Abstract
The objective of this study was to develop an understanding of the molecular mechanisms by which type IV fimbrial biogenesis, natural transformation, and protease secretion are linked in the ovine foot rot pathogen, Dichelobacter nodosus. We have shown that like the D. nodosus fimbrial subunit FimA, the pilin-like protein PilE and the FimN, FimO, and FimP proteins, which are homologs of PilB, PilC, and PilD from Pseudomonas aeruginosa, are essential for fimbrial biogenesis and natural transformation, indicating that transformation requires an intact type IV fimbrial apparatus. The results also showed that extracellular protease secretion in the fimN, fimO, fimP, and pilE mutants was significantly reduced, which represents the first time that PilB, PilC, and PilE homologs have been shown to be required for the secretion of unrelated extracellular proteins in a type IV fimbriate bacterium. Quantitative real-time PCR analysis of the three extracellular protease genes aprV2, aprV5, and bprV showed that the effects on protease secretion were not mediated at the transcriptional level. Bioinformatic analysis did not identify a classical type II secretion system, and the putative fimbrial biogenesis gene pilQ was the only outer membrane secretin gene identified. Based on these results, it is postulated that in D. nodosus, protease secretion occurs by a type II secretion-related process that directly involves components of the type IV fimbrial biogenesis machinery, which represents the only type II secretion system encoded by the small genome of this highly evolved pathogen.
Dichelobacter nodosus is a slow-growing, anaerobic, gram-negative rod that is the principal causative agent of foot rot in ruminants, especially sheep and goats. Ovine foot rot is characterized by the separation of the keratinous hoof from the underlying tissue, leading to lameness, loss of body weight, and reduced wool growth and quality. Therefore, the economic losses from this disease are very significant (72). The outcome of a D. nodosus infection is dependent upon the virulence properties of the D. nodosus isolate and the climatic conditions, with virulent disease occurring under warm, wet conditions. The virulence factors of D. nodosus include type IV fimbriae (34), extracellular serine proteases (36, 37, 39, 63), and potentially, the genomic islands vrl and vap, which are preferentially associated with virulent strains (9, 65).
Type IV fimbriae or pili are produced by many pathogenic gram-negative bacteria, including Neisseria gonorrhoeae, Pseudomonas aeruginosa, Pseudomonas stutzeri, Moraxella bovis, and D. nodosus (46). These fimbriae have a polar location on the surface of the cell and are comprised of a conserved structural subunit that generally has an N-terminal methylated residue, often phenylalanine, a conserved hydrophobic N-terminal domain, and a C-terminal disulfide bond (14). Type IV fimbriae mediate attachment and adherence to epithelial cells (50), twitching motility (46), gliding motility (77), cell agglutination, and biofilm and fruiting body formation (12, 59); they act as receptors for bacteriophages (11); and they are required for extracellular protein secretion (34, 42) and natural transformation (7, 26).
Type IV fimbriae are capable of extension and retraction, processes that are integral to twitching motility (70). Current models suggest that pilus extension and retraction reflect fimbrial subunit polymerization and depolymerization events (49) that are mediated by two antagonistic cytosolic hexameric ATPases (32, 43, 51). One of these proteins, known as PilB in P. aeruginosa (4, 57) and PilF in Neisseria spp. (22), promotes fimbrial polymerization. An inner membrane protein (known as PilC in P. aeruginosa [57] and PilG in N. gonorrhoeae [75]) appears to be involved in fimbrial assembly by interacting with PilB. The bifunctional prepilin peptidase PilD is encoded by a gene located in a cluster with pilB (pilF) and pilC (pilG) and is required for cleavage of the leader peptide and for methylation of the new N-terminal phenylalanine residue of the pilin subunit prior to its assembly into filamentous fimbriae (22, 57).
In addition to the major pilin subunit, several pilin-like proteins that contain an overall positively charged leader peptide preceding a conserved hydrophobic N-terminal domain are involved in type IV fimbrial biogenesis and its associated functions, such as natural transformability and epithelial cell adherence (2, 3, 5, 6, 27, 67, 81, 82). It has been postulated that these pilin-like proteins form the base of the fimbrial structure or initiate, modulate, or cap pilin assembly into the fimbriae (46, 80). Alternatively, as suggested for Neisseria, they may be inhibitors of fimbrial retraction (82). In type II secretion systems, their homologs (known as pseudopilins) are involved in pilus-like macromolecular secretin formation (21, 56).
Another important gene cluster, pilMNOPQ, is essential for type IV fimbrial biogenesis in P. aeruginosa (44, 45), N. gonorrhoeae (16, 17), and Myxococcus xanthus (78) and is also required for natural transformation in N. gonorrhoeae (16, 17). It has been suggested that the PilQ secretin forms a homododecameric outer membrane complex that is essential for the translocation of the fimbriae across the outer membrane (13).
Type IV fimbrial biogenesis in D. nodosus is poorly understood. Previous studies in this laboratory (30) identified three genes in the virulent D. nodosus strain A198, fimN, fimO, and fimP, which encode homologs of the P. aeruginosa PilB, PilC, and PilD proteins, respectively (57). At the time, due to the lack of a genetic manipulation system in D. nodosus, genetic studies were carried out with P. aeruginosa. The D. nodosus fimP gene complemented a pilD mutant of P. aeruginosa and was shown to have prepilin peptidase activity, but neither fimN nor fimO could complement their homologous pilB or pilC mutations (31). Our recent discovery of natural transformation in D. nodosus led to the construction of fimA mutants and the finding that the fimbrial subunit protein, FimA, is essential for virulence in sheep and for natural transformation and is involved in protease secretion (34).
In this study, we describe the identification and characterization of genes involved in type IV fimbrial biogenesis in D. nodosus and report the functional genetic analysis of the fimN, fimO, fimP, and pilE (encodes one of five putative pilin-like proteins) genes. The results demonstrate that D. nodosus has most of the genes known to be required for type IV fimbrial biogenesis but that there is a significant difference between D. nodosus and other type IV fimbriate organisms, including Pseudomonas and Neisseria spp. We provide experimental evidence to support the hypothesis that the type IV fimbrial assembly apparatus is part of the only type II secretion system in this bacterium.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. E. coli DH5α cells used for plasmid propagation and cloning experiments were grown at 37°C in 2× YT medium (68). The transformable virulent D. nodosus strain VCS1703A and its derivatives were grown in a Coy anaerobic chamber (Coy Laboratory Products Inc.) in an atmosphere of 10% H2, 10% CO2, and 80% N2 on Eugon (BBL) yeast extract (EYE) agar with 5% defibrinated horse blood (Bio-Lab) or in EYE broth, as described previously (34). When required, media were supplemented with the following antibiotics at the indicated concentrations: for E. coli, ampicillin, 100 μg/ml; erythromycin, 150 μg/ml; kanamycin, 20 μg/ml; and tetracycline, 10 μg/ml; and for D. nodosus, erythromycin, 1 μg/ml; tetracycline, 1 μg/ml; and kanamycin, 10 μg/ml. D. nodosus cells used for azocasein assays, transmission electron microscopy, twitching motility assays, elastase assays, and protease zymograms were grown in TAS media (71).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F−endA1 hsdR17(rK− mK−) thi-1 λ−recA1 gyrA96 relA1 rhoA supE44 deoR φ80dlacZΔM15 Δ(lacZYA-argF)U169 | Invitrogen |
| D. nodosus | ||
| VCS1703A | Serogroup G, transformable virulent isolate | 34 |
| JIR3885 | VCS1703A fimO Ω aphA-3 (fimO1) | Natural transformation with pJIR2618 |
| JIR3886 | VCS1703A fimO Ω aphA-3 (fimO2) | Natural transformation with pJIR2618 |
| JIR3889 | VCS1703A fimP Ωerm(B) (fimP1) | Natural transformation with pJIR2773 |
| JIR3890 | VCS1703A fimP Ωerm(B) (fimP2) | Natural transformation with pJIR2773 |
| JIR3895 | VCS1703A fimN ΩaphA-3 (fimN1) | Natural transformation with pJIR2798 |
| JIR3896 | VCS1703A fimN ΩaphA-3 (fimN2) | Natural transformation with pJIR2798 |
| JIR3910 | VCS1703A pilE Ωerm(B) (pilE1) | Natural transformation with pJIR3088 |
| JIR3911 | VCS1703A pilE Ωerm(B) (pilE2) | Natural transformation with pJIR3088 |
| JIR3913 | VCS1703A pilC Δ(405-2373) ΩaphA-3 (pilC1) | Natural transformation with pJIR3132 |
| JIR3914 | VCS1703A pilC Δ(405-2373) ΩaphA-3 (pilC2) | Natural transformation with pJIR3132 |
| Plasmids | ||
| pBluescript II SK(+) | AprlacZ+ cloning vector | Stratagene |
| pWSK29 | AprlacZ+ low-copy-no. cloning vector | 79 |
| pUC18K | pUC18 containing aphA-3 inserted into SmaI site, nonpolar cassette | 48 |
| pGEM7Zf(−) | AprlacZ+ cloning vector | Promega |
| pJIR1532 | pBluescript II SK(+) containing tet(M) located between D. nodosus rrnA promoter and terminator | Recombinant |
| pJIR2412 | pBluescript II SK(+) PstI Ωerm(B) | 60 |
| pJIR2614 | pJIR2412 XbaI/BamHIΩ(935-bp PCR product containing 5′ end of pilQ) XhoI/KpnI Ω(927-bp PCR product containing 3′ end of pilQ) | Recombinant |
| pJIR2617 | pBluescript II SK(+) XbaI/EcoRI Ω(2.1-kb PCR product containing fimO+) | Recombinant |
| pJIR2618 | pJIR2617 HpaI ΩaphA-3 | Recombinant |
| pJIR2691 | pWSK29 containing tet(M) located between D. nodosus rrnA promoter and terminator | Recombinant |
| pJIR2714 | pJIR2412 EcoRI/HindIII Ω(940-bp PCR product containing internal pilQ) | Recombinant |
| pJIR2769 | pBluescript II SK(+) XbaI/BamHI Ω(1.9-kb PCR product containing fimP+) | Recombinant |
| pJIR2770 | pGEM7Zf(−) XbaI/BamHI Ω(1.9-kb fimP from pJIR2769 XbaI/BamHI) | Recombinant |
| pJIR2773 | pJIR2770 HindII Ωerm(B) | Recombinant |
| pJIR2797 | pGEM7Zf(−) XbaI/EcoRI Ω(2.6-kb PCR product containing fimN+) | Recombinant |
| pJIR2798 | pJIR2797 EcoRV ΩaphA-3 | Recombinant |
| pJIR3087 | pGEM7Zf(−) BamHI/XbaI Ω(2-kb PCR product containing pilE+) | Recombinant |
| pJIR3088 | pJIR3087 T4-filled HindIII Ωerm(B) | Recombinant |
| pJIR3124 | pUC18K XbaI/BamHI Ω(1.4-kb PCR product containing 3′ fragment of pilC) | Recombinant |
| pJIR3132 | pJIR3124 T4-filled EcoRI Ω(1.3-kb PCR product containing 5′ fragment of pilC) | Recombinant |
DNA manipulations and molecular techniques.
