Abstract
Sequences that mediate the initiation of transcription in Flavobacterium species are not well known. The majority of identified Flavobacterium promoter elements show homology to those of other members of the phylum Bacteroidetes, but not of proteobacteria, and they function poorly in Escherichia coli. In order to analyze the Flavobacterium promoter structure systematically, we investigated the −33 consensus element, −7 consensus element, and spacer length of the Flavobacterium ompA promoter by measuring the effects of site-directed mutations on promoter activity. The nonconserved sequences in the spacer region and in regions close to the consensus motifs were randomized in order to determine their importance for promoter activity. Most of the base substitutions in these regions caused large decreases in promoter activity. The optimal −33/−7 motifs (TTTG/TANNTTTG) were identical to Bacteroides fragilis σABfr consensus −33/−7 promoter elements but lacked similarity to the E. coli σ70 promoter elements. The length of the spacer separating the −33 and −7 motifs of the ompA promoter also had a pronounced effect on promoter activity, with 19 bp being optimal. In addition to the consensus promoter elements and spacer length, the GC content of the core promoter sequences had a pronounced effect on Flavobacterium promoter activity. This information was used to conduct a scan of the Flavobacterium johnsoniae and B. fragilis genomes for putative promoters, resulting in 188 hits in B. fragilis and 109 hits in F. johnsoniae.
Studies of molecular regulatory mechanisms in bacteria of the phylum Bacteroidetes are underdeveloped because of a lack of genetic tools and the poorly characterized genetic elements (2, 40, 51). This is in spite of the ubiquitous distribution of members of the phylum Bacteroidetes (6, 14, 30, 31); their importance as pathogens of fish (15, 42), birds (54), and humans (7); and their roles in transforming high-molecular-weight carbon compounds in soil and aquatic environments (14). Most research on gene regulation in this phylum has targeted Bacteroides (4, 11, 32, 49, 55, 57) and, to a lesser extent, Porphyromonas (27, 43, 57, 60). Early examination of the transcriptional signals in Bacteroides fragilis indicated that its promoters exhibited features distinct from those of proteobacteria (4, 55). Similar promoter structures have also been reported in other members of the phylum Bacteroidetes, such as Porphyromonas gingivalis (27), Pedobacter heparinus (formerly Flavobacterium heparinum) (5), and Prevotella loescheii (37). Key findings were a unique pair of −33/−7 element consensus sequences (TTTG/TANNTTTG) separated by a spacer of variable length (generally 19 to 21 nucleotides) that are possibly involved in promoter recognition in members of the phylum Bacteroidetes and that these elements are not homologous to Escherichia coli σ70 promoters (21). Genome-wide analysis of the sigma factors from 240 bacteria revealed that, unlike proteobacteria, members of the phylum Bacteroidetes lack typical σ70 sequences, which account for transcriptional initiation of genes involved in basic metabolic functions of E. coli (59). Further investigation of the Bacteroides transcriptional machinery by reconstitution of homologous and heterologous RNA polymerase holoenzymes with Bacteroides, as well as E. coli, promoters allowed the identification of an unusual primary sigma factor that is confined to the phyla Bacteroidetes and Chlorobi (55). This primary sigma factor, σABfr, with some unique structural and functional characteristics, only recognized promoters from members of the phylum Bacteroidetes and thus explains the lack of functionality of E. coli promoters (55). Given that a similar sigma factor (72.1% identity) was also found in Flavobacterium johnsoniae (55), we believe that σABfr-like promoter sequences must also exist in members of the genus Flavobacterium.
Recently, we isolated and characterized strong Flavobacterium promoters by using a promoter trap system (12, 13). The analysis with mapped transcriptional start points (TSPs) revealed that putative consensus promoter sequences resemble those of Bacteroides, e.g., −7 (TANNTTTG) and −33 promoter (TTG) motifs, and the spacer region between them varied from 17 to 23 bp (12, 13). However, the optimal transcription initiation signals have not been defined. Here, the promoter for ompA (outer membrane protein A) was chosen as a model system because it is one of the strongest promoters of Flavobacterium known (S. Chen et al., unpublished data) and it resembles the typical Bacteroidetes promoter structure. The purposes of this study were to determine the relative nucleotide preference at each position in the −33 and −7 regions by site-directed mutagenesis and to analyze how variations in the spacer length and the nonconsensus core promoter sequences contribute to promoter strength.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The plasmids used in this study are listed in Table 1. Flavobacterium hibernum strain W22 was isolated from a water-filled tree hole in an American beech tree located near the Michigan State University campus. F. johnsoniae UW101 (ATCC 17061) was obtained from Mark McBride of the University of Wisconsin—Milwaukee. Strains of E. coli were routinely grown in Luria broth (LB) or on LB agar plates at 37°C. Flavobacterium strains were grown at 26°C in Casitone yeast extract (CYE) medium as previously described (40). Liquid cultures were incubated with shaking at 200 rpm. Solid CYE medium contained 20 g of agar per liter. Ampicillin was added (100 μg/ml) for plasmid selection in E. coli, and erythromycin was added (100 μg/ml) for plasmid selection in Flavobacterium.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristics and/or plasmid constructiona | Reference or source |
|---|---|---|
| pGEM-T Easy vector | Cloning vector; Apr | Promega |
| pCP29 | E. coli-Flavobacterium shuttle plasmid; Apr (Cfxr Emr) | 28 |
| pSCH03 | Promoter trap plasmid; promoterless gfpmut3 gene in pCP23; Apr (Tcr) | 12 |
| PSCH143 | E. coli-Flavobacterium shuttle plasmid; Apr (Emr) | This study |
| PSCH144 | Promoter trap plasmid; promoterless gfpmut3 gene in pSCH143; Apr (Emr) | This study |
| pFj29 | ompA trapped in pSCH144; Apr (Emr) | Unpublished data |
| OmpA.−54 | 356-bp fragment of ompA promoter fused to gfpmut3; Apr (Emr) | This study |
| OmpA.−48 | 350-bp fragment of ompA promoter fused to gfpmut3; Apr (Emr) | This study |
| OmpA.−32 | 334-bp fragment of ompA promoter fused to gfpmut3; Apr (Emr) | This study |
| OmpA.−5 | 307-bp fragment of ompA promoter fused to gfpmut3; Apr (Emr) | This study |
| OMPS-7 | Three-base change from TTT to AAA at positions −7 to −9; Apr (Emr) | This study |
| OMPS-33 | Three-base change from TTG to AAC at positions −33 to −35; Apr (Emr) | This study |
| OMPM-6A | Single-base change from G to A at position −6; Apr (Emr) | This study |
| OMPM-6T | Single-base change from G to T at position −6; Apr (Emr) | This study |
| OMPM-6C | Single-base change from G to C at position −6; Apr (Emr) | This study |
| OMPM-7A | Single-base change from T to A at position −7; Apr (Emr) | This study |
| OMPM-7G | Single-base change from T to G at position −7; Apr (Emr) | This study |
| OMPM-7C | Single-base change from T to C at position −7; Apr (Emr) | This study |
| OMPM-8A | Single-base change from T to A at position −8; Apr (Emr) | This study |
| OMPM-8G | Single-base change from T to G at position −8; Apr (Emr) | This study |
| OMPM-8C | Single-base change from T to C at position −8; Apr (Emr) | This study |
| OMPM-9A | Single-base change from T to A at position −9; Apr (Emr) | This study |
| OMPM-9G | Single-base change from T to G at position −9; Apr (Emr) | This study |
| OMPM-9C | Single-base change from T to C at position −9; Apr (Emr) | This study |
| OMPM-10A | Single-base change from T to A at position −10; Apr (Emr) | This study |
| OMPM-10G | Single-base change from T to G at position −10; Apr (Emr) | This study |
| OMPM-10C | Single-base change from T to C at position −10; Apr (Emr) | This study |
| OMPM-11A | Single-base change from C to A at position −11; Apr (Emr) | This study |
| OMPM-11G | Single-base change from C to G at position −11; Apr (Emr) | This study |
| OMPM-11T | Single-base change from C to T at position −11; Apr (Emr) | This study |
| OMPM-12T | Single-base change from A to T at position −12; Apr (Emr) | This study |
| OMPM-12G | Single-base change from A to G at position −12; Apr (Emr) | This study |
| OMPM-12C | Single-base change from A to C at position −12; Apr (Emr) | This study |
| OMPM-13A | Single-base change from T to A at position −13; Apr (Emr) | This study |
| OMPM-13G | Single-base change from T to G at position −13; Apr (Emr) | This study |
| OMPM-13C | Single-base change from T to C at position −13; Apr (Emr) | This study |
| OMPM-33A | Single-base change from G to A at position −33; Apr (Emr) | This study |
| OMPM-33T | Single-base change from G to T at position −33; Apr (Emr) | This study |
| OMPM-33C | Single-base change from G to C at position −33; Apr (Emr) | This study |
| OMPM-34A | Single-base change from T to A at position −34; Apr (Emr) | This study |
| OMPM-34G | Single-base change from T to G at position −34; Apr (Emr) | This study |
| OMPM-34C | Single-base change from T to C at position −34; Apr (Emr) | This study |
| OMPM-35A | Single-base change from T to A at position −35; Apr (Emr) | This study |
| OMPM-35G | Single-base change from T to G at position −35; Apr (Emr) | This study |
| OMPM-35C | Single-base change from T to C at position −35; Apr (Emr) | This study |
| OMPM-36A | Single-base change from T to A at position −36; Apr (Emr) | This study |
| OMPM-36G | Single-base change from T to G at position −36; Apr (Emr) | This study |
| OMPM-36C | Single-base change from T to C at position −36; Apr (Emr) | This study |
| OMPMS17 | Derivate of ompA promoter containing 17-bp spacer | This study |
| OMPMS18 | Derivate of ompA promoter containing 18-bp spacer | This study |
| OMPMS20 | Derivate of ompA promoter containing 20-bp spacer | This study |
| OMPMS21 | Derivate of ompA promoter containing 21-bp spacer | This study |
| OMPMS22 | Derivate of ompA promoter containing 22-bp spacer | This study |
| OMPMS23 | Derivate of ompA promoter containing 23-bp spacer | This study |
Antibiotic resistance phenotypes: ampicillin, Apr; tetracycline, Tcr; erythromycin, Emr; cefoxitin, Cfxr. Unless indicated otherwise, antibiotic resistance phenotypes are those expressed in E. coli. Antibiotic resistances in parentheses are those expressed in Flavobacterium strains but not in E. coli.
