Abstract
The length of the tandem repeat region of the Vsa protein of Mycoplasma pulmonis has previously been shown to modulate the susceptibility of mycoplasmas to killing by complement: cells that produce a short form of the Vsa protein are highly sensitive, and cells producing the long Vsa protein are resistant. In contrast to their differing susceptibilities to complement, the mycoplasmas were highly sensitive to gramicidin irrespective of the length of the Vsa protein produced. We show here that when encased within a biofilm, cells of M. pulmonis producing a short form of the Vsa protein were more resistant to complement and gramicidin than mycoplasmas that were dispersed. The resistance appeared to be localized to those mycoplasmas within tower structures of the biofilms. Biofilm formation may be a mechanism that protects mycoplasmas from host immunity.
Biofilms are communities of microorganisms encased in an extracellular matrix of polysaccharide, lipid, DNA, and protein (7). A major function of a biofilm is to protect the microbial cells from antimicrobial agents (3) and immune surveillance (15). Generally, biofilms are structurally organized into honeycombed regions and areas of outgrowths referred to as mushrooms or towers (16). Biofilms formed in vitro by the mycoplasmas contain all of the molecular and structural features found in biofilms that are formed by other bacteria (9, 11). The tower structures contain mycoplasmal cells that are densely packed together relative to the honeycombed region.
The variable surface antigens (Vsa proteins) of Mycoplasma pulmonis modulate numerous properties of the mycoplasma, including susceptibility to complement, susceptibility to phage, and the abilities to hemadsorb and to form a biofilm (11-13). Differences in the size of Vsa result from the loss or gain in the number of tandem repeat units via slipped-strand mispairing in the gene's 3′ repetitive region (10). Mycoplasmas that produce a Vsa protein containing about 40 tandem repeats do not form biofilms (11) and are resistant to killing by complement but susceptible to the antimicrobial protein gramicidin (12, 13). Mycoplasmas that produce a Vsa protein containing a few tandem repeat units, such as M. pulmonis strain CT182-R3, are efficiently killed by both complement and gramicidin. It has been proposed that the long Vsa proteins sterically hinder the access of larger molecules, such as complement, to the mycoplasma cell membrane while permitting the access of smaller molecules such as gramicidin (12).
The mycoplasmas used in these previous studies were dispersed into the reaction medium and were readily accessible to complement and gramicidin. M. pulmonis strain CT182-R3 grows as a biofilm (11). To determine whether the biofilm was protective, we incubated intact mycoplasma biofilms or mycoplasma cells that were dispersed from biofilms with complement or gramicidin. We found that the biofilm protected the mycoplasmal cells. Furthermore, the resistance appeared to be localized to the tower structures in the biofilms.
MATERIALS AND METHODS
Mycoplasma strains.
M. pulmonis strain CT182-R3 produces a VsaA protein containing three tandem repeats and has been shown to be sensitive to killing by complement (12, 13). The starter stocks of strain CT182-R3 used for these experiments are the stocks used in previous publications. The mycoplasmas were grown for 48 h on 22-mm by 22-mm glass coverslips (11) in 12-well polystyrene tissue culture plates, or in 6-well plates where indicated (Corning Incorporated, Corning, NY), in mycoplasma broth supplemented with 20% heat-inactivated (HIA) whole horse serum (HyClone).
Complement killing assays.
Mycoplasma biofilms that had adhered to tissue culture plates were washed three times with phosphate-buffered saline (PBS) and either left intact or dispersed by scraping and pipetting. The dispersed cells or the biofilms were incubated with guinea pig serum (GPS; Colorado Serum Company, Denver, CO) or HIA-GPS at a final concentration of 10% at 37°C for 30 min in the presence of Mg2+ and Ca2+ at final concentrations of 5 mM and 1 mM, respectively. The biofilms were then dispersed by scraping and pipetting. The mycoplasmas were gently sonicated to disrupt cell aggregates and serially diluted to determine the number of CFU recovered from the reactions. The percentage of CFU recovered for each replicate well was represented as the fraction (× 100) of CFU recovered after complement treatment relative to the CFU surviving treatment with HIA-GPS. Data were analyzed by the Student t test.
In some experiments, the GPS-treated biofilms were washed with PBS, either left intact or dispersed into PBS, and incubated a second time with a 10% concentration of GPS or HIA-GPS for 30 min at 37°C. After the second round of treatment, the mycoplasmas from the reactions were dispersed and sonicated, and the fraction of surviving CFU was determined. Data were analyzed by one-way analysis of variance, with pairwise multiple comparisons performed by the Student-Newman-Keuls method.