Unless otherwise stated, standard procedures were applied for DNA manipulations and molecular techniques (68). D. nodosus genomic DNA was prepared by using a QIAGEN DNeasy kit according to the manufacturer's instructions. Southern hybridizations were performed as described previously (33), with probes specific for the relevant antibiotic resistance genes, the target genes, or the 16S rrnA gene. PCR-restriction fragment length polymorphism analysis (24) of the omp1 gene, which encodes an outer membrane protein of D. nodosus (53), was conducted to confirm that all mutants were derivatives of the wild-type strain VCS1703A. Reverse transcriptase PCR (RT-PCR) was performed as previously described (34), except that the D. nodosus cells were grown in TAS broth. Quantitative RT-PCR (qRT-PCR) was carried out as previously described (60) using RNA prepared as for RT-PCR. DNA sequencing was performed using an A3730S capillary sequencer (Applied Biosystems) and analyzed by using Sequencher version 3.0 (Gene Codes Corporation).
Nucleotide and amino acid sequence comparisons were carried out using the National Center for Biotechnology Information BLAST server (http://www.ncbi.nlm.nih.gov/BLAST) and resources available at TIGR (http://www.tigr.org) and the Victorian Bioinformatics Consortium (http://vbc.med.monash.edu.au/). Homologous amino acid sequences were aligned with the CLUSTAL W program at the Network Protein Sequence Analysis (NPS@) server provided by Pole Bioinformatique Lyonnais (http://npsa-pbil.ibcp.fr). The prediction of subcellular localization was carried out using PSORTb (http://www.psort.org/psortb).
D. nodosus mutant construction.
To construct a fimN suicide vector, a 2.6-kb PCR product that contains the fimN gene was cloned into the XbaI/EcoRI sites of pGEM7Zf(−). An 800-bp aphA-3 kanamycin resistance cassette from pUC18K (48) was inserted into the EcoRV site of the resultant plasmid pJIR2797 (within fimN) to construct the fimN suicide vector pJIR2798, where the aphA-3 cassette was flanked on each side by 1.3-kb fragments of D. nodosus-derived DNA. The fimO suicide vector pJIR2618 was constructed by cloning a 2.1-kb fimO PCR product into the XbaI/EcoRI sites of pBluescript II SK(+) followed by introducing the aphA-3 cassette into the HpaI site, which was located in the middle of the 2.1-kb D. nodosus-derived sequence. To construct the fimP suicide vector pJIR2773, a 1.9-kb PCR fragment containing the fimP gene was cloned into pBluescript II SK(+) and then subcloned into pGEM7Zf(−) followed by the insertion of a 1.1-kb fragment containing the erm(B) erythromycin resistance cassette from pJIR2412 into the HindII site within fimP. In this construct, a 954-bp and a 967-bp fimP-derived fragment was located upstream and downstream, respectively, of erm(B). To construct the pilE suicide vector pJIR3088, a 2.1-kb PCR product containing the pilE gene was cloned into pGEM7Zf(−), and an erm(B) cassette was inserted into the T4 polymerase-filled HindIII site located within pilE, so that erm(B) was flanked by about 1 kb of D. nodosus DNA on each side. Since pilC is the first gene in an operon, a nonpolar cassette from pUC18K was used to construct the pilC mutants. A 1.42-kb PCR product comprising the 3′ end of pilC and its downstream region was amplified and cloned into the XbaI/BamHI sites of pUC18K. Then a 1.33-kb PCR fragment containing the 5′ end of pilC was cloned into the T4 polymerase-filled EcoRI site of the resultant plasmid pJIR3124 to construct the pilC suicide vector pJIR3132, in which the aphA-3 cassette was located between two pilC fragments and replaced a 1.9-kb internal part of the pilC gene.
Mutants were constructed by homologous recombination between these double-crossover suicide vectors and the D. nodosus chromosome, as described previously (34). Either circular or linearized plasmid DNA or PCR products derived from suicide vectors were introduced into D. nodosus by natural transformation. Transformants were initially screened by resistance to the appropriate antibiotics and then by capillary PCR analysis (33). Potential mutants were further analyzed by PCR with 16S rrnA gene primers to confirm they were derivatives of D. nodosus (38) and with antibiotic resistance gene primers to verify that the correct resistance genes were present. PCR analysis and Southern hybridization were used to confirm that the genes had been insertionally inactivated by double-crossover events.
SDS-PAGE and immunoblotting.
Whole-cell extracts of D. nodosus strains were prepared as previously described (34). Cell surface fimbriae were purified by isoelectric precipitation (47). The fimbrial subunits were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after staining with Coomassie brilliant blue and also by immunoblotting with serogroup G-specific fimbrial antisera (J. Egerton, University of Sydney) at a 1:1,000 dilution (34).
Analysis of fimbriae and twitching motility.
Transmission electron microscopy was performed on 3-day TAS agar cultures as previously described (34). Twitching motility assays were carried out with D. nodosus cells inoculated through the agar layer of 1% TAS medium plates, as previously described (34).
Extracellular protease assays.
Caseinase and elastase activities were detected qualitatively by growing D. nodosus colonies on EYE agar containing 2% skim milk powder or on TAS agar containing 0.3% (wt/vol) insoluble elastin (73), respectively. Total protease activity in the culture supernatant was determined quantitatively using azocasein (Sigma) as the substrate, as previously described (34). One unit of protease activity is defined as the amount of enzyme required to digest 1 μg of azocasein per min (34). Protease zymograms were carried out as previously described (40).
Proteomics, MS, and protein identification.
The proteins present in culture supernatants derived from the wild-type and mutant strains were TCA precipitated and analyzed by two-dimensional gel electrophoresis, as previously described (60). Protein spots of interest were excised from gels stained with colloidal Coomassie blue, and SDS was removed by incubation in 5% acetic acid in 40% methanol for 15 min at room temperature followed by washing for 5 min in deionized H2O. Dye was removed by incubation for 20 min in 50 mM ammonium bicarbonate in 50% acetonitrile (high-performance liquid chromatography grade; BDH); this step was repeated when necessary. The gel pieces were washed twice for 1 h in 20 mM ammonium bicarbonate, dehydrated for 10 min in acetonitrile, and dried under vacuum. After rehydration in 5 μl of 100 ng/μl sequencing grade trypsin (Promega) in 0.1% (vol/vol) acetic acid, 15 mM ammonium bicarbonate, and 1 mM CaCl2 for 10 min at 4°C, 20 mM ammonium bicarbonate was added to immerse the gel pieces followed by incubation at 37°C overnight. The supernatant was retained. Further extractions were performed by adding an equal volume of 5% trifluoroacetic acid (TFA; Pierce) and incubating for 30 min at room temperature with shaking, followed by a 5% TFA-50% acetonitrile extraction with a volume sufficient to cover the gel pieces. Both extracts were pooled with the earlier supernatant and then vacuum dried. The material was resuspended in a small volume of 50% acetonitrile-0.1% TFA. One microliter of Matrix solution (10 mg/ml of α-cyano-4-hydroxycinnamic acid [Laser Biolab, France] in 50% acetonitrile-0.1% TFA) was added to the target plate together with 1 μl of peptide sample prior to matrix-assisted laser desorption ionization-time of flight (mass spectrometry [MS]) analysis. MS and MS/MS were performed with an Applied Biosystems 4700 Proteomics Analyzer in reflection mode using standard procedures. Peptide mass fingerprint spectra and MS/MS spectra were submitted to the Mascot-based GPS explorer software version 3.0 and then searched against a D. nodosus database predicted from the then-unpublished genome sequence (http://www.tigr.org). The mass protein score was utilized to determine if the observed match was likely to be a random event. A match with a score greater than 100 was accepted as a positive hit.