Recombinant DNA methods.
Genomic DNA extractions were performed with a genomic DNA extraction kit (Promega, Madison, WI), and plasmid DNA was purified with the QIAprep spin miniprep kit (QIAGEN, Germantown, MD). DNA ligations, restriction endonuclease digestions, and agarose gel electrophoresis were performed according to standard techniques (48). DNA transformation experiments with E. coli were carried out by the calcium chloride method and with Flavobacterium strains by electroporation as described previously by Chen et al. (12). PCR amplifications were performed with the Failsafe PCR system (Epicenter Technology, Madison, WI). PCR products were separated on 0.7 to 1.0% (wt/vol) agarose gels, and the bands were purified with the QIAquick gel extraction system (QIAGEN). Ligation mixtures were transformed into E. coli JM109 (Promega), and transformants were plated on LB agar plates with ampicillin for selection. Resistant colonies were isolated and screened for the presence of plasmid DNA. The plasmids were then electroporated into Flavobacterium strains.
The pCP29 vector (Table 1) was digested with BamHI and SalI in order to remove the cfxA (cefoxitin) antibiotic resistance gene because it was of no use in this study. The digested vector was blunt ended with T4 DNA polymerase, gel purified, and self-ligated. The constructs were transformed into E. coli JM109, leading to plasmid pSCH143. Promoterless gfp including a multiple cloning site and a ribosome binding site (RBS) on its 5′ end was released from plasmid pSCH03 (12) by KpnI and SphI and ligated into the same sites on pSCH143, resulting in a promoter-probe vector herein designated pSCH144. This plasmid was used for isolating strong promoters from F. johnsoniae by the promoter trap technique as described in reference 12. One of the derivatives of pSCH144, designated plasmid pFj29 (Chen et al., unpublished) and exhibiting high promoter activity in F. johnsoniae, was chosen for further analysis. Sequence analysis of pSCH144 showed a genomic DNA fragment with a size of 734 bp containing part of the ompA gene transcriptionally fused with a gfp reporter.
To analyze the structure of the ompA promoter in detail, deletion derivatives of the promoter found upstream of ompA on plasmid pFj29 were constructed. The positions of N-terminal primers on the 5′ end of the ompA region were as follows, with the TSP assigned the +1 position: D1, −54 bp; D2, −48 bp; D3, −32 bp; D4, −5 bp. Amplification of the deletion derivatives was performed with N-terminal primers containing an N-terminal KpnI site (Table 2) and C-terminal primer OMPAR with a BamHI site (Table 2) complementary to the 3′ end of ompA at position +302 bp. The PCR products were inserted into the T Easy vector (Promega) and sequenced. The inserts were released from this vector by KpnI and BamHI and inserted into the same sites of promoter-probe vector pSCH143 to create the deletion plasmid series OmpA.−54, OmpA.−48, OmpA.−32, and OmpA.−5 (Table 2).
TABLE 2.
Primers used in this study
| Primer | Sequencea |
|---|---|
| MS-7 | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT AAA GCT TG 3′ |
| MS-33 | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TAA CTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-6A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TTT ACT TG 3′ |
| M-6T | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TTT TCT TG 3′ |
| M-6C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TTT CCT TG 3′ |
| M-7A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TTA GCT TG 3′ |
| M-7G | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TTG GCT TG 3′ |
| M-7C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TTC GCT TG 3′ |
| M-8A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TAT GCT TG 3′ |
| M-8G | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TGT GCT TG 3′ |
| M-8C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TCT GCT TG 3′ |
| M-9A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT ATT GCT TG 3′ |
| M-9G | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT GTT GCT TG 3′ |
| M-9C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT CTT GCT TG 3′ |
| M-10A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACA TTT GCT TG 3′ |
| M-10G | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACG TTT GCT TG 3′ |
| M-10C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACC TTT GCT TG 3′ |
| M-11A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT AAT TTT GCT TG 3′ |
| M-11G | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT AGT TTT GCT TG 3′ |
| M-11T | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ATT TTT GCT TG 3′ |
| M-12T | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT TCT TTT GCT TG 3′ |
| M-12G | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT GCT TTT GCT TG 3′ |
| M-12C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT CCT TTT GCT TG 3′ |
| M-13A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTA ACT TTT GCT TG 3′ |
| M-13G | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTG ACT TTT GCT TG 3′ |
| M-13C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTC ACT TTT GCT TG 3′ |
| M-33A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT ATA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-33T | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT TTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-33C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT CTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-34A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTA GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-34G | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTG GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-34C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTC GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-35A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TAT GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-35G | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TGT GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-35C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TCT GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-36A | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT ATT GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-36G | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT GTT GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| M-36C | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT CTT GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| OmpA.−54 | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| OmpA.−48 | 5′ CGG GGT ACC TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT GGT GTT ACT TTT GCT TG 3′ |
| OmpA.−32 | 5′ CGG GGT ACC TAT TTA AAA AAT TTG GTG TTA CTT TTG CTT G 3′ |
| OmpA.−5 | 5′ CGG GGT ACC CTT GTA ATT AAC TAA ATT G 3′ |
| S17 | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TGG TGT TAC TTT TGC TTG 3′ |
| S18 | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTG GTG TTA CTT TTG CTT G 3′ |
| S20 | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT TTG GTG TTA CTT TTG CTT G 3′ |
| S21 | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT TTT GGT GTT ACT TTT GCT TG 3′ |
| S21 | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT TTT TGG TGT TAC TTT TGC TTG 3′ |
| S22 | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT TTT TTG GTG TTA CTT TTG CTT G 3′ |
| S23 | 5′ CGG GGT ACC TTT TTT TTA ACA TTT GAT TTT GTA TTT AAA AAA TTT TTT TTT GGT GTT ACT TTT GCT TG 3′ |
| Random | 5′ CGG GGT ACC TTT TTT TTA ACA TNN NNN NTT GNN NNN NNN NNN NNN NNN NNT ANN TTT GNN NNN AAT TAA CTA AAT TG 3′ |
| OMPAR | 5′ CGC GGA TCC TTG TAA ACC AAC TGA GAA ACC 3′ |
Restriction sites used for cloning of PCR amplicons are underlined.
