris-HCl [pH 7.9], 0.5% bromophenol blue, 0.5% xylene cyanol). The reaction mixture was then loaded directly onto a preheated 6% denaturing polyacrylamide gel for electrophoresis (0.3% N,N-methylenebisacrylamide, 8.9 mM Tris, 8.9 mM boric acid, 20 mM EDTA, 8 M urea). The gels were imaged and analyzed using a Cyclone storage phosphor system (Perkin-Elmer). The results shown are representative of three similar experiments.
RESULTS
In vitro DNA binding by His6-RhaS-CTD and His6-RhaR-CTD.
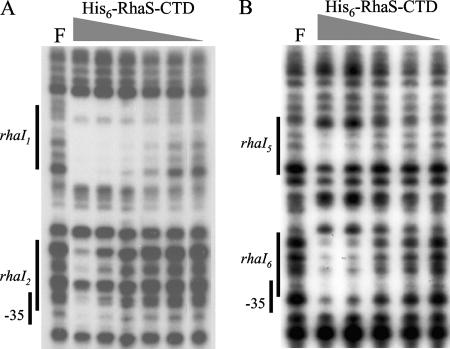
Specific residues in the CTDs of RhaS and RhaR had previously been shown to contribute to DNA binding and transcription activation (4, 5, 38, 42). In order to test whether the CTD of each protein was sufficient for DNA binding and transcription activation, we constructed plasmids expressing truncated versions of RhaS (encoding His6-RhaS-CTD, consisting of RhaS amino acids 163 to 278), and RhaR (encoding His6-RhaR-CTD, consisting of RhaR amino acids 196 to 312). We tested the in vitro DNA-binding activity of His6-RhaS-CTD and His6-RhaR-CTD by performing DNase I footprinting using the purified proteins. We found that His6-RhaS-CTD bound to the rhaBAD promoter region at two sites (Fig. 2A). The extent of the two footprinted regions corresponds very well with the two half-sites for RhaS binding previously predicted from the footprint of full-length RhaS and mutagenesis of the binding site region (12). There were two differences from the previously published footprints; however, both were consistent with the prediction that His6-RhaS-CTD is monomeric, whereas full-length RhaS is dimeric. First, His6-RhaS-CTD did not protect the DNA between the two half-sites, while full-length RhaS did. Second, it was possible to observe differences in the binding strengths to the two half-sites with His6-RhaS-CTD. We found that there were protein concentrations at which the footprint at rhaI1 (the promoter distal half-site) was detectable while the footprint at rhaI2 (the promoter proximal half-site) was no longer detectable, indicating that His6-RhaS-CTD bound more tightly to rhaI1 than to rhaI2 (Fig. 2A).
FIG. 2.
DNase I footprinting assay of His6-RhaS-CTD binding to the rhaBAD promoter (A) and the rhaT promoter (B). The DNA fragment used as the template for rhaBAD was generated by PCR with primers 2371 (32P labeled) and 2410, while that for rhaT was generated with primers 2096 (32P labeled) and 2097. The positions of the RhaS half-sites were determined based on a DNA sequencing ladder (not shown). The highest His6-RhaS-CTD concentration was 6 μM, and the dilution steps were threefold. F, free DNA.
We also performed DNase I footprinting with His6-RhaS-CTD at the rhaT promoter. DNA sequence inspection and previous results indicating that RhaS was required for activation of rhaT expression (40) suggested that RhaS binds to DNA at the rhaT promoter, but direct evidence of RhaS protein binding to rhaT promoter DNA had not been obtained. Our DNase I footprinting results provide direct evidence of His6-RhaS-CTD binding to the predicted RhaS half-sites at the rhaT promoter (Fig. 2B). In this case, His6-RhaS-CTD appeared to have a slightly higher affinity for the rhaI6 half-site (the promoter-proximal half-site) than the rhaI5 half-site (the promoter distal half-site). While the protection of rhaI5 was weak, we were unable to use higher protein concentrations in this experiment due to the tendency of His6-RhaS-CTD to aggregate.