RESULTS
Identification of genes potentially involved in fimbrial biogenesis in D. nodosus.
The availability of the D. nodosus strain VCS1703A genome sequence (54) enabled the putative identification of genes involved in fimbrial biogenesis by carrying out BLAST searches with known type IV fimbrial biogenesis components from P. aeruginosa. This analysis led to the identification of more than 20 potential homologs (Table 2), at several different genomic locations, in addition to the fimbrial genes previously identified (30, 61). There were several regions that contained more than two putative fimbrial genes, particularly the fimNOP, pilCVWX-fimU-pilE, pilMNOPQ, and pilGHIJ-chpA loci. The genetic organization of these loci was similar but not identical to that previously observed in P. aeruginosa (80).
TABLE 2.
Genes identified as being potentially involved in fimbrial biogenesis in D. nodosus
| Locusa | Designationa | No. of amino acids | Potential function | Rationaleb |
|---|---|---|---|---|
| DNO_0110 | fimA | 163 | Major pilin | 66% similarity to P. aeruginosa PilA/COG4969 |
| DNO_0238 | fimX | 684 | Signal transduction protein | 59% similarity to P. aeruginosa FimX/COG4943 |
| DNO_0344 | pilM | 337 | Fimbrial biogenesis | 60% similarity to P. aeruginosa PilM/COG4972 |
| DNO_0345 | pilN | 215 | Fimbrial biogenesis | 59% similarity to P. aeruginosa PilN/COG3166 |
| DNO_0346 | pilO | 222 | Fimbrial biogenesis | 61% similarity to P. aeruginosa PilO/COG3167 |
| DNO_0347 | pilP | 206 | Fimbrial biogenesis | 57% similarity to P. aeruginosa PilP/COG3168 |
| DNO_0348 | pilQ | 734 | Secretin | 67% similarity to P. aeruginosa PilQ/COG4796 |
| DNO_0434 | pilS | 581 | Two-component system/sensor protein | 59% similarity to P. aeruginosa PilS/COG0642 |
| DNO_0435 | pilR | 528 | Two-component system/response regulator | 70% similarity to P. aeruginosa PilR/COG2204 |
| DNO_0515 | pilF | 253 | Fimbrial assembly | 60% similarity to P. aeruginosa PilZ/COG3063 |
| DNO_0532 | rpoN | 469 | σ51 factor | 70% similarity to P. aeruginosa RpoN/COG1508 |
| DNO_0675 | pilT | 357 | Twitching motility ATPase | 86% similarity to P. aeruginosa PilT/COG2805 |
| DNO_0676 | pilU | 406 | Twitching motility ATPase | 76% similarity to P. aeruginosa PilU/COG5008 |
| DNO_0712 | fimV | 959 | Fimbrial biogenesis | 53% similarity to P. aeruginosa FimV/COG3170 |
| DNO_0890 | pilE | 151 | Pilin-like protein | 69% similarity to P. aeruginosa PilE/COG4968 |
| DNO_0891 | fimU | 159 | Pilin-like protein | 58% similarity to P. aeruginosa FimU/T/COG4970 |
| DNO_0892 | pilX | 221 | Pilin-like protein | 53% similarity to P. aeruginosa PilX/COG4726 |
| DNO_0893 | pilW | 381 | Pilin-like protein | 46% similarity to P. aeruginosa PilW/COG4966 |
| DNO_0894 | pilV | 189 | Pilin-like protein | 52% similarity to P. aeruginosa PilV/COG4967 |
| DNO_0895 | pilC | 1272 | Fimbrial biogenesis/adhesin | 52% similarity to N. gonorrhoeae PilC/COG3419 |
| DNO_1093 | chpA | 2554 | CheA/CheY hybrid, chemosensory | 55% similarity to P. aeruginosa ChpA/COG0643 |
| DNO_1094 | pilJ | 436 | PilJ homolog, chemosensory | 54% similarity to P. aeruginosa PilJ/COG0840 |
| DNO_1095 | pilI | 173 | CheW-like protein, chemosensory | 54% similarity to P. aeruginosa PilI/COG0835 |
| DNO_1096 | pilH | 120 | CheY-like protein, chemosensory | 87% similarity to P. aeruginosa PilH/COG0745 |
| DNO_1097 | pilG | 127 | CheY homolog, chemosensory | 82% similarity to P. aeruginosa PilG/COG0745 |
| DNO_1106 | ppk | 722 | Polyphosphate kinase | 70% similarity to P. aeruginosa Ppk/COG0855 |
| DNO_1124 | fimP | 286 | Prepilin peptidase | 77% similarity to P. aeruginosa PilD/XcpA/COG1989 |
| DNO_1125 | fimO | 407 | Fimbrial assembly | 78% similarity to P. aeruginosa PilC/COG1459 |
| DNO_1126 | fimN | 564 | Fimbrial assembly | 80% similarity to P. aeruginosa PilB/COG2804 |
| DNO_1215 | pilZ | 120 | Fimbrial assembly | 76% similarity to P. aeruginosa PilZ/COG3215 |
fimN, fimO, and fimP are required for type IV fimbrial assembly.
Comparative analysis revealed that the fimNOP cluster (equivalent to pilBCD from P. aeruginosa) of the transformable D. nodosus strain VCS1703A was identical to that previously observed in D. nodosus strain A198 (30) in terms of its genetic organization, nucleotide sequence, and the deduced amino acid sequences of the protein products. Two adjacent genes (DNO_1127 and DNO_1122), which correspond to orfM and orf197 from strain A198 (30), were part of this gene cluster. These genes appear to encode glutamine amidotransferase and dephosphocoenzyme A kinase, respectively. Although these genes are associated with pilBCD homologs in P. aeruginosa, N. gonorrhoeae, and P. putida (30), there is no evidence that they are involved in type IV fimbrial biogenesis (22). In addition to their similarity to their respective PilBCD homologs, the FimNOP proteins have similarity to the type II secretion components GspER (COG2804), GspFS (COG1459), and GspOA (COG1989), respectively.
Homologous recombination with suicide vectors containing insertionally inactivated genes was used to construct separate chromosomal fimN, fimO, and fimP mutants of strain VCS1703A after double-crossover events. The genotypes of these mutations were confirmed by PCR analysis and Southern hybridization (data not shown), and independently derived fimN (JIR3895 and JIR3896), fimO (JIR3885 and JIR3886), and fimP (JIR3889 and JIR3890) mutants were used for phenotypic characterization. Since orfM, fimNOP, and orf197 comprise an operon in strain A198 (31), it was important to ensure that the disruption of these genes did not result in polarity effects on the downstream genes. Accordingly, RNA from the fimN and fimO mutants was analyzed by RT-PCR, using primers specific for the fimOP and fimP-DNO_1122 intergenic regions. The results (data not shown) confirmed that the downstream genes were expressed in both mutants, either from read-through from the antibiotic resistance genes or from secondary promoters.
Compared to the large spreading colonies produced by the wild-type strain VCS1703A, the fimN, fimO, and fimP mutants all had small, nonspreading colonies (data not shown), which correlated with the loss of twitching motility as determined by an agar stab assay (Fig. 1) and the absence of surface fimbriae in transmission electron micrographs (Fig. 2). To determine the role of these gene products in fimbrial biogenesis, whole-cell lysates of these mutants were analyzed by SDS-PAGE and Western immunoblotting using antisera raised against D. nodosus type G fimbriae. The results showed that although these mutants did not have extracellular fimbriae, they still expressed the fimbrial subunit protein FimA (Fig. 3), indicating that these proteins are required for FimA polymerization. In addition, compared to the FimA proteins produced by the wild-type strain and the fimN and fimO mutants, the FimA subunits produced by the fimP mutants were larger (Fig. 3), consistent with the prediction that they represented uncleaved prepilin proteins. These results provided direct evidence in D. nodosus to support the previous finding that FimP is the prepilin peptidase responsible for the cleavage of the immature FimA prepilin (30, 31). In addition, these data confirmed the RT-PCR results since, if the fimN and fimO mutations did have polar effects, then all of the mutants would have unprocessed FimA protein.