For mutagenesis of the −33 and −7 elements, alteration of the spacer length between the two conserved elements, and construction of the randomized promoter library, we used PCR protocols described elsewhere (12). The desired mutation(s) was introduced on an oligonucleotide primer with a KpnI site (Table 2). OMPAR (Table 2) was used as the reverse primer. Each of the mutant templates contained the ompA promoter fragment comprising nucleotides encompassing −54 to +302 bp. Three base substitutions were introduced into the −33 region by changing the sequence from −33 to −35 (TTG → AAC) or into the −7 region by altering the sequence from −14 to −10 (TTT→ AAA) as the scanning tests. Next, we made 24 single-base mutations in the −7 region (from −6 to −13) and 12 single-base mutations in the −33 region (from −33 to −36). Spacer length alterations ranging from 17 to 23 bp, as well as the randomized mutations in the spacer, were generated by similar PCR-based methods. The fragments with mutant promoters were cloned into the T Easy vector for sequence verification. Mutant promoters were released with KpnI and BamHI and inserted into the same sites of vector pSCH144. The constructs were first propagated in E. coli. Finally, each of the verified mutant plasmids was introduced into Flavobacterium strains by electroporation.
Determination of promoter activity in Flavobacterium and in E. coli.
Promoter strength was evaluated by measurement of gfp reporter expression. Cells harboring a sequence-confirmed construct(s) were cultured in CYE medium and quantitative analysis of green fluorescent protein (GFP) production was performed with a SpectraMax M5 spectrophotometer (Molecular Devices, Sunnyvale, CA). Aliquots (200 μl) of cultures were centrifuged, washed with 0.1 M phosphate-buffered saline (pH 7.4), diluted in the same buffer to an optical density at 600 nm of ∼0.4, and subjected to fluorescence determination in a 96-well plate (Costar, Corning, NY) at an excitation wavelength of 490 nm, an emission wavelength of 530 nm, and a cutoff of 515 nm at 22°C. Cell densities were determined at 600 nm. To ensure that the values recorded were due to GFP, cultures of untransformed strains were used as the blanks for calculation of relative units of fluorescence.
RNA isolation.
Flavobacterium cells (2 ml) from exponentially growing cultures (turbidity at 650 nm of 0.3 to 0.4) were stabilized with 2 volumes of RNAprotect Bacteria Reagent (QIAGEN, Valencia, CA) for 5 to 10 min. Total RNA was extracted by using the RNeasy kit (QIAGEN) according to the protocol of the manufacturer. Following extraction, total RNA was treated with DNase I. The DNase I was later heat inactivated at 70°C for 15 min. To concentrate the samples, total RNA was precipitated with ethanol and resuspended in 30 μl of RNase-free water. Samples were stored at −80°C.
TSP.
The transcriptional start site was determined by using the 5′ rapid amplification of cDNA ends system as recommended by the supplier (Clontech, Palo Alto, CA), with 3 μg of total RNA (DNA free). A gfpmut3 gene-specific primer (gfpSphIR) was used to initiate first-strand cDNA synthesis for 1.5 h at 42°C. Small aliquots of the above cDNA as the template were amplified with SMART PCR primer USP and gene-specific primers (Table 2). The PCR products were cloned into the T Easy vector according to standard procedures and sequenced.
DNA sequence analysis.
Each construct was sequenced by the dideoxy termination method with an automated sequencing system (Applied Biosystems, Foster City, CA). GenBank database searches were carried out with the National Center for Biotechnology Information BLAST web server (http://www.ncbi.nlm.nih.gov/BLAST). Multiple sequence alignments were carried out with the ClustalW program (http://www.ebi.ac.uk/clustalw/) and later adjusted manually. Weblogo was used to create the sequence logos (http://weblogo.berkeley.edu/). The F. johnsoniae UW101, B. fragilis NCTC9434, and E. coli K-12 genomic sequences were obtained from GenBank (AAPM01000000 [version 17-JAN-2007], NC003228, and NC0009134, respectively). Genome scale DNA pattern searches were performed with PatScan (http://www-unix.mcs.anl.gov/compbio/PatScan/) (18) and regulatory sequence analysis tools (RSAT; http://rsat.scmbb.ulb.ac.be/rsat/) (53).
Nucleotide sequence accession number.
The sequence of the trapped genomic fragment in pSCH144 was deposited in GenBank and assigned accession no. EF571005.
RESULTS
Mapping of the ompA promoter.
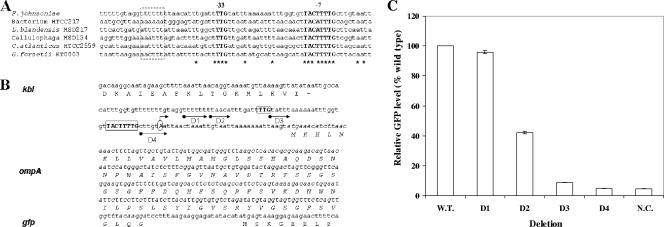
The TSP of the ompA gene, as determined by SMART rapid amplification of cDNA ends, was an A nucleotide 32 bp upstream from the start codon of ompA in F. johnsoniae (Fig. 1). The putative −33 (TTG) and −7 (TACTTTTG) elements matching the consensus Bacteroidetes promoter sequences (4) were found 32 and 5 bp upstream of the TSP, respectively. The spacer length between the −7 and −33 regions was 19 bp. Similar promoter elements were found within 50 bp upstream of the ompA genes from other flavobacteria, indicating that the regulatory regions of ompA genes are conserved (Fig. 1). A serial deletion on the 5′ end of the ompA promoter was conducted to determine the regions required for maximal ompA gene expression in Flavobacterium. The results (Fig. 1) showed that there was no significant difference in the levels of GFP production between the wild type (pFj29) and D1 (OmpA.−54). Deletion of TTTTTT upstream of the −33 region (D2, OmpA.−48, Fig. 1) decreased promoter activity by more than 50% compared to that of the wild type. D3 (OmpA.−32) exhibited a ninefold lower level of fluorescence compared to the wild type. D3 (OmpA.−32) contains a deletion that originates at bp −32 and thus has the −33 region (TTG) deleted. A further deletion (D4, OmpA.−5) that removed the entire −7 region (TACTTTTG) abolished promoter activity to the negative control level. These data indicated that at least 48 bp upstream of the TSP in the ompA promoter region are required for maximal ompA expression in log-phase Flavobacterium. Substitutions of 3 bp in either the −33 (−35TTG−33 → AAC) or the −7 (−14TTT−10 → AAA) conserved element of the ompA promoter eliminated its ability to drive GFP production in both F. hibernum strain W22 and F. johnsoniae (data not shown), indicating that these motifs are critical for Flavobacterium promoter activity.
FIG. 1.
Mapping of the ompA promoter. (A) Alignment analysis of putative ompA promoters from various flavobacteria. The accession numbers of ompA genes for F. johnsoniae, Flavobacteriales bacterium HTCC2170, “Leeuwenhoekiella blandensis” MED217, Cellulophaga sp. strain MED134, Croceibacter atlanticus HTCC2559, and “Gramella forsetii” KT0803 are AAPM01000000, ZP_01105836, NZ_AANC01000009, NZ_AAMZ01000002, AAMP00000000, and NC_008571, respectively. The putative UP sequences are boxed. The −33 and −7 motifs are capitalized and in boldface. Identical nucleotides are shown with asterisks. (B) Organization of the wild-type ompA promoter sequence used for deletion assay. Only the 3′ end of the kbl gene (2-amino-3-ketobutyrate coenzyme A ligase) is shown. The sequences of ompA transcriptionally fused with gfp are in italics. The putative −7 and −33 motifs are capitalized and boxed. The capital A with a curved arrow is the TSP. The forward dot arrows below the sequence represent positions of the deletion N-terminal primers. (C) Quantitative analysis of gfpmut3 expression of the ompA promoter deletion vector series in F. johnsoniae including the wild type (W.T.) as a positive control (pFj29) and a negative control (N.C.) (pSCH144). Reactions were performed in triplicate, and standard deviations are marked by error bars. Results for each deletion promoter clone have been normalized to a promoter activity of 100% for the wild-type ompA clone (Fj29).
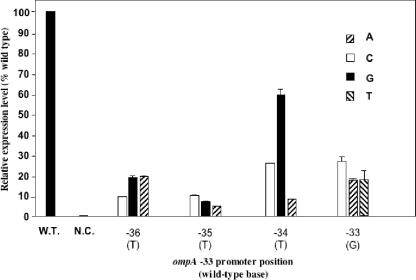
Point mutations in the −33 promoter region.
We next examined the effects of single-base substitutions in the proposed −33 region (−35TTG−33). At each position, we tested each of the three possible substitutions separately (Fig. 2). Each of the single-base alterations at positions −33, −34, and −35 resulted in a decrease in promoter activity (Fig. 2). The most pronounced effect in the core −33 promoter element was at position −35. Base substitution at this position caused gfp expression to drop below 5% of the wild-type level. Substitution −34T→G caused the most modest reduction of GFP production, to 63% of the wild-type level. This could be attributed to the fact that a new −33 consensus motif (−36TTG−34) was created by the mutation (T→G). The T nucleotide at position −36 has been proposed as the fourth conserved base (∼80%) in B. fragilis (4), but it was less conserved (∼35%) in Flavobacterium strains (12). Mutational analysis showed that replacement of −36T with any of the other three bases decreased the promoter activity to approximately 20% of the wild-type level.