Gramicidin killing assays.
Mycoplasmal biofilms adhering to 12-well plates were washed three times with PBS. Five hundred microliters of gramicidin from Bacillus aneurinilyticus (Sigma), at a concentration of 100 ng/ml in PBS, or PBS alone, was added to the wells. In one experimental group, the biofilm was scraped and dispersed by pipetting immediately after the addition of gramicidin. In another experimental group, the biofilm was left intact during the incubation period. After incubation for 30 min at 37°C, 1.5 ml of PBS was added to each well, and the biofilms were scraped and dispersed by pipetting. This fourfold dilution reduced the concentration of gramicidin to about the MIC for M. pulmonis (12). The reaction mixtures were immediately diluted 100-fold in mycoplasma broth, sonicated, and serially diluted to determine the number of CFU recovered. The percentage of CFU recovered was expressed as the fraction (× 100) of CFU from the wells treated with gramicidin relative to the CFU recovered from wells incubated with PBS only. The reactions were performed in triplicate for each group in an experiment, and the experiments were performed twice. Data were analyzed by the Student t test.
Fluorescence microscopy.
The biofilms that were grown on 22-mm by 22-mm glass coverslips were washed three times in sterile PBS. For the reactions with complement, the biofilms were incubated with a 10% concentration of GPS or HIA-GPS for 30 min at 37°C as described above. For reactions with gramicidin, the biofilms were incubated with gramicidin at a concentration of 500 ng/ml or with PBS for 30 min at 37°C.
After the biofilms were incubated with complement or gramicidin, the glass coverslips were washed three times in PBS and incubated in PBS containing 20 μg of Hoechst 33342 and 500 ng of propidium iodide (PI) per ml for 30 min. The coverslips were washed twice in PBS and mounted on glass slides with a solution containing 50% glycerol and 50% Prolong Gold mounting medium (Molecular Probes). Digital images were acquired at a magnification of ×1,600 with a Leica HC fluorescence microscope using the Chroma 86012v2 4′,6′-diamidino-2-phenylindole or Texas red filter set. Since intact cells are impermeable to PI but permeable to Hoechst 33342, mycoplasma cells that bound PI were interpreted as dead cells.
RESULTS
Mycoplasmas in a biofilm are relatively resistant to complement.
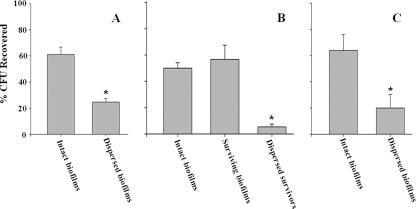
When mycoplasmas were incubated with GPS a single time, about 60% of the mycoplasmas were recovered from biofilms that were left intact (Fig. 1A) while 25% of the mycoplasmas were recovered from biofilms that were dispersed prior to incubation with GPS (P < 0.001). In separate experiments, the percentage of CFU recovered from GPS-treated biofilms that were left intact and incubated a second time with GPS did not differ from the percentage of CFU recovered from biofilms that were incubated with GPS only once (Fig. 1B). About 50% of the CFU were recovered. In contrast, when the GPS-treated biofilm was dispersed and incubated a second time with GPS, only 6% of the mycoplasmas were recovered (P < 0.001). Similar results were obtained using 6-well plates instead of 12-well plates. About 50% of the mycoplasmas were recovered from biofilms that were incubated a single time with GPS, while only 10% of the mycoplasmas that were dispersed from the first reaction were recovered when they were incubated a second time with GPS (data not shown).
FIG. 1.
Killing of mycoplasmal biofilms by complement or gramicidin. Results are mean percentages of CFU recovered from biofilms after incubation with GPS (A and B) or gramicidin (C). Error bars, standard errors of the means. Asterisks indicate statistical significance. (A) Intact biofilms (n = 10) or dispersed biofilms (n = 10) were incubated with GPS. (B) Intact biofilms were incubated with GPS (n = 9) as for panel A. Surviving biofilms were washed, left intact, and incubated again with GPS (n = 9). Dispersed survivors from the intact biofilms were washed, dispersed, and incubated again with GPS (n = 6). (C) Intact biofilms were incubated with gramicidin (n = 6). Dispersed biofilms were dispersed immediately after the addition of gramicidin (n = 6).
Mycoplasmas in a biofilm are relatively resistant to gramicidin.
The partial resistance of mycoplasmal biofilms to killing by gramicidin was similar to the resistance to killing by complement (Fig. 1C). About 64% of the mycoplasmas were recovered when intact biofilms of strain CT182-R3 were incubated with gramicidin. In contrast, only 20% of the mycoplasmas were recovered if the biofilm was scraped and dispersed immediately after the addition of gramicidin to the wells (P < 0.05).