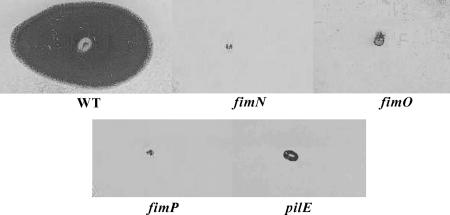
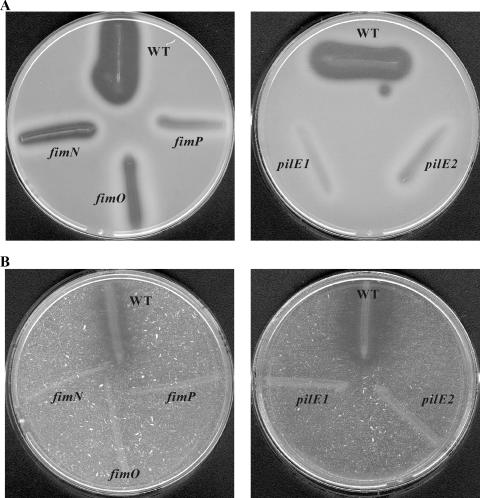
FIG. 1.
Analysis of twitching motility. Assays were carried out on 1% TAS agar with stab-inoculated D. nodosus cultures incubated for 5 to 7 days. After compression and staining with Coomassie brilliant blue, a large, dark zone around the point of inoculation is indicative of twitching motility. Assays of wild-type strain VCS1703A (WT), the fimN2 mutant JIR3896, the fimO2 mutant JIR3886, the fimP2 mutant JIR3890, and the pilE2 mutant JIR3911 are shown. The profile obtained from the second mutant of each gene was identical to that of each mutant represented here.
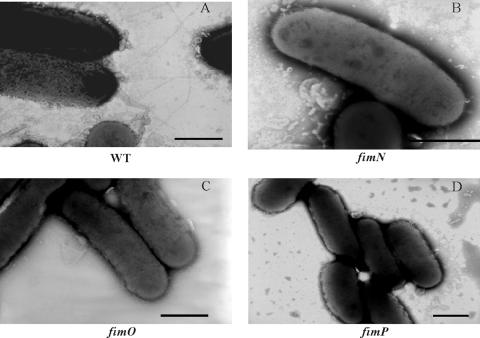
FIG. 2.
Absence of cell surface fimbriae in the fimN, fimO, and fimP mutants. Three-day-old cultures of the wild-type (WT) strain VCS1703A (A), the fimN1 mutant JIR3895 (B), the fimO2 mutant JIR3886 (C), and the fimP1 mutant JIR3889 (D) were removed from TAS agar with phosphate-buffered saline, negatively stained, and analyzed by transmission electron microscopy Bars = 1 μm. Note that the fimbriae, which were generally observed at one pole of the wild-type cells, were not observed in any of the cells examined in the fimN, fimO, or fimP mutants. The profile of the second mutant of each gene was identical to that of each mutant shown here.
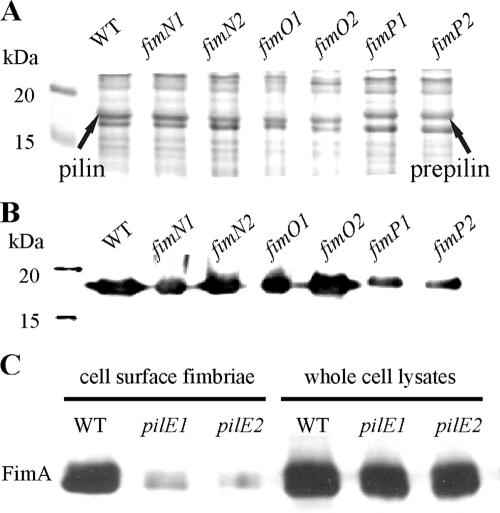
FIG. 3.
Western immunoblotting of whole-cell lysates and purified cell surface fimbriae. Shown is the production of FimA in whole-cell lysates from the wild-type strain VCS1703A (WT) and the mutants JIR3895 (fimN1), JIR3896 (fimN2), JIR3885 (fimO1), JIR3886 (fimO2), JIR3889 (fimP1), and JIR3890 (fimP2). Samples were separated by 12 to 15% SDS-PAGE and analyzed by Coomassie brilliant blue staining (A) and Western immunoblotting with fimbrial serogroup G-specific antisera at a 1:1,000 dilution (B). Note that the FimA-specific protein is larger in the fimP mutants. (C) Reduced extracellular fimbrial levels of pilE mutants. Purified cell surface fimbriae and whole-cell lysates of wild-type strain VCS1703A (WT) and the mutants JIR3910 (pilE1) and JIR3911 (pilE2) were analyzed.
The pilE gene is essential for type IV fimbrial biogenesis, and the pilC mutants are unstable.
The first gene product of the putative pilCVWX-fimU-pilE operon had similarity to PilC from N. gonorrhoeae and PilY1 from P. aeruginosa and was named after its N. gonorrhoeae homolog. The products of the last five genes of this operon had N-terminal sequence identity to the FimA fimbrial subunit (Fig. 4); these genes were designated after their homologs in P. aeruginosa. Many of the homologous genes in Pseudomonas (46) and Neisseria (82) species are also clustered at a single locus, but with a different genetic organization.
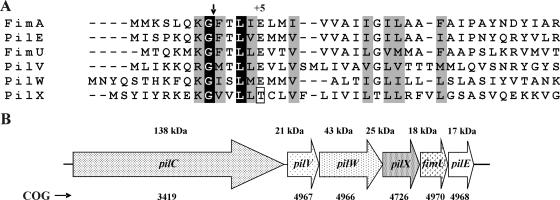
FIG. 4.
Sequence alignment of FimA and pilin-like proteins and genetic organization of the pilCVWX-fimU-pilE gene cluster. (A) N-terminal sequence alignment. The arrow indicates the prepilin processing site of FimA and the predicted processing sites of the other proteins. Identical residues are shaded in black, while residues with strong and weak similarity are shaded in gray. The atypical threonine residue at +5 in PilX is boxed. (B) Genetic organization of the pilC to pilE cluster. The direction of transcription, predicted molecular sizes of the corresponding proteins, and clusters of orthologous groups (COG) as determined by an NCBI (http://www.ncbi.nlm.nih.gov) conserved domain search are shown. The genes are shown to scale.
The putative PilE, FimU, PilV, and PilW proteins all have a conserved glutamate residue at the +5 position relative to the predicted prepilin peptidase cleavage site (Fig. 4), which is common for fimbrial subunits (14), pilin-like proteins (82), and pseudopilins from type II secretion systems (21). However, the putative PilX protein had a threonine residue at this position (Fig. 4); the absence of glutamate at +5 appears to be a conserved feature of PilX proteins (3, 82) and the type II secretion pseudopilin GspKX family (21). Since it has been shown that this conserved glutamate is required for fimbrial assembly (62), the incorporation of atypical pilins or pseudopilins into a growing fiber is thought to control its length by terminating fiber elongation (10).
To examine the biological function of at least some of these proteins in D. nodosus, we decided to mutate the first and last genes in this putative operon, pilC and pilE. Like its orthologs, the D. nodosus PilC protein was predicted by bioinformatic analysis to be located in the outer membrane and to have a conserved C-terminal region of unknown function. To determine the role of this protein, two independently derived nonpolar pilC mutants, JIR3913 and JIR3914, were constructed by natural transformation of the wild-type strain VCS1703A with the suicide vector pJIR3132 and shown by PCR analysis and Southern hybridization to be derived from double-crossover events (data not shown).
The colony morphology of these mutants was initially between that of the wild-type strain and nonfimbriate fimA mutants. The colonies had a domed center but only a moderate spreading edge, indicating that these mutants may have reduced levels of fimbriae. However, it was found that upon further subculture, the phenotypes of these mutants frequently switched to a nonfimbriate state, as indicated by the nonspreading domed colonies. SDS-PAGE analysis showed that these cells either had greatly reduced FimA production or no longer produced FimA (data no shown). Since there is no evidence from other bacteria that PilC may regulate fimbrial subunit expression, we concluded that the pilC mutants were unstable and that their continued subculture and growth led to the selection of secondary mutations that dramatically reduced FimA production. The instability of the pilC mutants meant that no further phenotypic analysis could be carried out on these strains.
Independently derived pilE mutants were constructed by natural transformation with the pilE suicide vector pJIR3088. Several potential pilE mutants were obtained, but they were all derived from a single transformation experiment. To obtain an independently derived mutant, additional transformation experiments were performed using both circular and linearized plasmid DNA, but unfortunately, no more mutants were obtained. Analysis of genomic DNA from two pilE mutants (JIR3910 and JIR3911) from the initial transformation experiment confirmed that they were derived from double-crossover events that insertionally inactivated the pilE gene (data not shown). The pilE mutants had slightly spreading to nonspreading domed colonies, which was consistent with the observation that they did not have detectable twitching motility zones in agar stab assays (Fig. 1). Further investigation using SDS-PAGE and Western immunoblotting with fimbrial antisera showed that the pilE mutants exhibited significantly reduced levels of surface fimbriae compared to the wild-type strain, although they produced approximately wild-type levels of FimA, as shown in whole-cell lysates (Fig. 3C). These results suggested that PilE was involved in fimbrial assembly and/or stabilization, with the latter possibility being supported by the presence of trace amounts of surface fimbriae in the pilE mutants.
FimN, FimO, FimP, and PilE are essential for natural transformation.