FIG. 2.
Effects of single-base-pair substitutions on the ompA promoter −33 region in F. johnsoniae. Promoter activities were determined as described in Materials and Methods. Reactions were performed in triplicate, and standard deviations are marked by error bars. Results for each deletion promoter clone were normalized to a promoter activity of 100% for the wild-type (W.T.) ompA clone (Fj29). The sequences and the numbers indicate the base pairs at the specific positions in the wild-type ompA promoter. N.C., negative control.
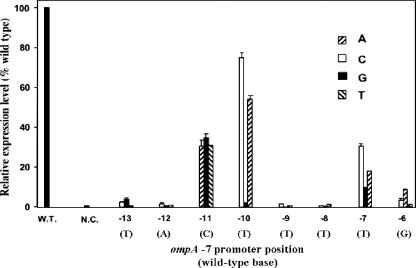
Point mutations in the −7 promoter region.
To determine whether the TACTTTTG sequence of the ompA promoter (positions −6 to −13) functions as an important element, we investigated how specific mutations in this sequence affect promoter activity. Most of the base substitutions within the core conserved −7 motif (TANNTTTG) produced dramatic effects on promoter activity (Fig. 3). Any substitutions at positions −8, −9, and −12 nearly abolished promoter activity, reducing it to the negative control level, indicating that the bases in these specific positions are extremely important. Substitutions at −6 caused more-than-10-fold reductions in promoter activity (Fig. 3). In contrast, the effects of substitutions at less-conserved positions −11 and −10 were generally more modest, with the exception of the T-to-G mutation at −10, which abolished promoter activity (Fig. 3).
FIG. 3.
Effects of single-base-pair substitutions on the ompA promoter −7 region in F. johnsoniae. Promoter activities were determined as described in Materials and Methods. Reactions were performed in triplicate, and standard deviations are marked by error bars. Results for each deletion promoter clone were normalized to a promoter activity of 100% for the wild-type (W.T.) ompA clone (Fj29). The sequences and the numbers indicate the base pairs at the specific positions in the wild-type ompA promoter. N.C., negative control.
Determination of optimum spacer length between the −33 and −7 sequences of the Flavobacterium ompA promoter.
Alignment analysis of promoters in Flavobacterium showed that the spacer length between the −7 and −33 regions varied in a broad range of 17 to 23 bp (12). In E. coli, deviation from optimal spacer length by insertion or deletion of only one base impaired promoter function (56). In Flavobacterium promoters, the optimal spacer length has not been clearly determined. We altered the spacer length of the ompA promoter by site-directed mutagenesis by inserting or deleting T nucleotides within the promoter spacer as shown in Table 3. We systematically tested mutant ompA promoters with spacers of 17, 18, 19, 20, 21, 22, and 23 bp and compared the fluorescence of GFP produced to that of cells harboring the wild-type promoter. In F. johnsoniae, any insertions or deletions of spacer bases dramatically decreased GFP production, indicating that the optimal promoter spacer length in Flavobacterium strains is 19 bp (Table 3). Similar effects of spacer length on promoter activity were recorded in F. hibernum. The F. johnsoniae ompA promoter was inefficient in E. coli and did not exhibit significant variation in strength even if the spacer length was decreased to 17 bp, which is the length observed in most of the σ70 promoters (21).
TABLE 3.
Effect of ompA promoter spacer length on promoter activity in E. coli, F. johnsoniae, and F. hibernum
| Clone | Promoter structurea | Relative promoter activityd
|
||
|---|---|---|---|---|
| E. coli | F. johnsoniae | F. hibernum | ||
| OmpA 17 | TTGATTTTGTATTTAAAAAATGGTGTTACTTTTGCTTGT | 282 ± 23 (91) | 831 ± 47 (10) | 160 ± 9 (3.9) |
| OmpA 18 | TTGATTTTGTATTTAAAAAATTGGTGTTACTTTTGCTTGT | 284 ± 26 (92) | 946 ± 17 (11) | 996 ± 12 (25) |
| OmpA 20 | TTGATTTTGTATTTAAAAAATTTTGGTGTTACTTTTGCTTGT | 285 ± 14 (92) | 1,932 ± 72 (23) | 901 ± 17 (22) |
| OmpA 21 | TTGATTTTGTATTTAAAAAATTTTTGGTGTTACTTTTGCTTGT | 301 ± 14 (98) | 1,256 ± 43 (15) | 835 ± 13 (21) |
| OmpA 22 | TTGATTTTGTATTTAAAAAATTTTTTGGTGTTACTTTTGCTTGT | 279 ± 11 (91) | 343 ± 2 (4.0) | 448 ± 7 (11) |
| OmpA 23 | TTGATTTTGTATTTAAAAAATTTTTTTGGTGTTACTTTTGCTTGT | 284 ± 19 (92) | 300 ± 3 (3.5) | 322 ± 3 (7.9) |
| Wild type | TTGATTTTGTATTTAAAAAATTTGGTGTTACTTTTGCTTGT | 311 ± 31 (100) | 8,479 ± 43 (100) | 4,088 ± 73 (100) |
| Negative controlb | NAc | 197 ± 10 (64) | 57 ± 2 (0.7) | 36 ± 3 (0.9) |
The −33 (left) and −7 (right) sequences are in boldface. The spacer length was varied by inserting or deleting a T nucleotide(s) in the spacer region of the ompA promoter.
Strains carrying pSCH144 (promoterless gfp) were used as a negative control.
NA, not applicable.
Relative GFP fluorescence in different strains was determined as described in Materials and Methods, and the promoter activity was normalized to that of the wild-type ompA promoter clone (defined as 100%), as shown in parentheses. Triplicate samples were used, and the standard deviations are shown.
Mutational analysis of the spacer and the sequences close to the −33 and −7 consensus motifs.
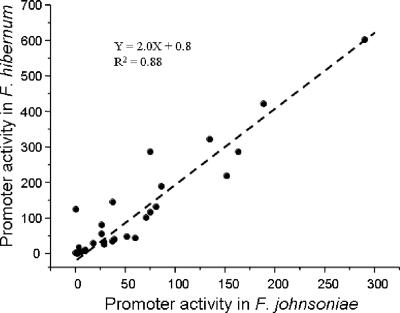
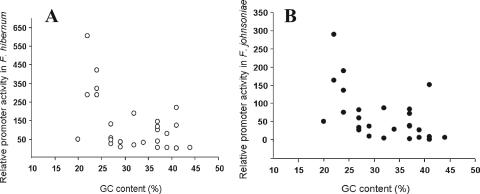
To determine the importance of sequences adjacent to the −33 and −7 consensus regions, a synthetic promoter library was constructed. Twenty-seven plasmids carrying unique randomized sequences of the spacer were transformed into F. johnsoniae and F. hibernum. The relative fluorescence levels of log-phase E. coli, F. johnsoniae, and F. hibernum were determined as shown in Table 4. Promoter clone DL98 carried the same promoter sequence as the wild type. Twenty-seven clones with the intact −7/−33 promoter motifs exhibited different fluorescence intensities varying from 1- to 290-fold in F. johnsoniae and from 1.2- to 603-fold in F. hibernum compared to the respective negative controls. The strengths of individual promoters in F. johnsoniae and F. hibernum were strongly correlated (r = 0.94, df = 26, P < 0.001) (Fig. 4). The Flavobacterium promoter-like collections did not boost GFP production significantly in E. coli. This fact further supports the presumption that a sigma factor different from that of E. coli exists in members of the phylum Bacteroidetes. The randomized promoter collections carried sequences with various GC contents ranging from 22% to 44% (Table 4). Promoter clones with more AT-rich sequences (GC content less than the average GC content of 34%) showed a stronger ability to drive expression of the reporter gene in F. johnsoniae and F. hibernum (Fig. 5). It should be noted that the strength of the promoter varied significantly among promoters with the same GC content, indicating that the sequence in the less-conserved promoter regions was also influential in determining promoter strength.
TABLE 4.