Mycoplasmas in towers of biofilms are resistant to complement and gramicidin.
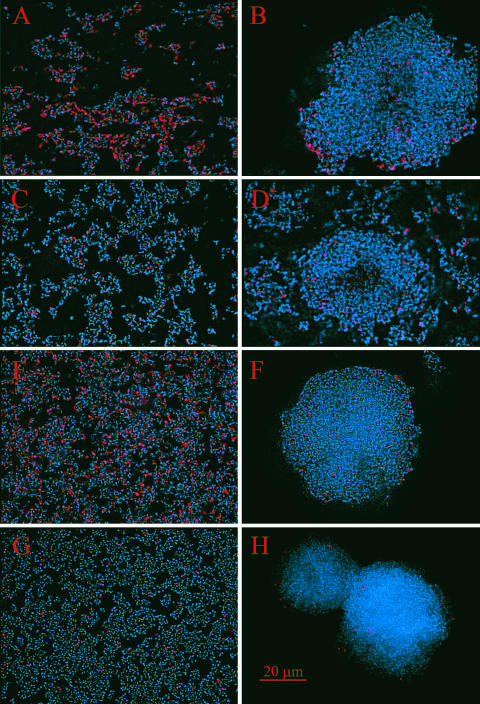
The relative resistance of the biofilm to lysis by complement or gramicidin appeared to reside in the tower structures (Fig. 2). Fluorescence microscopy of biofilms that were incubated with GPS or gramicidin and stained with PI and Hoechst 33342 revealed a large number of cells in the honeycombed region of the biofilm stained with PI (Fig. 2A and E). Very few cells in the honeycombed region stained with PI in control biofilms that were incubated with HIA-GPS or PBS (Fig. 2C and G). Little uptake of PI was detected in the cells within the tower structures of the biofilms that were incubated with either GPS (Fig. 2B), HIA-GPS (Fig. 2D), gramicidin (Fig. 2F), or PBS (Fig. 2H). However, some of the cells within the towers stained with PI, indicating that the cells within the towers were accessible to PI.
FIG. 2.
Fluorescent images of biofilms of M. pulmonis. Red, DNA of cells stained with PI; blue, DNA of cells stained with Hoechst 33342. Images on the left show the honeycomb regions of the biofilms. Images on the right show the tower structures of the biofilms. Biofilms were incubated with either GPS (A and B), HIA-GPS (C and D), gramicidin (E and F), or PBS (G and H). The scale bar is in red.
DISCUSSION
The length of the Vsa tandem repeat region modulates susceptibility to complement (12, 13), and all strains of M. pulmonis tested to date are sensitive to gramicidin when the cells are dispersed (5, 6, 12). The results presented here indicate that biofilm formation protects mycoplasmas from antimicrobial agents and the innate immune system. Within the honeycomb region of the biofilm, cells are sensitive to lysis by complement or gramicidin, but cells in the towers are relatively resistant. Although gramicidin is produced by Bacillus aneurinilyticus, the murine host of M. pulmonis produces other antimicrobial peptides. Mycoplasmas can form aggregates on epithelial or subepithelial surfaces in vivo (14). It follows that if such aggregates constitute a biofilm, the mycoplasmas will be partially protected from complement and antimicrobial peptides. Since other mycoplasmas can grow as biofilms (9), our findings may be applicable to a wide range of mycoplasma species and perhaps other bacterial pathogens.
The mechanisms that contribute to the resistance of the biofilms are unknown. The amount of information available on the interactions of complement and biofilms is limited. Biofilms protect Pseudomonas aeruginosa from killing by serum (1), and the level of complement activation is lower than that by planktonic cells (8). Complement is clearly being activated by the mycoplasmal biofilm, since the cells in the honeycomb region are lysed. Additionally, the resistance of the mycoplasmas encased within a biofilm to killing by GPS did not result from individual cells acquiring resistance, because 90% of the mycoplasmas from GPS-treated biofilms were killed when they were dispersed and incubated with GPS a second time. Therefore, the resistance imparted by the biofilm resides in the structure of the biofilm.