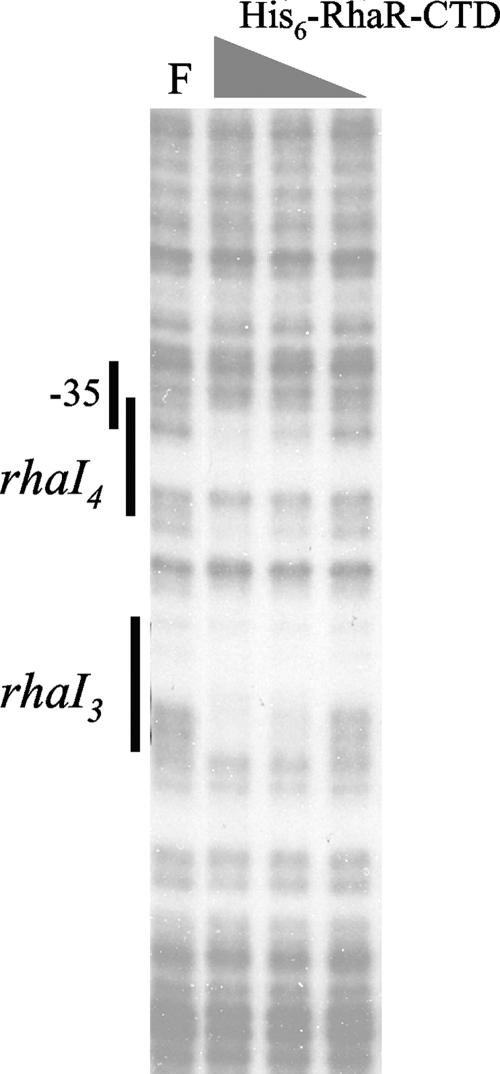
Finally, we tested in vitro DNA binding by His6-RhaR-CTD by DNase I footprinting. We found that His6-RhaR-CTD showed specific binding to two sites within rhaSR promoter DNA (Fig. 3). There was somewhat more ambiguity than usual in the exact extent of the protected regions in this case due to the relative lack of DNase I cleavage sites within the A tracts, especially in the region of rhaI3; however, the two protected regions appear to correspond well with the previously demonstrated RhaR half-sites (38, 44). There was not any substantial difference in the apparent strength of His6-RhaR-CTD binding to the two half-sites. These results indicate that our purified His6-RhaS-CTD and His6-RhaR-CTD protein preparations contained active proteins that were capable of specifically binding to DNA.
FIG. 3.
DNase I footprinting assay of His6-RhaR-CTD binding to the rhaSR promoter. The DNA fragment used as the template was generated by PCR with primers 2371 (32P labeled) and 2409. The positions of the RhaR half-sites were determined based on a DNA sequencing ladder (not shown). The highest His6-RhaR-CTD concentration was 5 μM, and the dilution steps were threefold. F, free DNA.
The CTD of RhaS but not RhaR is sufficient for transcription activation.
His6-RhaS-CTD was tested for in vivo activation of lacZ fusions to the rhaBAD and rhaT promoters, while His6-RhaR-CTD was tested for in vivo activation of a lacZ fusion to the rhaSR promoter (Fig. 1). We found that plasmid-expressed His6-RhaS-CTD could activate transcription to high levels, with 1,000-fold activation of the rhaBAD promoter and 180-fold activation of the rhaT promoter (Table 2). In contrast, plasmid-expressed His6-RhaR-CTD could only activate expression of the rhaSR promoter by twofold (Table 2). Given that the activation by full-length, chromosomally expressed RhaS at rhaBAD is approximately 33-fold higher than that of chromosomally expressed RhaR at rhaSR, comparable efficiencies of activation by the CTDs to their full-length counterparts would have resulted in His6-RhaR-CTD activating rhaSR by approximately 30-fold. This value is much higher than the twofold value measured for His6-RhaR-CTD. To test whether the very poor activation might be due to an artifact of our His6-RhaR-CTD-expressing construct, we made a number of different RhaR-CTD constructs; however, none were capable of significant transcription activation. Our results suggest that although His6-RhaR-CTD is capable of specific DNA binding, it is not capable of activating transcription well. Interestingly, Tobin and Schleif previously found that in the absence of l-rhamnose, full-length RhaR was able to bind to DNA but was not able to activate transcription (38, 39), suggesting that His6-RhaR-CTD (which lacks an l-rhamnose-binding domain) may be similar in its activity to full-length RhaR in the absence of l-rhamnose.