Relative promoter strengths of various promoter collections in F. johnsoniae, F. hibernum, and E. coli
| Promoter | Sequencea | % GC | Relative promoter activity in arbitrary fluorescence units (n-fold change)d
|
||
|---|---|---|---|---|---|
| F. johnsoniae | F. hibernum | E. coli | |||
| Consensus | NNNNNNTTGNNNNNNNNNNNNNNNNNNNTANNTTTGNNNNN | ||||
| DL69 | TTAAGGTTGCGGGCGAGATGGTTTTATTTAGGTTTGGTTGA | 41 | 0 ± 0 (0) | 21 ± 1 (2.1) | 113 ± 4 (0.3) |
| DL15 | TGCTGGTTGTAGTGTCAGTAAATTTTGGTAAGTTTGTCCGG | 41 | 6 ± 1 (0.2)v | 1,248 ± 42 (124.8) | 265 ± 8 (0.8) |
| DL82 | GGGTTCTTGTCACGCGAGGTCATCATTTTATTTTTGTGCTT | 41 | 40 ± 1 (1.2) | 12 ± 1 (1.2) | 475 ± 3 (1.5) |
| DL65 | TTGCGGTTGTCCTATTGTTGGTTACTGATACATTTGTTATC | 37 | 81 ± 4 (2.4) | 29 ± 2 (2.9) | 542 ± 11 (1.7) |
| DL79 | TTTATGTTGTGACGTGTTGTCTCTGATTTAATTTTGGTGAA | 32 | 100 ± 2 (3) | 190 ± 4 (19.0) | 257 ± 7 (0.8) |
| DL63 | TGCATGTTGTAGAGTCTCTGCATCTAAGTAAATTTGTAGGG | 39 | 161 ± 2 (4.9) | 38 ± 2 (3.8) | 318 ± 7 (1) |
| DL05 | GGGGTATTGCTGTCTTAATAGACGGGTTTACCTTTGTACAG | 44 | 172 ± 3 (5.1) | 51 ± 1 (5.1) | 329 ± 7 (1) |
| DL53 | TACGTTTTGGTTTTTTGATATAAGCCGATACTTTTGTATGT | 29 | 323 ± 4 (9.6) | 73 ± 2 (7.3) | 490 ± 3 (1.5) |
| DL76 | TTGGTTTTGCTGGGTAATACATATTAAATACATTTGTAACT | 27 | 875 ± 82 (26.1) | 557 ± 4 (55.7) | 334 ± 17 (1) |
| DL19 | GGCGAGTTGGTTGAACGTATAATTTTAGTATTTTTGGCCAG | 39 | 881 ± 101 (26.2) | 808 ± 24 (80.8) | 499 ± 4 (1.5) |
| DL64 | TACGTTTTGTGCAAATTCTAATGATTTATATTTTTGTGGGT | 27 | 1,005 ± 31 (28.7) | 260 ± 4 (26.0) | 205 ± 4 (0.6) |
| DL52 | TCAGCGTTGTTATAGTGTTCAAGTTTTGTATTTTTGGTTGG | 34 | 963 ± 31 (28.7) | 317 ± 3 (31.7) | 525 ± 6 (1.6) |
| DL58 | TTCGGTTTGTTGTGAATATTTTTCTTGGTAAATTTGTGTGA | 29 | 1,238 ± 13 (36.9) | 351 ± 1 (35.1) | 110 ± 1 (0.3) |
| DL04 | TTCGCTTTGATATTTAGTCTATGGTCGGTATCTTTGTGATC | 37 | 1,246 ± 19 (37.2) | 1,453 ± 39 (145.3) | 1,180 ± 26 (3.6) |
| DL97 | ACGTTGTTGTTAAGGGATGGTTTTTTTGTATGTTTGCCTGA | 37 | 1,309 ± 85 (39) | 403 ± 9 (40.3) | 541 ± 7 (1.7) |
| DL88 | TTATATTTGAGATTAGGTATAATCTTATTATTTTTGCTGTT | 20 | 1,726 ± 52 (51.5) | 479 ± 4 (47.9) | 352 ± 8 (1.1) |
| DL06 | CTTATTTTGCTTATTGTTTTCTAGTTAGTACTTTTGTTCGT | 27 | 594 ± 0 (59.8) | 439 ± 4 (43.9) | 324 ± 2 (1) |
| DL16 | TAGATCTTGCTGTTTGGGATAGAATTAGTAAATTTGCGGGT | 37 | 2,371 ± 31 (70.7) | 1,016 ± 18 (101.6) | 519 ± 10 (1.6) |
| DL29 | GAATTTTTGTTTTTTGTCGTGAAATTTGTATATTTGTAGGT | 24 | 2,512 ± 33 (74.9) | 2,870 ± 91 (287.0) | 298 ± 12 (0.9) |
| DL84 | TACCTTTTGGATTCTGGCCGTTTTTGAGTATGTTTGTATTG | 37 | 2,515 ± 16 (75) | 1,167 ± 12 (116.7) | 354 ± 6 (1.1) |
| DL35 | TAGTTTTTGGAGTAACTAAAATAAGTAGTAGATTTGTGGAT | 27 | 2,710 ± 12 (80.8) | 1,320 ± 11 (132.0) | 287 ± 2 (0.9) |
| DL01 | TTTCGTTTGTGGGAGAAAAATGTCTTTGTATATTTGTATGG | 32 | 2,892 ± 103 (86) | 1,895 ± 36 (189.5) | 166 ± 7 (0.5) |
| DL21 | TTGATTTTGTATTTAAAAAATTTGGTGTTACTTTTGCTCTG | 24 | 4,515 ± 43 (134.6) | 3,221 ± 22 (322.1) | 353 ± 7 (1.1) |
| DL93 | ACGGGGTTGCGAAACTGATAAATTATTGTACCTTTGGAAGG | 41 | 5,085 ± 41 (151.6) | 2,190 ± 43 (219.0) | 803 ± 38 (2.5) |
| DL68 | TTTCCTTTGTATGTTTACTAAAAGTTTTTAAATTTGTTTGC | 22 | 5,475 ± 67 (163.2) | 2,869 ± 105 (286.9) | 683 ± 15 (2.1) |
| DL22 | TTGATTTTGTATTTAAAAAATTTGGTGTTACTTTTGCCTGT | 24 | 6,324 ± 162 (188.6) | 4,221 ± 91 (422.1) | 1,180 ± 13 (3.6) |
| DL98 | TTGATTTTGTATTTAAAAAATTTGGTGTTACTTTTGCTTGT | 22 | 9,727 ± 175 (290) | 6,030 ± 55 (603.0) | 1,527 ± 21 (4.7) |
| Negative controlb | NAc | NA | 34 ± 2 (1.0) | 10 ± 4 (1.0) | 327 ± 9 (1.0) |
The clones are ordered according to promoter strength in F. johnsoniae. Matches to the oligonucleotide consensus sequences −33 and −7 regions are in boldface.
Strains carrying pSCH144 (promoterless gfp) were used as the negative control.
NA, not applicable.
The promoter activity was normalized to that of the promoter clones carrying pSCH144 (defined as 1.0), as shown in parentheses. Triplicate samples were used, and the standard deviations are shown.
FIG. 4.
Correlation between promoter activities in F. hibernum and F. johnsoniae. The relative promoter strength in Flavobacterium was normalized to that of the clone carrying pSCH144 (defined as 1.0) and was plotted as a function of the relative promoter strength measured in F. johnsoniae.
FIG. 5.
Relative promoter strength as a function of the GC content of the core ompA promoter element in F. hibernum (A) and F. johnsoniae (B).
Genome-wide analysis of putative F. johnsoniae and B. fragilis promoters.
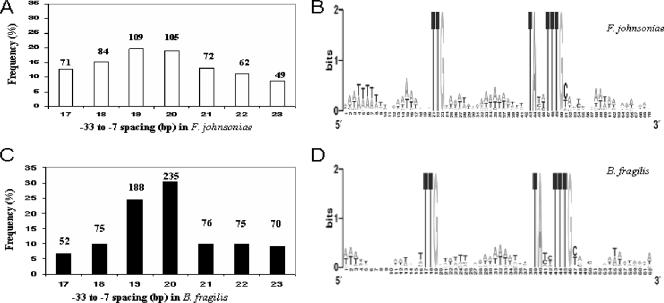
Genome-wide computational promoter discovery is a realistic way to mine regulatory sequences when bacterial genome sequences are available. We used Patscan (18) and RSAT (53) to perform a string pattern search of the F. johnsoniae and B. fragilis genomes with the following criteria. (i) Both strands of DNA sequences were scanned, (ii) TTG was the conserved −33 motif, (iii) TANNTTTG was the conserved −7 motif, (iv) the spacer length was set to 19 bp, and (v) only hits located in intergenic regions (noncoding sequences) were counted. One hundred eighty-eight hits within ∼300 bp on the coding strand upstream of the open reading frames (ORFs) were found in B. fragilis, and 109 hits were found in F. johnsoniae. Many of these likely function as promoters for the following reasons. (i) The sequences are usually located upstream within 300 bp of the ORFs. (ii) Many of the ORFs encode housekeeping proteins involved in transcription, translation, rRNA synthesis, or ribosomal structure and function (Chen et al., unpublished). These are likely to be highly expressed and are thus likely to have promoters that are close to the consensus sequence. (iii) The average GC content is 25%, lower than that in the genome. (iv) Alignment analysis of these putative promoters showed that similar clustered AT-rich sequences were predominant in the spacer region and UP-like sequences were found centered at approximately the −50 bp region in both F. johnsoniae and B. fragilis (Fig. 6). By the same strategy, we investigated the putative promoters with various spacer lengths (17 to 23 bp). The results indicated that promoters with a 19- or 20-bp spacer are dominant in members of the phylum Bacteroidetes (Fig. 6). A search of the E. coli genome with the same criteria resulted in fewer than 10 hits.