To determine the effect of the fimN, fimO, fimP, and pilE mutations on natural competence, we carried out transformation experiments using the rrnA-specific D. nodosus suicide vector pJIR2691, which contains a tet(M) tetracycline resistance cassette (Table 1). Natural transformation of the wild-type strain VCS1703A consistently produced tetracycline-resistant colonies that resulted from homologous recombination between pJIR2691 and one of the three chromosomal rrnA operons, as confirmed by PCR analysis. However, despite repeated attempts, no transformants were obtained from the fimN, fimO, fimP, or pilE mutants. The ability to undergo natural transformation is a prerequisite for the complementation of chromosomal mutants of D. nodosus, since there are no electroporation or conjugation methods available for this bacterium (34). Therefore, for technical reasons, it was not possible to complement these mutants by introducing a wild-type copy of the genes onto the chromosome.
FimN, FimO, FimP, and PilE are involved in extracellular protease secretion.
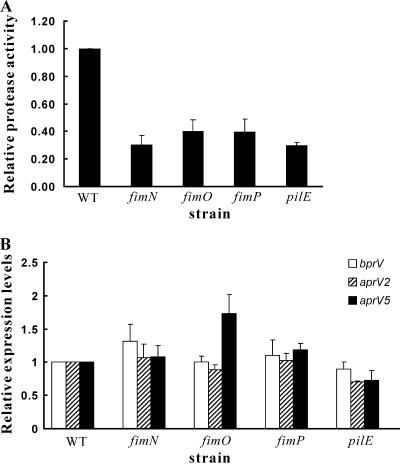
Previous studies have shown that the fimbrial subunit protein FimA is required for efficient extracellular protease secretion in D. nodosus (34), indicating that there may be a relationship between the type IV fimbrial system and the protein secretion apparatus in this organism. By performing qualitative caseinase and elastase assays on agar plates, it was initially observed that the fimN, fimO, fimP, and pilE mutants had reduced extracellular protease and elastase levels (Fig. 5). These results were confirmed by quantitative protease assays carried out on culture supernatants, using azocasein as the substrate. Each of the mutants had significantly reduced (P < 0.05) extracellular protease activity compared to the wild-type strain VCS1703A (Fig. 6A).
FIG. 5.
Qualitative protease activity. (A) Caseinase activity was determined by growing the strains indicated on skim milk agar for 2 days. (B) Elastase activity was determined by growing the strains indicated on TAS agar containing 0.3% elastin for 28 days. The wild-type strain VCS1703A (WT), the fimN2 mutant JIR3896 (fimN), the fimO2 mutant JIR3886 (fimO), the fimP2 mutant JIR3890 (fimP), and the pilE mutants JIR3910 (pilE1) and JIR3911(pilE2) are shown. The profile of each of the second fimN, fimO, and fimP mutants was identical to that of each mutant shown here.
FIG. 6.
Quantitative analysis of protease activity and protease gene expression. (A) Protease activity in culture supernatants was determined with an azocasein substrate. (B) Expression of the extracellular protease genes aprV2, bprV, and aprV5 was analyzed by qRT-PCR. All values were obtained from at least three independent biological samples and are expressed relative to the wild-type (WT) strain. Strains used were WT (VCS1703A), the fimN2 mutant (JIR3896), the fimO2 mutant (JIR3886), the fimP2 mutant (JIR3890), and the pilE2 mutant (JIR3911).
The proteases encoded by the aprV2, aprV5, and bprV genes (34) are responsible for all of the extracellular protease activity observed in D. nodosus (R. M. Kennan and J. I. Rood, unpublished results). To determine whether downregulation of protease gene expression was responsible for the reduction in extracellular protease activity, qRT-PCR experiments were performed using primers specific for each individual protease gene. The results showed that there were no significant differences in aprV2, aprV5, or bprV transcript levels between the wild-type strain and the various mutants (Fig. 6B), with the exception of a slight reduction in the aprV2 transcript level in the pilE mutant. This reduction would not account for the reduction in protease and elastase activity observed in the pilE mutants. Therefore, it was concluded that the FimN, FimO, FimP, and PilE proteins are all involved in extracellular protease secretion.
Proteomic analysis confirms that the mutants secrete reduced amounts of the AprV2, AprV5, and BprV proteases.
To determine whether there was a reduction in the secretion of all three extracellular proteases, protease zymogram analysis was carried out with culture supernatants from the fimN, fimO, and fimP mutants. The results showed that each of these mutants had reduced AprV2 and AprV5 activity (data not shown). Note that the basic protease BprV cannot be observed by this method (R. M. Kennan and J. I. Rood, unpublished results).
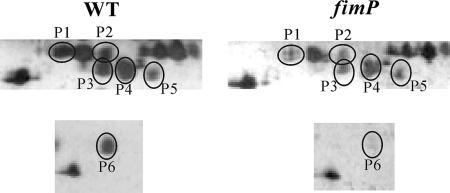
To confirm these results, and to see whether the secretion of any other proteins was affected by these mutations, proteomic studies were carried out. Culture supernatants from the wild-type and mutant strains were TCA precipitated and subjected to two-dimensional gel electrophoresis. Comparative analysis identified six protein spots (P1 to P6) that were present at reduced levels in each of the mutants (Fig. 7). Analysis by MS revealed that these spots were derived from the AprV5 (P1 and P2), AprV2 (P3 to P5), and BprV (P6) proteases, providing evidence that the secretion of BprV was also affected by the mutations (Table 3). No other consistent differences in protein spot intensity were observed, suggesting that in the culture supernatants, only the extracellular protease levels were affected by mutations in the fimbrial biogenesis system.
FIG. 7.
Two-dimensional gel electrophoresis of extracellular extracts of wild type (WT) and mutants. Culture supernatants were concentrated by TCA precipitation and subjected to two-dimensional gel electrophoresis. Sections of representative two-dimensional gels of the wild-type strain VCS1703A and the fimP2 mutant JIR3890 are shown. Proteins with reduced intensity in the mutant are circled and numbered from P1 to P6, as indicated.
TABLE 3.
Identification of extracellular proteins with altered expression in the fimP2 mutant
| Spot | Protein identification | Mascot protein scorea | Peptide count (% sequence coverage)b |
|---|---|---|---|
| P1 | AprV5, acidic serine protease | 244 | 7 (20) |
| P2 | AprV5, acidic serine protease | 147 | 7 (16) |
| P3 | AprV2, acidic serine protease | 433 | 11 (36) |
| P4 | AprV2, acidic serine protease | 805 | 13 (43) |
| P5 | AprV2, acidic serine protease | 407 | 12 (45) |
| P6 | BprV, basic serine protease | 193 | 9 (31) |
A score of greater than 100 was accepted as a positive hit.
Number of peptides obtained from MS + MS/MS spectra that match to the protein (degree of matched residues).
pilQ may be an essential gene in D. nodosus.
The 2,205-bp pilQ gene was identified by searching the products of the D. nodosus genome with the PilQ sequence from P. aeruginosa, and the gene encoded a putative 81-kDa (734 amino acids) protein that was predicted to be located in the outer membrane. Analysis of the putative D. nodosus PilQ protein showed that it contained the typical functional domains of secretins involved in type IV fimbrial biogenesis and type II secretion systems. The pilQ gene region also had a similar genetic organization to its homologs in P. aeruginosa and N. gonorrhoeae, being clustered with four other type IV fimbrial component genes in a putative pilMNOPQ operon.
It was postulated that the pilQ gene encoded an outer membrane protein that could be polymerized into a secretin complex that had a role in fimbrial extrusion and potentially in natural transformation and protease secretion. To examine this hypothesis, attempts were made to construct a chromosomal pilQ mutant in D. nodosus. A pilQ-specific suicide vector, pJIR2614, was constructed and introduced into VCS1703A by natural transformation. However, despite repeated attempts, when using this vector and PCR products derived from it, only single crossovers that did not mutate pilQ were observed. No double-crossover events that mutated pilQ were obtained. An alternative strategy that involved transformation with pJIR2714, which contained a 940-bp PCR product internal to pilQ, was then employed. Incorporation of this plasmid into the chromosome by a single-crossover event would lead to the mutation of the pilQ gene. Again, despite repeated attempts, no mutants were obtained. In this study and several others carried out in this laboratory, pilQ remains the only gene that we have been unable to mutate in D. nodosus. Based on these experiments and our analysis of the genome sequence, where pilQ is the only outer membrane secretin gene that can be identified, it is postulated that pilQ is an essential gene in D. nodosus.
DISCUSSION
D. nodosus is unique in that, not only does it exhibit the twitching motility that is typical among type IV fimbriate bacteria, but also it is virulent and competent for natural transformation, and it secretes extracellular proteases, all by processes that have been shown to be dependent on the amount of the fimbrial subunit protein that is produced (34, 61). In this study we have shown, to our knowledge for the first time in any one bacterium, that fimbrial biogenesis, not just the presence of the fimbrial subunit FimA, is required not only for twitching motility, but also for natural competence and extracellular protease secretion. Both the experimental and bioinformatics data are consistent with the hypothesis that the extracellular proteases of D. nodosus are secreted primarily by the fimbrial biogenesis system, which represents the only type II secretion system in this organism.