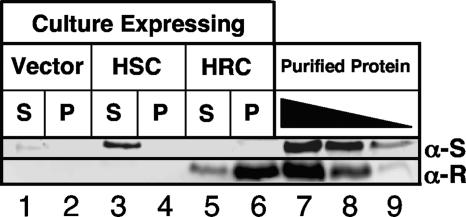
To test whether the low level of activation by His6-RhaR-CTD could be due to a low level of His6-RhaR-CTD protein expression or stability compared with the His6-RhaS-CTD protein, we performed Western blots with samples of the same strains assayed in Table 2. We found that both soluble His6-RhaS-CTD (Fig. 4, top blot, lane 3) and soluble His6-RhaR-CTD (Fig. 4, bottom blot, lane 5) were present in substantial amounts, based on comparisons with known amounts of the respective purified proteins. However, soluble His6-RhaS-CTD (5.5 ng/μg of soluble protein) was present at a level approximately 5.5-fold higher than that of soluble His6-RhaR-CTD (1.0 ng/μg of soluble protein). This 5.5-fold difference in soluble protein levels could explain some of the decrease in activation by His6-RhaR-CTD at rhaSR relative to His6-RhaS-CTD at rhaBAD; however, the similarity in the activity of His6-RhaR-CTD to that of full-length RhaR in the absence of l-rhamnose suggests that His6-RhaR-CTD may simply be unable to activate transcription well. Our results also showed that His6-RhaS-CTD and His6-RhaR-CTD did not respond to l-rhamnose availability (data not shown), which was expected since, based on sequence similarity with AraC, l-rhamnose binding is predicted to be a function of the RhaS and RhaR N-terminal domains.
FIG. 4.
Western blots comparing in vivo levels of expression of His6-RhaS-CTD and His6-RhaR-CTD. Soluble (S, supernatant) and insoluble (P, pellet) fractions after sonication were loaded as indicated. The vector-only sample was pSU18. His6-RhaS-CTD (HSC) was expressed from pSE271, and His6-RhaR-CTD (HRC) was expressed from pSE272. Lanes 7 to 9 on each gel contained known amounts of purified His6-RhaS-CTD (top blot) or His6-RhaR-CTD (bottom blot). The amounts of His6-RhaS-CTD were 737 (lane 7), 368 (lane 8), and 184 (lane 9) ng. The amounts of His6-RhaR-CTD were 162 (lane 7), 54 (lane 8), and 18 (lane 9) ng. Two gels were prepared, with identical culture samples on each gel, and each blot was probed with the primary antibody corresponding to the purified protein samples loaded, as indicated to the right. α-S, anti-RhaS antibody; α-R, anti-RhaR antibody.
Comparison of RhaS and RhaS-CTD activation of rhaB-lacZ fusions.
The above results (Table 2) indicate that RhaS-CTD was capable of activating transcription from a rhaB-lacZ fusion that includes the full RhaS binding site. We next tested whether both or only one of the RhaS half-sites contribute to RhaS-CTD activation and whether RhaS-CTD is sufficient to allow CRP to contribute to rhaBAD activation. Given that it lacks its dimerization domain, we predicted that RhaS-CTD would function as a monomer, similar to MarA (28). We expected that the RhaS-CTD monomer that bound to the half-site adjacent to RNAP would contribute to transcription activation based on our previous finding that RhaS contacts with σ70 contribute to transcription activation (5, 42). We also have some evidence that activation by RhaS may involve contacts with α-CTD (17); therefore, it was possible that RhaS-CTD bound to the promoter-distal RhaS half-site might further contribute to transcription activation. Previous in vivo experiments also indicate that CRP is not capable of activating rhaBAD expression in the absence of RhaS (11), perhaps suggesting that RhaS must bend the DNA to allow CRP to activate, although other possibilities exist as well. Therefore, we also tested whether RhaS-CTD was capable of fulfilling the function of RhaS that allows CRP activation.
To address these questions, we compared transcription activation by full-length RhaS and RhaS-CTD (no His6 tag) at three different truncations of the rhaBAD promoter (each fused to lacZ and carried as a single-copy λ lysogen) (Fig. 1). At Φ(rhaB-lacZ)Δ66, which carries only one half-site of the full RhaS binding site, RhaS-CTD was capable of more than 2,000-fold activation (Table 3). Interestingly, this was a 10-fold-higher level than when similarly expressed full-length RhaS activated this fusion. At the Φ(rhaB-lacZ)Δ84 fusion, which carries the full RhaS binding site, there was no increase in the activation by RhaS-CTD, indicating that RhaS-CTD activation occurs from the promoter proximal half-site and consistent with the prediction that RhaS-CTD functions as a monomer. In contrast, full-length RhaS activated this fusion to a level more than 30-fold higher than its activation of the fusion containing only a single RhaS half-site and 3-fold higher than the activation by RhaS-CTD. Finally, at the Φ(rhaB-lacZ)Δ110 fusion, which contains the CRP site required for full rhaBAD activation as well as the full RhaS binding site, there was a twofold contribution to activation by CRP when in combination with RhaS-CTD and a fivefold contribution to activation by CRP when in combination with full-length RhaS. This suggests that RhaS-CTD can fulfill at least part of the function of RhaS that allows CRP activation at rhaBAD.