FIG. 6.
Distribution of the predicted promoters with 17 to 23 bp separating the −33 and −7 consensus motifs in F. johnsoniae and B. fragilis and alignment analysis of putative promoters with a 19-bp spacer. The noncoding regions located upstream of ORFs in the F. johnsoniae and B. fragilis genomes were scanned by PatScan or RSAT. Please note that we conducted a string pattern search as a primary study with the consensus promoter motifs which may not be necessary for other native promoters to be functional in members of the phylum Bacteroidetes. (A) Distribution of putative promoters with various spacer lengths (17 to 23 bp) in F. johnsoniae. The number of promoters in each group is indicated above the bar. (B) Alignment of the putative promoters with a 19-bp spacer in F. johnsoniae. (C) Distribution of putative promoters with various spacer lengths (17 to 23 bp) in B. fragilis. The number of promoters in each group is indicated above the bar. (D) Alignment of the putative promoters with a 19-bp spacer in B. fragilis.
DISCUSSION
Our mutational analysis of the sequences upstream of ompA confirmed the importance of the consensus sequences TTG in the −33 region (Fig. 2) and TANNTTTG in the −7 region (Fig. 3). In addition, the exchanges of individual bases in the consensus promoter sequences have established preferences of the bases at specific positions. In the −33 region, most of the substitutions drastically decreased promoter activity. Even a less-conserved T immediately upstream of the TTG sequence was shown to be important for ompA promoter activity (Fig. 3). This base has been proposed as part of the promoter consensus sequence in B. fragilis (4). A TTG motif can be found in the −35 region of many promoters in prokaryotes, but the −33 consensus motif in members of the phylum Bacteroidetes is relatively small compared to the conserved −35 motifs in proteobacteria (4).
In the −7 promoter element, the consensus TANNTTTG, identified in our previous work (12, 13) and confirmed by these studies, is unlike any of the known −10 sequences found in other bacterial groups (Table 5), although the general AT-rich character present in most promoters is also prominent here (Fig. 5 and 6). The distinct feature in the −7 region described here is the octamer sequence, longer than the common −10 hexamer (TATAAT) predominant in other bacteria (Table 5). Replacement of individual bases in the −7 region showed that several bases are essential for promoter activity (Fig. 3). Exchange of bases in positions −8, −9, −12, and −13 abolished promoter activity almost completely; indicating that these bases are highly conserved. In addition to characterizing the −33 and −7 motifs, we also determined that sequences upstream of the −33 element are important for the activity of the ompA promoter. Deletion of TTTTTT, an UP-like sequence located at approximately the −50 position, reduced the expression of the gfp reporter gene by approximately 50% (Fig. 1). UP-like fragments are recognized as common components of many prokaryotic promoters (19, 20, 46, 47) (∼3% of the promoters for mRNA and ∼15% of the promoters for stable RNAs in E. coli). They function as binding sites for the RNA polymerase α subunit (αCTD) to stimulate transcription (46, 47). The most extensively characterized UP element is an AT-rich sequence located between −40 and −60 in the rrnB P1 promoter of E. coli (19, 20), which stimulates promoter activity at least 30-fold (19). UP-like elements found upstream of Bacteroidetes promoters have been recognized in B. fragilis (1) and P. gingivalis (27). A genome-wide survey of putative promoters in members of the phylum Bacteroidetes also showed that UP-like sequences occur at ∼20 bp upstream of the −33 promoter motifs (Fig. 6). How the UP elements function in members of the phylum Bacteroidetes is not fully understood, and further studies are required to characterize them.
TABLE 5.
Summary of the consensus promoter sequences in different bacterial promotersa
| Genome taxonomy | Species | −35 sequence | Spacing (bp) | −10 sequence | Reference |
|---|---|---|---|---|---|
| Alphaproteobacteria | Sinorhizobium meliloti | TTGACW | ∼17 | TATAAT | 35 |
| C. crescentus | TTGACGS | 10-14 | GCTANAWC | 36 | |
| Betaproteobacteria | Neisseria sp. | TTGACA | ∼17 | TATAAT | 8 |
| Gammaproteobacteria | A. pleuropneumoniae | TTRAA | 13-16 | TATAAT | 17 |
| E. coli | TTGACA | 17 ± 1 | TATAAT | 21 | |
| Epsilonproteobacteria | Campylobacter jejuni | TTTAAGT | 15-19 | TATAAT | 58 |
| Firmicutes | B. subtilis | TTGACA | 17 ± 1 | TATAAT | 22 |
| Actinobacteria | Streptomyces sp. | TTGACR | 16-18 | TASRRT | 50 |
| Corynebacterium glutamicum | TTGANA | 16-18 | TANAAT | 44 | |
| Mycobacterium paratuberculosis | TGMCGT | 16-20 | CGGCCS | 3 | |
| Bacteroidetes | B. fragilis | TTTG | 19-21 | TANNTTTG | 4 |
| Flavobacterium sp. | TTG | 17-23 | TANNTTTG | 12 |
Promoter sequences were aligned by using the −35 and −10 regions. Consensus nucleotides are defined as present at a given position in more than 50% of the sequences. An M indicated an A or a C base, an N indicated any nucleotide, an R indicates A or G, an S indicates C or G, and a W indicates A or C. The spacer lengths of the housekeeping gene promoters in the representative strains were estimated from the literature.
Spacers between the −7 and −33 motifs in promoters of B. fragilis have been found to contain 19 to 21 bp (4, 55). In Flavobacterium, the reported range of lengths is even broader, 17 to 23 bp (12, 23-25), but the optimal spacer length for promoter activity in either of these species has not been determined. The mutagenesis studies reported here found that for the ompA promoter a spacer of 18 to 21 bp is recognized (Table 3). In addition, not only the length of the spacer but its base composition is of paramount importance (Table 4). The significance of spacer length and composition for promoter activity has been determined in several other bacterial groups (1, 34, 52, 56). Statistical analysis indicated that more than 50% of the native σ70 promoters carried a spacer length of 17 bp and the gene transcription level in vitro with σ70 was dramatically decreased for promoters with suboptimal spacer lengths (52, 56). The spacer length is believed to provide optimal binding of the 2.4 and 4.2 domains of the σ factors, and therefore one of its roles has been proposed to be in determination of the sigma factor's selection (16, 33). This is reflected in the differences of the spacer lengths recognized by different sigma factors in different bacteria (Table 5). For instance, the promoters for housekeeping genes in Caulobacter crescentus have a spacer of 10 to 14 bp (44), the promoters for housekeeping genes in Actinobacillus pleuropneumoniae have a spacer of 16 bp, and the promoters for housekeeping genes Bacteroides have a spacer of 19 bp (4). Even in the same bacteria, different environmental stimuli or growth phases cause bacteria to exchange the sigma subunit in the RNA polymerase and thereby initiate the expression of new sets of genes in accordance with the emerging needs (16, 59). Spacer length and composition, as promoter DNA determinants, may be involved in this process (52). Since members of the phylum Bacteroidetes are phylogenetically distinct from other bacteria and have an unusual essential sigma factor, it is not surprising that in bacteria in the phylum Bacteroidetes we find a spacer considerably different from that associated with σ70 promoters of other bacteria (Table 5).