We have shown that in D. nodosus, FimN, FimO, and PilE are required for both fimbrial biogenesis and protease secretion, unlike their counterparts in P. aeruginosa, PilB, PilC, and PilE, respectively, which appear to be exclusively involved in type IV fimbrial biogenesis (67, 74, 76). Apart from the fimbrial subunit protein PilA and the prepilin peptidase PilD (8, 42), no other Pseudomonas proteins involved in fimbrial biogenesis have been reported to be required for extracellular protein secretion. In Vibrio cholerae, the colonization factor TcpF is secreted by the Tcp pathway, but unlike the D. nodosus proteases, this protein is encoded within the Tcp fimbrial locus and is part of the Tcp system, although it is not essential for fimbrial biogenesis (35, 58). A recent study with Francisella demonstrated that several type IV fimbrial homologs mediate protein secretion, but there is no evidence to show that this bacterium produces surface fimbriae (25). A role for FimP in protease secretion in D. nodosus was more predictable, since its homolog in P. aeruginosa, PilD (XcpA or GspOA), cleaves the signal sequences of both the pilin subunit PilA and the pseudopilins involved in type II secretion (21).
The fact that both FimP and FimA (34) were required for extracellular protease secretion in D. nodosus provided evidence that these proteases may be secreted via a type II secretion pathway. Type II secretion systems have 12 to 16 components (21, 29, 64, 69) that include several proteins with similarity to components of the type IV fimbrial biogenesis pathway, such as GspDQ (PilQ homolog), GspER (FimN/PilB homolog), GspFS (FimO/PilC homolog), GspOA (PilD/FimP homolog), GspGT-KX (pilin homologs), and several proteins (GspLY, GspMZ, and GspCP) with no homologs in fimbrial biogenesis. P. aeruginosa has both systems, which share a prepilin peptidase, PilD/XcpA, and presumably the fimbrial subunit PilA, whose function in type II secretion is not known, although together with the pseudopilins, it has been predicted to form an intermediate structure of the secretion apparatus (42). Bioinformatic analysis indicated that the seemingly essential pilQ gene appeared to encode the only outer membrane secretin in D. nodosus and that apart from the genes involved in fimbrial biogenesis, no other type II secretion genes were present. There were no identifiable homologs of the type IV fimbria-independent components and no FimN, FimO, FimP, or pseudopilin-like paralogs. D. nodosus does have an entire complement of Sec components, which are mainly utilized for the inner membrane translocation of proteins targeted for type II secretion (64). Taken together, these data suggest that proteases may be secreted by a novel type II secretion-related pathway, which utilizes the components of the type IV fimbrial apparatus but does not require GspLY, GspMZ, and GspCP homologs. In support of this view is the fact that the novel type II-related secretion pathway Xcm, which is required for the secretion of a manganese-oxidizing factor in P. putida strain GB-1, also lacks GspLY, GspMZ, and GspCP (15).
We postulate that the growing periplasmic fimbrial polymer may act to push the proteases through the PilQ secretin in the outer membrane, a process that resembles the extrusion of proteins through GspDQ by the pseudopilus in the type II secretion pathway (21, 29, 69). Therefore, we suggest that in D. nodosus, the PilQ secretin is utilized by both the fimbrial biogenesis and protein secretion systems, which may explain why it is difficult, if not impossible, to insertionally inactivate. It follows that retraction of the fimbriae might also be required for protease secretion, being required to release PilQ secretin complexes that are blocked by protruding fimbriae. Since there are no amino acid biosynthesis pathways encoded on the D. nodosus genome (54), the extracellular proteases may have an essential physiological role in the provision of the amino acids that are required for growth. Therefore, our inability to construct a pilQ mutant may be related to the key role of PilQ in protease secretion.
It was previously suggested, based purely on bioinformatic analysis, that AprV2, AprV5, and BprV may be autotransporter proteins whose passage through the outer membrane is dependent upon their carboxy-terminal domains and does not require any other proteins (28). However, there is no experimental evidence to support this hypothesis. We have now provided clear evidence that protease secretion does require other proteins, specifically, those involved in fimbrial biogenesis. It is possible that the putative autotransporter properties of these enzymes may have been responsible for the residual levels of extracellular proteases that were observed in the various mutants. Alternatively, this activity may simply have resulted from the lysis of some cells in the bacterial population.
PilE is one of five pilin-like proteins identified from the D. nodosus genome sequence. In P. aeruginosa, mutation of pilE leads to a loss of type IV fimbriae (67), whereas in N. gonorrhoeae, the PilE homologs PilV and ComP appear to play a more important role in specific DNA binding during natural transformation (PilV and ComP) and adherence (PilV) than in fimbrial biogenesis (1, 81, 83). In D. nodosus, the pilE mutant had greatly reduced levels of surface fimbriae, although wild-type levels of FimA were still produced. We suggest that PilE is essential for fimbrial assembly and/or stabilization, perhaps by being incorporated into the fimbrial polymer as a minor but critical subunit.
The pilC gene encodes a putative outer membrane protein with similarity to PilC from Neisseria spp., which appears to be a fimbrial tip adhesin and is also involved in fimbrial biogenesis and fimbrial retraction (52, 55, 66), and PilY1 from P. aeruginosa, which is required for fimbrial structure and twitching motility (3) and is suggested to be involved in the regulation of fimbrial retraction (80). Although we were able to construct pilC mutants in D. nodosus, and these mutants were altered in colony morphology, they were highly unstable and rapidly lost the ability to produce FimA. These results suggest that PilC is involved in fimbrial biogenesis, but further research would be required to confirm this hypothesis.
Other studies have shown that type IV fimbrial biogenesis and natural transformation are closely related, with some proteins having a dual function in both processes (7, 26). Our findings, both in this study and previously (34), have shown that mutation of any of the fimbrial biogenesis genes eliminates the ability of D. nodosus to undergo natural transformation. Natural transformation has been intensely studied in N. gonorrhoeae and a model involving type IV fimbrial components and some type IV fimbriae-associated proteins proposed (26). Comparative studies on the potential DNA transformation components demonstrated that despite their common properties, such as three ComEA homologs and one ComEC homolog (18, 20), which are presumably involved in DNA uptake, and a lipoprotein ComL homolog, which is thought to be involved in penetrating the peptidoglycan (19, 23), there are differences between D. nodosus and N. gonorrhoeae. Apart from the different roles of the PilE homologs, which have already been discussed, pilR and rpoN mutants of D. nodosus express residual levels of FimA but do not elaborate surface fimbriae and are not transformable (61), whereas in N. gonorrhoeae, limited amounts of pilin that do not produce fully extended fimbriae are sufficient for natural transformation (41). Although the D. nodosus results may reflect the low level of natural transformability in this organism, it appears that in D. nodosus, the same apparatus may be responsible for natural transformation and fimbrial protrusion and retraction. Current models suggest that natural transformation utilizes the PilQ secretin pore for DNA uptake (7, 26). We postulate that in D. nodosus, DNA uptake occurs through the PilQ secretin and also is dependent upon extension or retraction of its type IV fimbriae.
In conclusion, we have demonstrated that in D. nodosus, several proteins that are required for fimbrial biogenesis, FimN, FimO, FimP, and PilE, are also required for both natural transformation and extracellular protease secretion. It is postulated that in D. nodosus, natural transformation requires an intact type IV fimbrial apparatus and that the extracellular proteases AprV2, AprV5, and BprV are secreted via a type II secretion-like pathway that also utilizes the type IV fimbrial apparatus. Further genetic and biochemical studies will be required to determine the molecular mechanisms by which type IV fimbrial biogenesis, natural transformation, and protease secretion are mediated.
Acknowledgments
We sincerely thank Khim Hoe for assistance with electron microscopy; Ian Smith, David Steer, and Shane Reeve of the Department of Biochemistry and Molecular Biology, Monash University, for help with proteomics and MS analysis; and Cynthia Whitchurch for helpful discussions.
This research was supported by the Australian Research Council through funding to the Australian Research Council Centre of Excellence in Structural and Functional Microbial Genomics. Xiaoyan Han was supported by a postgraduate scholarship provided by the Centre of Excellence.
Footnotes
Published ahead of print on 18 May 2007.