TABLE 3.
Transcription activation by RhaS-CTD compared to full-length RhaS
| Promoter fusiona | β-Galactosidase sp act (Miller units)b
|
Activation (fold) with:c
|
|||
|---|---|---|---|---|---|
| Vector | RhaS-CTD | RhaS | RhaS-CTD | RhaS | |
| Φ(rhaB-lacZ)Δ66 | 0.015 | 34 | 3.5 | 2,300 | 230 |
| Φ(rhaB-lacZ)Δ84 | 0.015 | 36 | 110 | 2,400 | 7,300 |
| Φ(rhaB-lacZ)Δ110 | 0.018 | 91 | 700 | 5,100 | 39,000 |
Each strain carried a single-copy lacZ fusion integrated into the chromosome as a λ lysogen and also Δ(rhaSR).
β-Galactosidase activity was determined as described in Materials and Methods. All cultures were grown in the presence of l-rhamnose. The vector-only sample was pSE262. RhaS-CTD was expressed from pSE268, and RhaS was expressed from pSE265.
Activation (fold) values were calculated by dividing the activity in the presence of RhaS-CTD or RhaS by the activity in the presence of vector only.
In vivo comparison of different RhaS half-sites.
The DNase I footprinting assays indicated that there were differences in the relative strengths of the RhaS half-sites at the rhaBAD and rhaT promoters. In order to further compare RhaS binding to the RhaS half-sites, each half-site was placed at the same position in the context of the Φ(rhaB-lacZ)Δ66 promoter (referred to as “half-site fusions”). In these constructs, each of the half-sites replaces rhaI2, the wild-type promoter-proximal half-site, at this promoter (Fig. 1). In addition to the four previously identified RhaS half-sites, we also tested two additional DNA sequences that were identified using a string-matching program (written in the computer language Perl) to identify potential RhaS half-sites within the entire rha region. The program identified only two DNA sequences with perfect matches in sequence and spacing to the 6 bp previously identified as most important for RhaS binding (12) (Fig. 5C). One of the potential half-sites (rhaO1) is located within the rhaR gene and is centered at −1153 relative to the rhaBAD transcription start site or at +914 relative to the rhaSR transcription start site, while the other potential site (rhaO2) is centered at −499 relative to the rhaT transcription start site.
We first tested the ability of each of the half-site fusions to be activated in vivo by His6-RhaS-CTD (Fig. 5A). Among the previously identified RhaS half-sites, we found the greatest activation (fold) at rhaI1, followed by rhaI6, then rhaI2, and finally rhaI5. These results confirm the relative half-site strengths identified by DNase I footprinting and further provide information about the relative strengths of the rhaBAD versus rhaT half-sites. We also found that His6-RhaS-CTD could activate transcription from the fusion carrying the rhaO1 half-site to an extent comparable with that of the previously identified half-sites, while there was only a very low level of activation from rhaO2. The same order of relative half-site strengths was also determined using electrophoretic mobility shift assays with purified His6-RhaS-CTD (data not shown). The ability of His6-RhaS-CTD to activate transcription to a high level from rhaO1 confirms that the 6 bp used to identify this site are important for RhaS binding; however, the low level of activation with rhaO2 suggests that the context of these 6 bp is also important. We also tested the same set of half-site fusions for activation by full-length RhaS expressed from the chromosome (Fig. 5B). We found a very similar order of apparent half-site strengths in this experiment, although the position of rhaI2 in the order was different and the magnitude of activation by RhaS from these fusions was much lower than that by His6-RhaS-CTD. In this case, there was no activation from the rhaO2 half-site, further supporting the idea that it is, at best, a marginal RhaS half-site.
Transcription activation by His6-RhaS-CTD in vitro.