Many naturally occurring promoters do not exhibit maximal efficiency (51, 59). The nonconsensus domains of these promoters undoubtedly contribute to the determination of their efficiency. The synthetic promoter construction method (SPCM) has been widely used to address questions arising in the analysis of the complex machinery of gene transcription (1, 34). Our results obtained by mutational analysis of the ompA promoter by SPCM (Table 4) showed that promoter strength may be greatly affected by the GC content of its sequence, particularly if the GC content is increased above that found in the chromosome (34% in F. johnsoniae). Of particular interest among the SPCM data presented here are those indicating that nonconserved promoter sequences contribute significantly to promoter activity (Table 4). In particular, the GC content of the nonconserved regions may have a profound effect (Fig. 5). Similar effects have been noted in other species. Agarwal and Tyagi (1) demonstrated that housekeeping promoters of Mycobacterium, despite exhibiting −10 and −35 sequence similarities to those of E. coli, differ in their requirement of spacer sequences between these two positions. Similarly, Liu et al. (34) reported that the presence of GC-rich sequences in the promoter spacer (specifically around position −15) dramatically decreased promoter activity in E. coli. In contrast, replacement with AT-rich sequences (GC content, <25%) resulted in an up-to-40-fold increase in the level of transcription. The increase in promoter activity with the increase in AT content in the spacer was attributed to the enhancement of RNA polymerase binding and of open complex formation (34). Genome sequence analysis showed that the Bacteroidetes promoter-like sequences with characteristics identified by this and previous studies (4, 9, 10, 12, 13, 23-25, 27, 38, 39, 41, 55) are widely distributed in both the F. johnsoniae and B. fragilis genomes (Fig. 6). A bioinformatic study indicated that members of the phylum Bacteroidetes lack typical σ70 genes (29). On the other hand, extracytoplasmic function σ factors are generally far more frequently represented than the other classes of σ factors (59). These unique promoters for housekeeping genes have been linked to an unusual sigma factor, σABfr, in members of the phylum Bacteroidetes (55). Compared with the σ70 factor, the σABfr factor has many distinct features (55). It completely lacks region 1.1 and the nonconserved segment (NCR) connecting regions 1.2 and 2.1 present exclusively in primary sigma factors. Region 1.1 has been shown to have modulatory effects on RNA polymerase function, and NCR is believed to contact the β′ subunit of the polymerase (26). Several amino acids reported to play critical roles in σ70 function, such as 11 residues in regions 2.3 to 3.0 and 2 residues in region 4.2, are not found in similar positions in σABfr. As expected, σABfr did not support the transcription of any promoters of either B. fragilis or E. coli in association with the E. coli RNA polymerase (55). In contrast, it formed an active holoenzyme with its cognate core polymerase, and this complex recognized only Bacteroidetes promoters (55). We postulate that distinct promoters described here and previously (12, 13) are recognized in their native species with the aid of distinct σABfr-like factors of Flavobacterium strains.
Acknowledgments
We gratefully acknowledge Mark McBride for generous advice and supplying plasmids pCP23 and pCP29 and Flavobacterium strains. We also acknowledge the technical assistance of William Morgan and Blair Bullard.
This project was funded by NIH grant AI21884.
Footnotes
Published ahead of print on 4 May 2007.
REFERENCES
- 1.Agarwal, N., and A. K. Tyagi. 2006. Mycobacterial transcriptional signals: requirements for recognition by RNA polymerase and optimal transcriptional activity. Nucleic Acids Res. 34:4245-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez, B., P. Secades, M. J. McBride, and J. A. Guijarro. 2004. Development of genetic techniques for the psychrotrophic fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 70:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannantine, J. P., R. G. Barletta, C. O. Thoen, and R. E. Andrews, Jr. 1997. Identification of Mycobacterium paratuberculosis gene expression signals. Microbiology 143:921-928. [DOI] [PubMed] [Google Scholar]
- 4.Bayley, D. P., E. R. Rocha, and C. J. Smith. 2000. Analysis of cepA and other Bacteroides fragilis genes reveals a unique promoter structure. FEMS Microbiol. Lett. 193:149-154. [DOI] [PubMed] [Google Scholar]
- 5.Blain, F., A. L. Tkalec, Z. Shao, C. Poulin, M. Pedneault, K. Gu, B. Eggimann, J. Zimmermann, and H. Su. 2002. Expression system for high levels of GAG lyase gene expression and study of the hepA upstream region in Flavobacterium heparinum. J. Bacteriol. 184:3242-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borriss, M., E. Helmke, R. Hanschke, and T. Schweder. 2003. Isolation and characterization of marine psychrophilic phage-host systems from Arctic sea ice. Extremophiles 7:377-384. [DOI] [PubMed] [Google Scholar]
- 7.Bostanci, N., R. Allaker, U. Johansson, M. Rangarajan, M. A. Curtis, F. J. Hughes, and I. J. McKay. 2007. Interleukin-1α stimulation in monocytes by periodontal bacteria: antagonistic effects of Porphyromonas gingivalis. Oral Microbiol. Immunol. 22:52-60. [DOI] [PubMed] [Google Scholar]
- 8.Braun, D. C., and D. C. Stein. 2004. The lgtABCDE gene cluster, involved in lipooligosaccharide biosynthesis in Neisseria gonorrhoeae, contains multiple promoter sequences. J. Bacteriol. 186:1038-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun, T. F., M. K. Khubbar, D. A. Saffarini, and M. J. McBride. 2005. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J. Bacteriol. 187:6943-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun, T. F., and M. J. McBride. 2005. Flavobacterium johnsoniae GldJ is a lipoprotein that is required for gliding motility. J. Bacteriol. 187:2628-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerdeño-Tárraga, A. M., S. Patrick, L. C. Crossman, G. Blakely, V. Abratt, N. Lennard, I. Poxton, B. Duerden, B. Harris, M. A. Quail, A. Barron, L. Clark, C. Corton, J. Doggett, M. T. G. Holden, N. Larke, A. Line, A. Lord, H. Norbertczak, D. Ormond, C. Price, E. Rabbinowitsch, J. Woodward, B. Barrell, and J. Parkhill. 2005. Extensive DNA inversions in the B. fragilis genome control variable gene expression. Science 307:1463-1465. [DOI] [PubMed] [Google Scholar]
- 12.Chen, S., M. Bagdasarian, M. G. Kaufman, and E. D. Walker. 2007. Characterization of strong promoters from an environmental Flavobacterium hibernum strain using a green fluorescent protein-based reporter system. Appl. Environ. Microbiol. 73:1089-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, S., M. Bagdasarian, M. G. Kaufman, and E. D. Walker. 2007. Organization of a partial S10 operon and its transcriptional analysis in Flavobacterium hibernum strain W22. FEMS Microbiol. Lett. 267:38-45. [DOI] [PubMed] [Google Scholar]
- 14.Cottrell, M. T., and D. L. Kirchman. 2000. Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl. Environ. Microbiol. 66:1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decostere, A., F. Haesebrouck, G. Charlier, and R. Ducatelle. 1999. The association of Flavobacterium columnare strains of high and low virulence with gill tissue of black mollies (Poecilia sphenops). Vet. Microbiol. 67:287-298. [DOI] [PubMed] [Google Scholar]
- 16.Dombroski, A. J., B. D. Johnson, M. Lonetto, and C. A. Gross. 1996. The sigma subunit of Escherichia coli RNA polymerase senses promoter spacing. Proc. Natl. Acad. Sci. USA 93:8858-8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doree, S. M., and M. H. Mulks. 2001. Identification of an Actinobacillus pleuropneumoniae consensus promoter structure. J. Bacteriol. 183:1983-1989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Dsouza, M., N. Larsen, and R. Overbeek. 1997. Searching for patterns in genomic data. Trends Genet. 13:497-498. [DOI] [PubMed] [Google Scholar]
- 19.Estrem, S. T., T. Gaal, W. Ross, and R. L. Gourse. 1998. Identification of an UP element consensus sequence for bacterial promoters. Proc. Natl. Acad. Sci. USA 95:9761-9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gourse, R. L., W. Ross, and T. Gaal. 2000. UPs and downs in bacterial transcription initiation: the role of the alpha subunit of RNA polymerase in promoter recognition. Mol. Microbiol. 37:687-695. [DOI] [PubMed] [Google Scholar]