The resistance to complement and gramicidin was limited to the tower structures, a region where the cell density is highest and abundant amounts of polysaccharide are detected (11). Plausibly, either the cell density per se or the polysaccharides in the towers of the mycoplasma biofilms could contribute to the resistance. Extracellular polysaccharide can protect bacteria from the lytic effects of antimicrobial peptides (2, 15). Nevertheless, the integrity of the mycoplasmal cells in the honeycombed regions was compromised by the complement terminal lytic complex, while the towers hindered the access of at least one of the complement components to the cells within the towers. The towers also hindered the access of gramicidin, a small pore-forming peptide, to the mycoplasmas. Consistent with these results, antibodies to VsaA do not penetrate the towers of the biofilms, and it seems likely that antibody-mediated killing of the mycoplasmas would be hindered (11). These results suggest that the formation of towers in vivo could modulate resistance to complement and antimicrobial peptides secreted by host immune cells and could also protect the mycoplasmas from adaptive immune responses. In support of this, M. pulmonis cells that produce a short form of the Vsa protein (VsaH) and grow as a biofilm (11) have been isolated from the lungs of experimentally infected mice (4).
Acknowledgments
We thank Portia Caldwell and Abha Soni for expert technical assistance.
This work was supported by Public Health Service grant A164848 from the National Institutes of Health.
Editor: A. Camilli
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Anwar, H., J. L. Strap, and J. W. Costerton. 1992. Susceptibility of biofilm cells of Pseudomonas aeruginosa to bactericidal actions of whole blood and serum. FEMS Microbiol. Lett. 71:235-241. [DOI] [PubMed] [Google Scholar]
- 2.Campos, M. A., M. A. Vargas, V. Regueiro, C. M. Llompart, S. Alberti, and J. A. Bengoechea. 2004. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect. Immun. 72:7107-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 4.Denison, A. M., B. Clapper, and K. Dybvig. 2005. Avoidance of the host immune system through phase variation in Mycoplasma pulmonis. Infect. Immun. 73:2033-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fassi-Fehri, L., H. Wroblewski, and A. Blanchard. 2007. Activities of antimicrobial peptides and synergy with enrofloxacin against Mycoplasma pulmonis. Antimicrob. Agents Chemother. 51:468-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehri, L. F., P. Sirand-Pugnet, G. Gourgues, G. Jan, H. Wroblewski, and A. Blanchard. 2005. Resistance to antimicrobial peptides and stress response in Mycoplasma pulmonis. Antimicrob. Agents Chemother. 49:4154-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greiner, L. L., J. L. Edwards, J. Shao, C. Rabinak, D. Entz, and M. A. Apicella. 2005. Biofilm formation by Neisseria gonorrhoeae. Infect. Immun. 73:1964-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen, E. T., A. Kharazmi, P. Garred, G. Kronborg, A. Fomsgaard, T. E. Mollnes, and N. Hoiby. 1993. Complement activation by Pseudomonas aeruginosa biofilms. Microb. Pathog. 15:377-388. [DOI] [PubMed] [Google Scholar]
- 9.McAuliffe, L., R. J. Ellis, K. Miles, R. D. Ayling, and R. A. J. Nicholas. 2006. Biofilm formation by mycoplasma species and its role in environmental persistence and survival. Microbiology 152:913-922. [DOI] [PubMed] [Google Scholar]
- 10.Shen, X., J. Gumulak, H. Yu, C. T. French, N. Zou, and K. Dybvig. 2000. Gene rearrangements in the vsa locus of Mycoplasma pulmonis. J. Bacteriol. 182:2900-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons, W. L., J. R. Bolland, J. M. Daubenspeck, and K. Dybvig. 2007. A stochastic mechanism for biofilm formation by Mycoplasma pulmonis. J. Bacteriol. 189:1905-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons, W. L., A. M. Denison, and K. Dybvig. 2004. Resistance of Mycoplasma pulmonis to complement lysis is dependent on the number of Vsa tandem repeats: shield hypothesis. Infect. Immun. 72:6846-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons, W. L., and K. Dybvig. 2003. The Vsa proteins modulate susceptibility of Mycoplasma pulmonis to complement, hemadsorption, and adherence to polystyrene. Infect. Immun. 71:5733-5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stadtländer, C. T., H. L. Watson, J. W. Simecka, and G. H. Cassell. 1991. Cytopathic effects of Mycoplasma pulmonis in vivo and in vitro. Infect. Immun. 59:4201-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vuong, C., J. M. Voyich, E. R. Fischer, K. R. Braughton, A. R. Whitney, F. R. DeLeo, and M. Otto. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell. Microbiol. 6:269-275. [DOI] [PubMed] [Google Scholar]
- 16.Webb, J. S., L. S. Thompson, S. S. James, T. Charlton, T. Tolker-Nielsen, B. Koch, M. Givskov, and S. Kjelleberg. 2003. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]