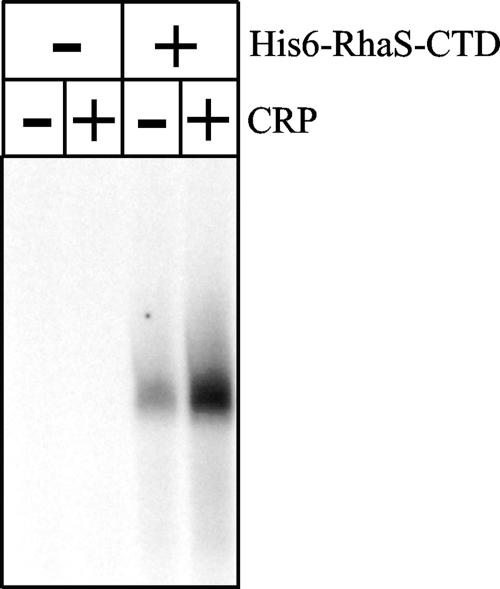
Given that RhaS-CTD (both with and without a His6 tag) was capable of activating transcription in vivo and was capable of specifically binding to DNA at RhaS half-sites in vitro, we investigated its ability to activate transcription in a purified in vitro transcription assay. We have never been able to carry out in vitro transcription with full-length RhaS due to its insolubility. In preliminary experiments, we found that His6-RhaS-CTD activated transcription much more efficiently from linear DNA templates than from supercoiled DNA templates (data not shown), in contrast to full-length RhaR, which required supercoiled DNA templates for efficient in vitro transcription activation (39, 44). We found that CRP alone was not capable of activating rhaBAD expression, similar to previous findings in vivo, and that His6-RhaS-CTD alone substantially activated rhaBAD expression (Fig. 6). We also found that CRP and His6-RhaS-CTD together could activate transcription to a level that was threefold higher than that by His6-RhaS-CTD alone. The threefold contribution to activation by CRP in this experiment is very similar to the twofold contribution found in the above in vivo experiment (Table 3), confirming that RhaS-CTD is sufficient to allow at least partial CRP activation of rhaBAD expression. This in vitro transcription system mimics the in vivo activation of rhaBAD by RhaS-CTD and also, to a great extent, the in vivo activation of rhaBAD by full-length RhaS, each in the presence and absence of CRP. Consistent with the very low level of activation in our in vivo results, we were not able to detect transcription activation by His6-RhaR-CTD (data not shown).
FIG. 6.
In vitro transcription activation by His6-RhaS-CTD. The linear template DNA containing the rhaBAD promoter was generated by PCR using primers 744 and 2654 with pSE283 as the template. When present (as indicated above the gel), the His6-RhaS-CTD concentration was 10 μM and the CRP concentration was 1 μM. cAMP was present in all reactions.
DISCUSSION
In vivo transcription activation by RhaS-CTD.
Our results indicate that RhaS-CTD (with and without a His6 tag) could activate transcription very well and that purified His6-RhaS-CTD protein was able to bind to DNA at the previously identified or predicted RhaS half-sites at rhaBAD and rhaT. Based on the amino acid sequence alignment of RhaS with AraC, as well as our studies of RhaS (Kolin and Egan, unpublished), we predicted that the dimerization interface of RhaS would be located in its NTD. Several pieces of evidence in this study are consistent with the prediction that RhaS-CTD functions as a monomer. His6-RhaS-CTD did not footprint the DNA between the two RhaS half-sites, nor did binding by His6-RhaS-CTD exhibit much if any cooperative binding to the two half-sites at rhaBAD and rhaT, in both cases, unlike full-length RhaS (12). The level of transcription activation by RhaS-CTD also did not increase with the addition of a second RhaS half-site, again unlike full-length RhaS.
We were also not surprised to find that RhaS-CTD was capable of equivalent transcription activation in the absence and the presence of l-rhamnose (data not shown), since amino acid sequence alignment with AraC suggests that the RhaS N-terminal domain likely binds l-rhamnose. The differential activation of transcription by full-length RhaS in the absence and presence of l-rhamnose could be due either to inhibition of activity in the absence of ligand or stimulation of activity in the presence of ligand. The finding that RhaS-CTD activates transcription very well in the absence of RhaS-NTD suggests that there may be inhibition of full-length RhaS activity in the absence of ligand. The light-switch mechanism used by AraC to respond to its ligand arabinose also involves inhibition in the absence of ligand (14, 27, 29, 31, 45, 46). However, our more recent results suggest that the l-rhamnose response of RhaS most likely involves an active stimulation of activity in the presence of l-rhamnose (Kolin and Egan, unpublished [see below]).
In vitro DNA binding and transcription activation by His6-RhaS-CTD.