REFERENCES
- 1.Aas, F. E., C. Lovold, and J. M. Koomey. 2002. An inhibitor of DNA binding and uptake events dictates the proficiency of genetic transformation in Neisseria gonorrhoeae: mechanism of action and links to type IV pilus expression. Mol. Microbiol. 46:1441-1450. [DOI] [PubMed] [Google Scholar]
- 2.Aas, F. E., M. Wolfgang, S. Frye, S. Dunham, C. Lovold, and M. Koomey. 2002. Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression. Mol. Microbiol. 46:749-760. [DOI] [PubMed] [Google Scholar]
- 3.Alm, R. A., J. P. Hallinan, A. A. Watson, and J. S. Mattick. 1996. Fimbrial biogenesis genes of Pseudomonas aeruginosa: pilW and pilX increase the similarity of type 4 fimbriae to the GSP protein-secretion systems and pilY encodes a gonococcal PilC homologue. Mol. Microbiol. 22:161-173. [DOI] [PubMed] [Google Scholar]
- 4.Alm, R. A., and J. S. Mattick. 1997. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene 192:89-98. [DOI] [PubMed] [Google Scholar]
- 5.Alm, R. A., and J. S. Mattick. 1995. Identification of a gene, pilV, required for type 4 fimbrial biogenesis in Pseudomonas aeruginosa, whose product possesses a pre-pilin-like leader sequence. Mol. Microbiol. 16:485-496. [DOI] [PubMed] [Google Scholar]
- 6.Alm, R. A., and J. S. Mattick. 1996. Identification of two genes with prepilin-like leader sequences involved in type 4 fimbrial biogenesis in Pseudomonas aeruginosa. J. Bacteriol. 178:3809-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Averhoff, B. 2004. DNA transport and natural transformation in mesophilic and thermophilic bacteria. J. Bioenerg. Biomembr. 36:25-33. [DOI] [PubMed] [Google Scholar]
- 8.Bally, M., G. Ball, A. Badere, and A. Lazdunski. 1991. Protein secretion in Pseudomonas aeruginosa: the xcpA gene encodes an integral inner membrane protein homologous to Klebsiella pneumoniae secretion function protein PulO. J. Bacteriol. 173:479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billington, S. J., J. L. Johnston, and J. I. Rood. 1996. Virulence regions and virulence factors of the ovine footrot pathogen, Dichelobacter nodosus. FEMS Microbiol. Lett. 145:147-156. [DOI] [PubMed] [Google Scholar]
- 10.Bleves, S., R. Voulhoux, G. Michel, A. Lazdunski, J. Tommassen, and A. Filloux. 1998. The secretion apparatus of Pseudomonas aeruginosa identification of a fifth pseudopilin, XcpX (GspK family). Mol. Microbiol. 27:31-40. [DOI] [PubMed] [Google Scholar]
- 11.Bradley, D. E. 1973. A pilus dependent Pseudomonas aeruginosa bacteriophage with a long noncontractile tail. Virology 51:489-492. [DOI] [PubMed] [Google Scholar]
- 12.Chiang, P., M. Habash, and L. I. Burrows. 2005. Disparate subcellular localization patterns of Pseudomonas aeruginosa type IV pilus ATPases involved in twitching motility. J. Bacteriol. 187:829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins, R. F., S. A. Frye, A. Kitmitto, R. C. Ford, T. Tonjum, and J. P. Derrick. 2004. Structure of the Neisseria meningitidis outer membrane PilQ secretin complex at 12 A resolution. J. Biol. Chem. 279:39750-39756. [DOI] [PubMed] [Google Scholar]
- 14.Craig, L., M. E. Pique, and J. A. Tainer. 2004. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2:363-378. [DOI] [PubMed] [Google Scholar]
- 15.De Vrind, J., A. De Groot, G. J. Brouwers, J. Tommassen, and E. De Vrind-De Jong. 2003. Identification of a novel Gsp-related pathway required for secretion of the manganese-oxidizing factor of Pseudomonas putida strain GB-1. Mol. Microbiol. 47:993-1006. [DOI] [PubMed] [Google Scholar]
- 16.Drake, S. L., and M. Koomey. 1995. The product of the pilQ gene is essential for the biogenesis of pili in Neisseria gonorrhoeae. Mol. Microbiol. 18:975-986. [DOI] [PubMed] [Google Scholar]
- 17.Drake, S. L., S. A. Sandstedt, and M. Koomey. 1997. PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol. Microbiol. 23:657-668. [DOI] [PubMed] [Google Scholar]
- 18.Draskovic, I., and D. Dubnau. 2005. Biogenesis of a putative channel protein, ComEC, required for DNA uptake: membrane topology, oligomerization and formation of disulphide bonds. Mol. Microbiol. 55:881-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Facius, D., M. Fussenegger, and T. F. Meyer. 1996. Sequential action of factors involved in natural competence for transformation of Neisseria gonorrhoeae. FEMS Microbiol. Lett. 137:159-164. [DOI] [PubMed] [Google Scholar]
- 20.Facius, D., and T. F. Meyer. 1993. A novel determinant (comA) essential for natural transformation competence in Neisseria gonorrhoeae and the effect of a comA defect on pilin variation. Mol. Microbiol. 10:699-712. [DOI] [PubMed] [Google Scholar]
- 21.Filloux, A. 2004. The underlying mechanisms of type II protein secretion. Biochim. Biophys. Acta 1694:163-179. [DOI] [PubMed] [Google Scholar]
- 22.Freitag, N. E., H. S. Seifert, and M. Koomey. 1995. Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol. Microbiol. 16:575-586. [DOI] [PubMed] [Google Scholar]
- 23.Fussenegger, M., D. Facius, J. Meier, and T. F. Meyer. 1996. A novel peptidoglycan-linked lipoprotein (ComL) that functions in natural transformation competence of Neisseria gonorrhoeae. Mol. Microbiol. 19:1095-1105. [DOI] [PubMed] [Google Scholar]
- 24.Ghimire, S. C., and J. R. Egerton. 1999. PCR-RFLP of outer membrane proteins gene of Dichelobacter nodosus: a new tool in the epidemiology of footrot. Epidemiol. Infect. 122:521-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hager, A. J., D. L. Bolton, M. R. Pelletier, M. J. Brittnacher, L. A. Gallagher, R. Kaul, S. J. Skerrett, S. I. Miller, and T. Guina. 2006. Type IV pili-mediated secretion modulates Francisella virulence. Mol. Microbiol. 62:227-237. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton, H. L., and J. P. Dillard. 2006. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol. Microbiol. 59:376-385. [DOI] [PubMed] [Google Scholar]
- 27.Helaine, S., E. Carbonnelle, L. Prouvensier, J. L. Beretti, X. Nassif, and V. Pelicic. 2005. PilX, a pilus-associated protein essential for bacterial aggregation, is a key to pilus-facilitated attachment of Neisseria meningitidis to human cells. Mol. Microbiol. 55:65-77. [DOI] [PubMed] [Google Scholar]
- 28.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, T. L., J. Abendroth, W. G. Hol, and M. Sandkvist. 2006. Type II secretion: from structure to function. FEMS Microbiol. Lett. 255:175-186. [DOI] [PubMed] [Google Scholar]
- 30.Johnston, J. L., S. J. Billington, V. Haring, and J. I. Rood. 1995. Identification of fimbrial assembly genes from Dichelobacter nodosus: evidence that fimP encodes the typeIV prepilin peptidase. Gene 161:21-26. [DOI] [PubMed] [Google Scholar]
- 31.Johnston, J. L., S. J. Billington, V. Haring, and J. I. Rood. 1998. Complementation analysis of the Dichelobacter nodosus fimN, fimO, and fimP genes in Pseudomonas aeruginosa and transcriptional analysis of the fimNOP gene region. Infect. Immun. 66:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiser, D. 2000. Bacterial motility: how do pili pull? Curr. Biol. 10:R777-R780. [DOI] [PubMed] [Google Scholar]
- 33.Kennan, R. M., S. J. Billington, and J. I. Rood. 1998. Electroporation-mediated transformation of the ovine footrot pathogen Dichelobacter nodosus. FEMS Microbiol. Lett. 169:383-389. [DOI] [PubMed] [Google Scholar]
- 34.Kennan, R. M., O. P. Dhungyel, R. J. Whittington, J. R. Egerton, and J. I. Rood. 2001. The type IV fimbrial subunit gene (fimA) of Dichelobacter nodosus is essential for virulence, protease secretion, and natural competence. J. Bacteriol. 183:4451-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirn, T. J., N. Bose, and R. K. Taylor. 2003. Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae. Mol. Microbiol. 49:81-92. [DOI] [PubMed] [Google Scholar]
- 36.Kortt, A. A., J. E. Burns, J. A. Vaughan, and D. J. Stewart. 1994. Purification of the extracellular acidic proteases of Dichelobacter nodosus. Biochem. Mol. Biol. Int. 34:1157-1166. [PubMed] [Google Scholar]
- 37.Kortt, A. A., and D. J. Stewart. 1994. Properties of the extracellular acidic proteases of Dichelobacter nodosus. Stability and specificity of peptide bond cleavage. Biochem. Mol. Biol. Int. 34:1167-1176. [PubMed] [Google Scholar]
- 38.La Fontaine, S., and J. I. Rood. 1996. Organization of ribosomal RNA genes from the footrot pathogen Dichelobacter nodosus. Microbiology 142:889-899. [DOI] [PubMed] [Google Scholar]
- 39.Lilley, G. G., M. C. Riffkin, D. J. Stewart, and A. A. Kortt. 1995. Nucleotide and deduced protein sequence of the extracellular, serine basic protease gene bprB from Dichelobacter nodosus strain 305: comparison with the basic protease gene bprV from virulent strain 198. Biochem. Mol. Biol. Int. 36:101-111. [PubMed] [Google Scholar]