- 21.Harley, C. B., and R. P. Reynolds. 1987. Analysis of E. coli promoter sequences. Nucleic Acids Res. 15:2343-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helmann, J. D. 1995. Compilation and analysis of Bacillus subtilis σA-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 23:2351-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunnicutt, D. W., M. J. Kempf, and M. J. McBride. 2002. Mutations in Flavobacterium johnsoniae gldF and gldG disrupt gliding motility and interfere with membrane localization of GldA. J. Bacteriol. 184:2370-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunnicutt, D. W., and M. J. McBride. 2000. Cloning and characterization of the Flavobacterium johnsoniae gliding-motility genes gldB and gldC. J. Bacteriol. 182:911-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunnicutt, D. W., and M. J. McBride. 2001. Cloning and characterization of the Flavobacterium johnsoniae gliding motility genes gldD and gldE. J. Bacteriol. 183:4167-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer, L. M., E. V. Koonin, and L. Aravind. 2004. Evolution of bacterial RNA polymerase: implications for large-scale bacterial phylogeny, domain accretion, and horizontal gene transfer. Gene 335:73. [DOI] [PubMed] [Google Scholar]
- 27.Jackson, C. A., B. Hoffmann, N. Slakeski, S. Cleal, A. J. Hendtlass, and E. C. Reynolds. 2000. A consensus Porphyromonas gingivalis promoter sequence. FEMS Microbiol. Lett. 186:133-138. [DOI] [PubMed] [Google Scholar]
- 28.Kempf, M. J., and M. J. McBride. 2000. Transposon insertions in the Flavobacterium johnsoniae ftsX gene disrupt gliding motility and cell division. J. Bacteriol. 182:1671-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kill, K., T. T. Binnewies, T. Sicheritz-Ponten, H. Willenbrock, P. F. Hallin, T. M. Wassenaar, and D. W. Ussery. 2005. Genome update: sigma factors in 240 bacterial genomes. Microbiology 151:3147-3150. [DOI] [PubMed] [Google Scholar]
- 30.Kirchman, D. L. 2002. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 39:91-100. [DOI] [PubMed] [Google Scholar]
- 31.Kirchman, D. L., L. Y. Yu, and M. T. Cottrell. 2003. Diversity and abundance of uncultured Cytophaga-like bacteria in the Delaware Estuary. Appl. Environ. Microbiol. 69:6587-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuwahara, T., A. Yamashita, H. Hirakawa, H. Nakayama, H. Toh, N. Okada, S. Kuhara, M. Hattori, T. Hayashi, and Y. Ohnishi. 2004. Genomic analysis of Bacteroides fragilis reveals extensive DNA inversions regulating cell surface adaptation. Proc. Natl. Acad. Sci. USA 101:14919-14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuznedelov, K., L. Minakhin, A. Niedziela-Majka, S. L. Dove, D. Rogulja, B. E. Nickels, A. Hochschild, T. Heyduk, and K. Severinov. 2002. A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science 295:855-857. [DOI] [PubMed] [Google Scholar]
- 34.Liu, M., M. Tolstorukov, V. Zhurkin, S. Garges, and S. Adhya. 2004. A mutant spacer sequence between −35 and −10 elements makes the Plac promoter hyperactive and cAMP receptor protein-independent. Proc. Natl. Acad. Sci. USA 101:6911-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLellan, S. R., A. M. MacLean, and T. M. Finan. 2006. Promoter prediction in the rhizobia. Microbiology 152:1751-1763. [DOI] [PubMed] [Google Scholar]
- 36.Malakooti, J., S. P. Wang, and B. Ely. 1995. A consensus promoter sequence for Caulobacter crescentus genes involved in biosynthetic and housekeeping functions. J. Bacteriol. 177:4372-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manch-Citron, J. N., P. J. Shahani, and R. Schneider. 2001. Cloning, characterization, and possible origin of the Prevotella loescheii dnaK homolog. Curr. Microbiol. 42:82-88. [DOI] [PubMed] [Google Scholar]
- 38.McBride, M. J., and T. F. Braun. 2004. GldI is a lipoprotein that is required for Flavobacterium johnsoniae gliding motility and chitin utilization. J. Bacteriol. 186:2295-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McBride, M. J., T. F. Braun, and J. L. Brust. 2003. Flavobacterium johnsoniae GldH is a lipoprotein that is required for gliding motility and chitin utilization. J. Bacteriol. 185:6648-6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McBride, M. J., and M. J. Kempf. 1996. Development of techniques for the genetic manipulation of the gliding bacterium Cytophaga johnsonae. J. Bacteriol. 178:583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson, S. S., and M. J. McBride. 2006. Mutations in Flavobacterium johnsoniae secDF result in defects in gliding motility and chitin utilization. J. Bacteriol. 188:348-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nematollahi, A., A. Decostere, F. Pasmans, and F. Haesebrouck. 2003. Flavobacterium psychrophilum infections in salmonid fish. J. Fish Dis. 26:563-574. [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa, K., F. Yoshimura, and M. J. Duncan. 2004. A regulation cascade controls expression of Porphyromonas gingivalis fimbriae via the FimR response regulator. Mol. Microbiol. 54:546-560. [DOI] [PubMed] [Google Scholar]
- 44.Pátek, M., B. J. Eikmanns, J. Pátek, and H. Sahm. 1996. Promoters from Corynebacterium glutamicum: cloning, molecular analysis and search for a consensus motif. Microbiology 142:1297-1309. [DOI] [PubMed] [Google Scholar]
- 45.Prouty, M. G., N. E. Correa, and K. E. Klose. 2001. The novel σ54- and σ28-dependent flagellar gene transcription hierarchy of Vibrio cholerae. Mol. Microbiol. 39:1595-1609. [DOI] [PubMed] [Google Scholar]
- 46.Ross, W., and R. L. Gourse. 2005. Sequence-independent upstream DNA-αCTD interactions strongly stimulate Escherichia coli RNA polymerase-lacUV5 promoter association. Proc. Natl. Acad. Sci. USA 102:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross, W., D. A. Schneider, B. J. Paul, A. Mertens, and R. L. Gourse. 2003. An intersubunit contact stimulating transcription initiation by E. coli RNA polymerase: interaction of the alpha C-terminal domain and sigma region 4. Genes Dev. 17:1293-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Smith, C. J., M. B. Rogers, and M. L. McKee. 1992. Heterologous gene expression in Bacteroides fragilis. Plasmid 27:141-154. [DOI] [PubMed] [Google Scholar]
- 50.Strohl, W. R. 1992. Compilation and analysis of DNA sequences associated with apparent Streptomycete promoters. Nucleic Acids Res. 20:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su, H. S., Z. Q. Shao, L. Tkalec, F. Blain, and J. Zimmermann. 2001. Development of a genetic system for the transfer of DNA into Flavobacterium heparinum. Microbiology 147:581-589. [DOI] [PubMed] [Google Scholar]
- 52.Typas, A., and R. Hengge. 2006. Role of the spacer between the −35 and −10 regions in σs promoter selectivity in Escherichia coli. Mol. Microbiol. 59:1037-1051. [DOI] [PubMed] [Google Scholar]
- 53.van Helden, J. 2003. Regulatory sequence analysis tools. Nucleic Acids Res. 31:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Veen, L., J. Nieuwenhuizen, D. Mekkes, M. Vrijenhoek, and P. van Empel. 2005. Diagnosis and incidence of Ornithobacterium rhinotracheale infections in commercial broiler chickens at slaughter. Vet. Rec. 156:315-317. [DOI] [PubMed] [Google Scholar]
- 55.Vingadassalom, D., A. Kolb, C. Mayer, T. Rybkine, E. Collatz, and I. Podglajen. 2005. An unusual primary sigma factor in the Bacteroidetes phylum. Mol. Microbiol. 56:888-902. [DOI] [PubMed] [Google Scholar]
- 56.Warne, S. E., and P. L. deHaseth. 1993. Promoter recognition by Escherichia coil RNA polymerase. Effects of single base pair deletions and insertions in the spacer DNA separating the −10 and −35 regions are dependent on spacer DNA sequence. Biochemistry 32:6134-6140. [DOI] [PubMed] [Google Scholar]
- 57.Whitehead, T. R. 1997. Development of a bifunctional xylosidase/arabinosidase gene as a reporter gene for the gram-negative anaerobes Bacteroides and Porphyromonas, and Escherichia coli. Curr. Microbiol. 35:282-286. [DOI] [PubMed] [Google Scholar]
- 58.Wösten, M., M. Boeve, M. G. A. Koot, A. C. van Nuenen, and B. A. M. van der Zeijst. 1998. Identification of Campylobacter jejuni promoter sequences. J. Bacteriol. 180:594-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wösten, M. M. S. M. 1998. Eubacterial sigma-factors. FEMS Microbiol. Rev. 22:127-150. [DOI] [PubMed] [Google Scholar]
- 60.Xie, H., W. O. Chung, Y. Park, and R. J. Lamont. 2000. Regulation of the Porphyromonas gingivalis fimA (fimbrillin) gene. Infect. Immun. 68:6574-6579. [DOI] [PMC free article] [PubMed] [Google Scholar]