We found that purified His6-RhaS-CTD was capable of specific DNA binding and also activation of transcription in purified in vitro reactions. Prior to this work, in vitro studies of the l-rhamnose regulon have been severely hampered by the strong tendency of full-length RhaS to aggregate. Our only previously published in vitro studies involving RhaS utilized partially purified protein that was refolded on a “per-reaction” basis in the presence of specific DNA (12). We have never been able to obtain full-length RhaS that is both soluble and active by refolding of protein purified under denaturing conditions nor by fusion with proteins that promote solubility. Therefore, our finding that His6-RhaS-CTD is capable of both specific DNA binding and activation of transcription in vitro represents a major breakthrough in our studies of the l-rhamnose regulon. While His6-RhaS-CTD is not entirely free of aggregation problems, its aggregation is substantially more manageable than that of full-length RhaS.
RhaS-CTD binding to rhaI half-sites.
Our DNase I footprinting and in vivo transcription assays both indicated that His6-RhaS-CTD bound to the rhaI1 half-site at rhaBAD significantly more strongly than to the rhaI2 half-site. This finding is similar to previous findings with the AraC and MelR proteins in which the activator binds to its upstream half-site much more tightly than its downstream half-site (8, 9, 18, 31) and in the absence of ligand forms a DNA loop that represses transcription (30, 41). To begin to address the question of whether RhaS regulation might involve DNA looping, we used a bioinformatics approach to look for other potential RhaS half-sites in the rha region. One of the two sites we identified, rhaO1, is located within the rhaR gene at −1153 relative to the rhaBAD transcription start site or +914 relative to the rhaSR transcription start site. The finding that RhaS binds to rhaO1 with an apparent strength that is comparable to that of the known RhaS half-sites suggests the possibility that it has some in vivo function, although whether there is a role and whether that role might involve DNA looping remain to be determined.
Differences between RhaS-CTD and RhaR-CTD.
Given that RhaS-CTD and RhaR-CTD share 34% amino acid sequence identity, we were surprised to find that His6-RhaS-CTD efficiently activated transcription, while His6-RhaR-CTD only barely activated transcription. Although the presence of low protein levels may partially account for the lower activation by His6-RhaR-CTD, we would argue it is not the full explanation. Purified His6-RhaR-CTD protein was capable of binding to DNA, indicating that this fusion protein contained the necessary determinants for DNA binding and was capable of folding correctly. One hypothesis to explain the very low activation by His6-RhaR-CTD might be that its DNA-binding motifs are correctly folded but that its transcription activation determinants are not properly folded. This hypothesis seems highly unlikely given that RhaR residue D276 is located within the stabilizing helix of one of the HTH DNA-binding motifs and that this σ70-contacting residue is responsible for approximately two-thirds of the transcription activation by RhaR (42). Also, as mentioned above, Tobin and Schleif (38, 39) previously found that, similar to His6-RhaR-CTD, full-length RhaR in the absence of l-rhamnose was capable of binding to DNA but not capable of activating transcription. It seems likely, therefore, that RhaR-CTD requires a signal from RhaR-NTD in the presence of l-rhamnose in order to activate transcription and therefore is unable to activate in the absence of RhaR-NTD. RhaS, therefore, provides an additional example of an AraC/XylS family protein whose DNA-binding domain is capable of efficient transcription activation in the absence of a second domain. In contrast, RhaR may be an additional example of an AraC/XylS family protein whose DNA-binding domain alone is capable of little or no transcription activation.
Acknowledgments
We would like to thank Peter Gegenheimer for use of his Cyclone storage phosphor system and members of the laboratory of William Picking, especially Marianela Espina and Andrew Olive, for help with Western blots and image analysis. Western blot images were obtained on an Odyssey Infrared Imager through collaboration with LI-COR, Inc.
This work was supported by NIH grant GM55099 from the National Institute of General Medical Sciences and NIH grant P20 RR17708 from the Institutional Development Award (IDeA) Program of the National Center for Research Resources (both to S.M.E.), NIH grant P20 RR016475 (to J.F.), and the Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research (to D.J.J.).
Footnotes
Published ahead of print on 18 May 2007.