- 40.Liu, D., and W. K. Yong. 1993. Use of elastase test, gelatin gel test and electrophoretic zymogram to determine virulence of Dichelobacter nodosus isolated from ovine foot rot. Res. Vet. Sci. 55:124-129. [DOI] [PubMed] [Google Scholar]
- 41.Long, C. D., D. M. Tobiason, M. P. Lazio, K. A. Kline, and H. S. Seifert. 2003. Low-level pilin expression allows for substantial DNA transformation competence in Neisseria gonorrhoeae. Infect. Immun. 71:6279-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu, H. M., S. T. Motley, and S. Lory. 1997. Interactions of the components of the general secretion pathway: role of Pseudomonas aeruginosa type IV pilin subunits in complex formation and extracellular protein secretion. Mol. Microbiol. 25:247-259. [DOI] [PubMed] [Google Scholar]
- 43.Maier, B. 2005. Using laser tweezers to measure twitching motility in Neisseria. Curr. Opin. Microbiol. 8:344-349. [DOI] [PubMed] [Google Scholar]
- 44.Martin, P. R., M. Hobbs, P. D. Free, Y. Jeske, and J. S. Mattick. 1993. Characterization of pilQ, a new gene required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 9:857-868. [DOI] [PubMed] [Google Scholar]
- 45.Martin, P. R., A. A. Watson, T. F. McCaul, and J. S. Mattick. 1995. Characterization of a five-gene cluster required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 16:497-508. [DOI] [PubMed] [Google Scholar]
- 46.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56:289-314. [DOI] [PubMed] [Google Scholar]
- 47.Mattick, J. S., B. J. Anderson, M. R. Mott, and J. R. Egerton. 1984. Isolation and characterization of Bacteroides nodosus fimbriae: structural subunit and basal protein antigens. J. Bacteriol. 160:740-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merz, A. J., and K. T. Forest. 2002. Bacterial surface motility: slime trails grappling hooks and nozzles. Curr. Biol. 12:R297-R303. [DOI] [PubMed] [Google Scholar]
- 50.Merz, A. J., and M. So. 2000. Interactions of pathogenic Neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423-457. [DOI] [PubMed] [Google Scholar]
- 51.Merz, A. J., M. So, and M. P. Sheetz. 2000. Pilus retraction powers bacterial twitching motility. Nature 407:98-102. [DOI] [PubMed] [Google Scholar]
- 52.Morand, P. C., E. Bille, S. Morelle, E. Eugene, J. L. Beretti, M. Wolfgang, T. F. Meyer, M. Koomey, and X. Nassif. 2004. Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins. EMBO J. 23:2009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moses, E. K., R. T. Good, M. Sinistaj, S. J. Billington, C. J. Langford, and J. I. Rood. 1995. A multiple site-specific DNA inversion model for the control of Omp1 phase and antigenic variation in Dichelobacter nodosus. Mol. Microbiol. 17:183-196. [DOI] [PubMed] [Google Scholar]
- 54.Myers, G. S. A., D. Parker, K. Al-Hasani, R. M. Kennan, T. Seemann, Q. Ren, J. H. Bagder, J. D. Selengut, R. T. DeBoy, H. Tettelin, J. D. Boyce, V. P. McCarl, X. Han, W. C. Nelson, R. Madupu, Y. Mohamoud, T. Holley, N. Fedorova, H. Khouri, S. P. Bottomley, R. J. Whittington, B. Adler, J. G. Songer, J. I. Rood, and I. T. Paulsen. 2007. Genome sequence and identification of candidate vaccine antigens from the animal pathogen Dichelobacter nodosus. Nat. Biotechnol. 25:569-575. doi: 10.1038/nbt1302. [DOI] [PubMed] [Google Scholar]
- 55.Nassif, X., J. L. Beretti, J. Lowy, P. Stenberg, P. O'Gaora, J. Pfeifer, S. Normark, and M. So. 1994. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc. Natl. Acad. Sci. USA 91:3769-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nunn, D. 1999. Bacterial type II protein export and pilus biogenesis: more than just homologies? Trends Cell Biol. 9:402-408. [DOI] [PubMed] [Google Scholar]
- 57.Nunn, D., S. Bergman, and S. Lory. 1990. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J. Bacteriol. 172:2911-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogierman, M. A., S. Zabihi, L. Mourtzios, and P. A. Manning. 1993. Genetic organization and sequence of the promoter-distal region of the tcp gene cluster of Vibrio cholerae. Gene 126:51-60. [DOI] [PubMed] [Google Scholar]
- 59.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 60.Parker, D., R. M. Kennan, G. S. Myers, I. T. Paulsen, and J. I. Rood. 2005. Identification of a Dichelobacter nodosus ferric uptake regulator and determination of its regulatory targets. J. Bacteriol. 187:366-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parker, D., R. M. Kennan, G. S. Myers, I. T. Paulsen, J. G. Songer, and J. I. Rood. 2006. Regulation of type IV fimbrial biogenesis in Dichelobacter nodosus. J. Bacteriol. 188:4801-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasloske, B. L., D. G. Scraba, and W. Paranchych. 1989. Assembly of mutant pilins in Pseudomonas aeruginosa: formation of pili composed of heterologous subunits. J. Bacteriol. 171:2142-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riffkin, M. C., L. F. Wang, A. A. Kortt, and D. J. Stewart. 1995. A single amino-acid change between the antigenically different extracellular serine proteases V2 and B2 from Dichelobacter nodosus. Gene 167:279-283. [DOI] [PubMed] [Google Scholar]
- 64.Robert, V., A. Filloux, and G. P. Michel. 2005. Subcomplexes from the Xcp secretion system of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 252:43-50. [DOI] [PubMed] [Google Scholar]
- 65.Rood, J. I. 2002. Genomic islands of Dichelobacter nodosus. Curr. Top. Microbiol. Immunol. 264:47-60. [PubMed] [Google Scholar]
- 66.Rudel, T., I. Scheuerpflug, and T. F. Meyer. 1995. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature 373:357-359. [DOI] [PubMed] [Google Scholar]
- 67.Russell, M. A., and A. Darzins. 1994. The pilE gene product of Pseudomonas aeruginosa, required for pilus biogenesis, shares amino acid sequence identity with the N-termini of type 4 prepilin proteins. Mol. Microbiol. 13:973-985. [DOI] [PubMed] [Google Scholar]
- 68.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 69.Sandkvist, M. 2001. Biology of type II secretion. Mol. Microbiol. 40:271-283. [DOI] [PubMed] [Google Scholar]
- 70.Skerker, J. M., and H. C. Berg. 2001. Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. USA 98:6901-6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skerman, T. M. 1975. Determination of some in vitro growth requirements of Bacteroides nodosus. J. Gen. Microbiol. 87:107-119. [DOI] [PubMed] [Google Scholar]
- 72.Stewart, D. J. 1989. Footrot of sheep, p. 5-45. In J. R. Egerton, W. K. Yong, and G. G. Riffkin (ed.), Footrot and foot abscess of ruminants. CRC Press, Boca Raton, FL.
- 73.Stewart, D. J. 1979. The role of elastase in the differentiation of Bacteroides nodosus infections in sheep and cattle. Res. Vet. Sci. 27:99-105. [PubMed] [Google Scholar]
- 74.Strom, M. S., D. Nunn, and S. Lory. 1991. Multiple roles of the pilus biogenesis protein PilD: involvement of PilD in excretion of enzymes from Pseudomonas aeruginosa. J. Bacteriol. 173:1175-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tonjum, T., N. E. Freitag, E. Namork, and M. Koomey. 1995. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol. Microbiol. 16:451-464. [DOI] [PubMed] [Google Scholar]
- 76.Turner, D. H., and J. C. Lara. 1993. Mutations in the consensus ATP-binding sites of XcpR and PilB eliminate extracellular protein secretion and pilus biogenesis in Pseudomonas aeruginosa. J. Bacteriol. 175:4962-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wall, D., and D. Kaiser. 1999. Type IV pili and cell motility. Mol. Microbiol. 32:1-10. [DOI] [PubMed] [Google Scholar]
- 78.Wall, D., P. E. Kolenbrander, and D. Kaiser. 1999. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pilus biogenesis, social motility, and development. J. Bacteriol. 181:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 80.Whitchurch, C. B. 2006. Type IV pili in Pseudomonas species, p. 139-188. In J. L. Ramos and R. J. Levesque (ed.), Pseudomonas, vol. 4. Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 81.Winther-Larsen, H. C., F. T. Hegge, M. Wolfgang, S. F. Hayes, and J. P. van Putten, and M. Koomey. 2001. Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence. Proc. Natl. Acad. Sci. USA 98:15276-15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winther-Larsen, H. C., M. Wolfgang, S. Dunham, J. P. van Putten, D. Dorward, C. Lovold, F. E. Aas, and M. Koomey. 2005. A conserved set of pilin-like molecules controls type IV pilus dynamics and organelle-associated functions in Neisseria gonorrhoeae. Mol. Microbiol. 56:903-917. [DOI] [PubMed] [Google Scholar]
- 83.Wolfgang, M., J. P. van Putten, S. F. Hayes, and M. Koomey. 1999. The comP locus of Neisseria gonorrhoeae encodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation. Mol. Microbiol. 31:1345-1357. [DOI] [PubMed] [Google Scholar]