REFERENCES
- 1.Backman, K., Y.-M. Chen, and B. Magasanik. 1981. Physical and genetic characterization of the gln A-glnG region of the Escherichia coli chromosome. Proc. Natl. Acad. Sci. USA 78:3743-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badia, J., L. Baldoma, J. Aguilar, and A. Boronat. 1989. Identification of the rhaA, rhaB and rhaD gene products from Escherichia coli K-12. FEMS Microbiol. Lett. 65:253-258. [DOI] [PubMed] [Google Scholar]
- 3.Bartolome, B., Y. Jubete, E. Martinez, and F. de la Cruz. 1991. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102:75-78. [DOI] [PubMed] [Google Scholar]
- 4.Bhende, P. M., and S. M. Egan. 1999. Amino acid-DNA contacts by RhaS: an AraC family transcription activator. J. Bacteriol. 181:5185-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhende, P. M., and S. M. Egan. 2000. Genetic evidence that transcription activation by RhaS involves specific amino acid contacts with sigma 70. J. Bacteriol. 182:4959-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Bustos, S. A., and R. F. Schleif. 1993. Functional domains of the AraC protein. Proc. Natl. Acad. Sci. USA 90:5638-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carra, J. H., and R. F. Schleif. 1993. Variation of half-site organization and DNA looping by AraC protein. EMBO J. 12:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caswell, R., C. Webster, and S. Busby. 1992. Studies on the binding of the Escherichia coli MelR transcription activator protein to operator sequences at the MelAB promoter. Biochem. J. 287:501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan, S. M. 2002. Growing repertoire of AraC/XylS activators. J. Bacteriol. 184:5529-5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan, S. M., and R. F. Schleif. 1993. A regulatory cascade in the induction of rhaBAD. J. Mol. Biol. 234:87-98. [DOI] [PubMed] [Google Scholar]
- 12.Egan, S. M., and R. F. Schleif. 1994. DNA-dependent renaturation of an insoluble DNA binding protein. Identification of the RhaS binding site at rhaBAD. J. Mol. Biol. 243:821-829. [DOI] [PubMed] [Google Scholar]
- 13.Gallegos, M.-T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh, M., and R. F. Schleif. 2001. Biophysical evidence of arm-domain interactions in AraC. Anal. Biochem. 295:107-112. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman, M. E., and M. B. Yarmolinsky. 1968. The integration and excision of the bacteriophage lambda genome. Cold Spring Harbor Symp. Quant. Biol. 33:735-747. [DOI] [PubMed] [Google Scholar]
- 16.Holcroft, C. C., and S. M. Egan. 2000. Interdependence of activation at rhaSR by cyclic AMP receptor protein, the RNA polymerase alpha subunit C-terminal domain, and RhaR. J. Bacteriol. 182:6774-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holcroft, C. C., and S. M. Egan. 2000. Roles of cyclic AMP receptor protein and the carboxyl-terminal domain of the α subunit in transcription activation of the Escherichia coli rhaBAD operon. J. Bacteriol. 182:3529-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard, V. J., T. A. Belyaeva, S. J. Busby, and E. I. Hyde. 2002. DNA binding of the transcription activator protein MelR from Escherichia coli and its C-terminal domain. Nucleic Acids Res. 30:2692-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaldalu, N., U. Toots, V. de Lorenzo, and M. Ustav. 2000. Functional domains of the TOL plasmid transcription factor XylS. J. Bacteriol. 182:1118-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon, H. J., M. H. J. Bennik, B. Demple, and T. Ellenberger. 2000. Crystal structure of the Escherichia coli Rob transcription factor in complex with DNA. Nat. Struct. Biol. 7:424-430. [DOI] [PubMed] [Google Scholar]
- 21.Martin, R. G., and J. L. Rosner. 2001. The AraC transcriptional activators. Curr. Opin. Microbiol. 4:132-137. [DOI] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 23.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poore, C. A., C. Coker, J. D. Dattelbaum, and H. L. T. Mobley. 2001. Identification of the domains of UreR, an AraC-like transcriptional regulator of the urease gene cluster in Proteus mirabilis. J. Bacteriol. 183:4526-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Power, J. 1967. The L-rhamnose genetic system in Escherichia coli K-12. Genetics 55:557-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prouty, M. G., C. R. Osorio, and K. E. Klose. 2005. Characterization of functional domains of the Vibrio cholerae virulence regulator ToxT. Mol. Microbiol. 58:1143-1156. [DOI] [PubMed] [Google Scholar]
- 27.Reed, W. L., and R. F. Schleif. 1999. Hemiplegic mutations in AraC protein. J. Mol. Biol. 294:417-425. [DOI] [PubMed] [Google Scholar]
- 28.Rhee, S., R. G. Martin, J. L. Rosner, and D. R. Davies. 1998. A novel DNA-binding motif in MarA: the first structure for an AraC family transcriptional activator. Proc. Natl. Acad. Sci. USA 95:10413-10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saviola, B., R. Seabold, and R. F. Schleif. 1998. Arm-domain interactions in AraC. J. Mol. Biol. 278:539-548. [DOI] [PubMed] [Google Scholar]
- 30.Schleif, R. 2000. Regulation of the L-arabinose operon of Escherichia coli. Trends Genet. 16:559-565. [DOI] [PubMed] [Google Scholar]
- 31.Seabold, R. R., and R. F. Schleif. 1998. Apo-AraC actively seeks to loop. J. Mol. Biol. 278:529-538. [DOI] [PubMed] [Google Scholar]
- 32.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 33.Soisson, S. M., B. MacDougall-Shackleton, R. Schleif, and C. Wolberger. 1997. Structural basis for ligand-regulated oligomerization of AraC. Science 276:421-425. [DOI] [PubMed] [Google Scholar]
- 34.Stewart, G. S., S. Lubinsky-Mink, C. G. Jackson, A. Cassel, and J. Kuhn. 1986. pHG165: a pBR322 copy number derivative of pUC8 for cloning and expression. Plasmid 15:172-181. [DOI] [PubMed] [Google Scholar]
- 35.Tate, C. G., J. A. R. Muiry, and P. J. F. Henderson. 1992. Mapping, cloning, expression, and sequencing of the rhaT gene which encodes a novel L-rhamnose-H+ transport protein in Salmonella typhimurium and Escherichia coli. J. Biol. Chem. 287:6923-6932. [PubMed] [Google Scholar]
- 36.Timmes, A., M. Rodgers, and R. Schleif. 2004. Biochemical and physiological properties of the DNA binding domain of AraC protein. J. Mol. Biol. 340:731-738. [DOI] [PubMed] [Google Scholar]
- 37.Tobin, J. F., and R. F. Schleif. 1987. Positive regulation of the Escherichia coli L-rhamnose operon is mediated by the products of tandemly repeated regulatory genes. J. Mol. Biol. 196:789-799. [DOI] [PubMed] [Google Scholar]
- 38.Tobin, J. F., and R. F. Schleif. 1990. Purification and properties of RhaR, the positive regulator of the L-rhamnose operons of Escherichia coli. J. Mol. Biol. 211:75-89. [DOI] [PubMed] [Google Scholar]
- 39.Tobin, J. F., and R. F. Schleif. 1990. Transcription from the rha operon psr promoter. J. Mol. Biol. 211:1-4. [DOI] [PubMed] [Google Scholar]
- 40.Via, P., J. Badia, L. Baldoma, N. Obradors, and J. Aguilar. 1996. Transcriptional regulation of the Escherichia coli rhaT gene. Microbiology 142:1833-1840. [DOI] [PubMed] [Google Scholar]
- 41.Wade, J. T., T. A. Belyaeva, E. I. Hyde, and S. J. Busby. 2000. Repression of the Escherichia coli melR promoter by MelR: evidence that efficient repression requires the formation of a repression loop. Mol. Microbiol. 36:223-229. [DOI] [PubMed] [Google Scholar]
- 42.Wickstrum, J. R., and S. M. Egan. 2004. Amino acid contacts between sigma 70 domain 4 and the transcription activators RhaS and RhaR. J. Bacteriol. 186:6277-6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wickstrum, J. R., and S. M. Egan. 2002. Ni+-affinity purification of untagged cyclic AMP receptor protein. BioTechniques 33:728-730. [DOI] [PubMed] [Google Scholar]
- 44.Wickstrum, J. R., T. J. Santangelo, and S. M. Egan. 2005. Cyclic AMP receptor protein and RhaR synergistically activate transcription from the l-rhamnose-responsive rhaSR promoter in Escherichia coli. J. Bacteriol. 187:6708-6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu, M., and R. Schleif. 2001. Mapping arm-DNA-binding domain interactions in AraC. J. Mol. Biol. 307:1001-1009. [DOI] [PubMed] [Google Scholar]
- 46.Wu, M., and R. Schleif. 2001. Strengthened arm-dimerization domain interactions in AraC. J. Biol. Chem. 276:2562-2564. [DOI] [PubMed] [Google Scholar]
- 47.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 48.Zhi, H., and D. J. Jin. 2003. Purification of highly-active and soluble Escherichia coli sigma 70 polypeptide overproduced at low temperature. Methods Enzymol. 370:174-180. [DOI] [PubMed] [Google Scholar]
- 49.Zhi, H., W. Yang, and D. J. Jin. 2003. Escherichia coli proteins eluted from mono Q chromatography, a final step during RNA polymerase purification procedure. Methods Enzymol. 370:291-300. [DOI] [PubMed] [Google Scholar]