Abstract
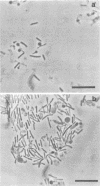
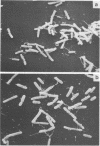
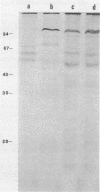
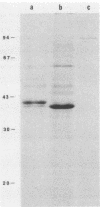
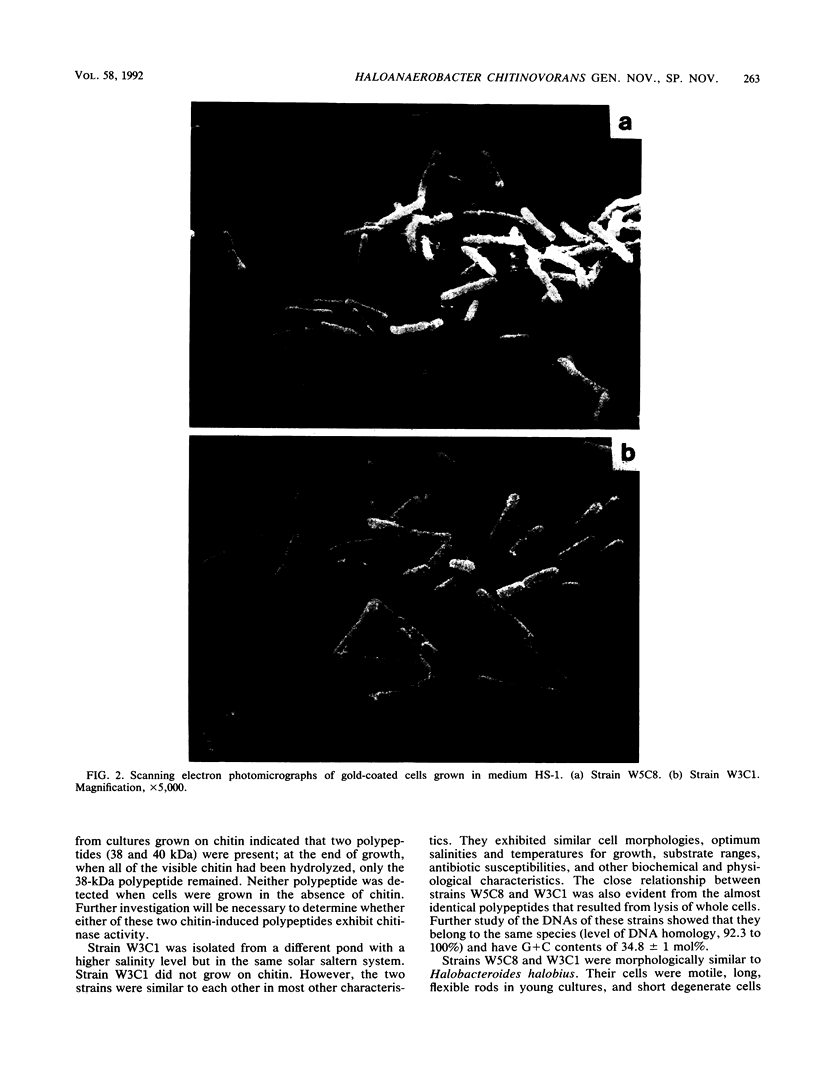
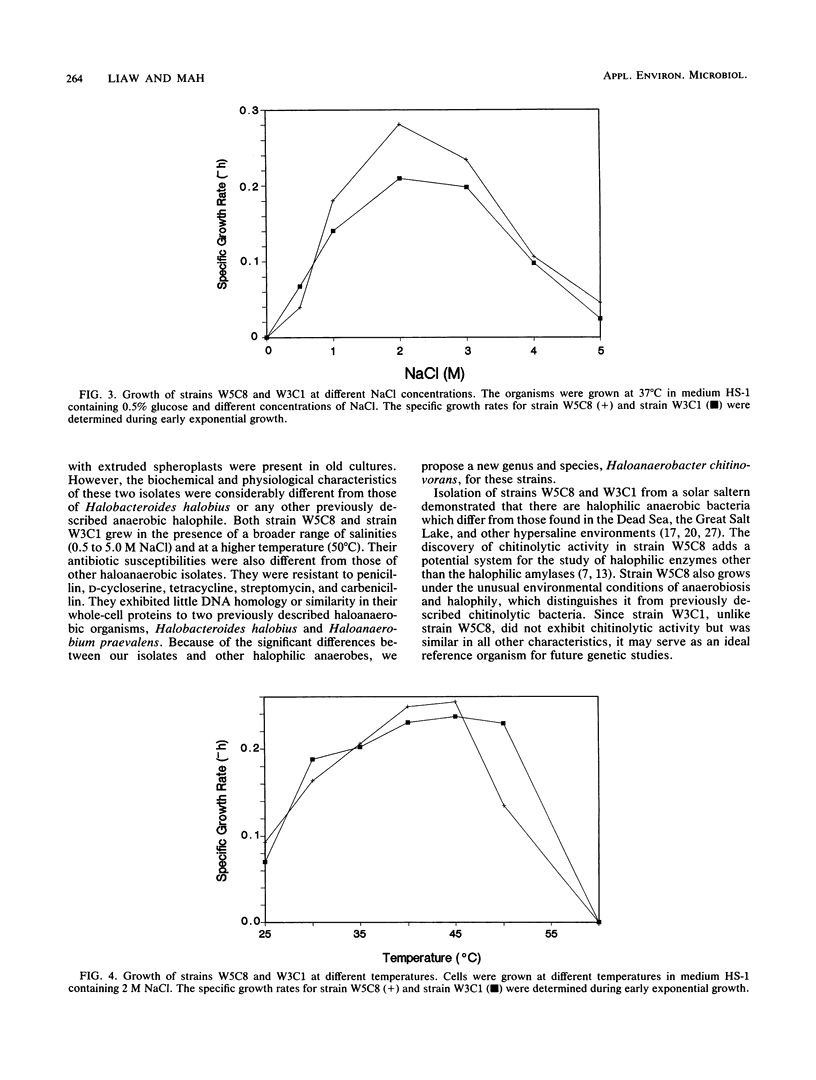
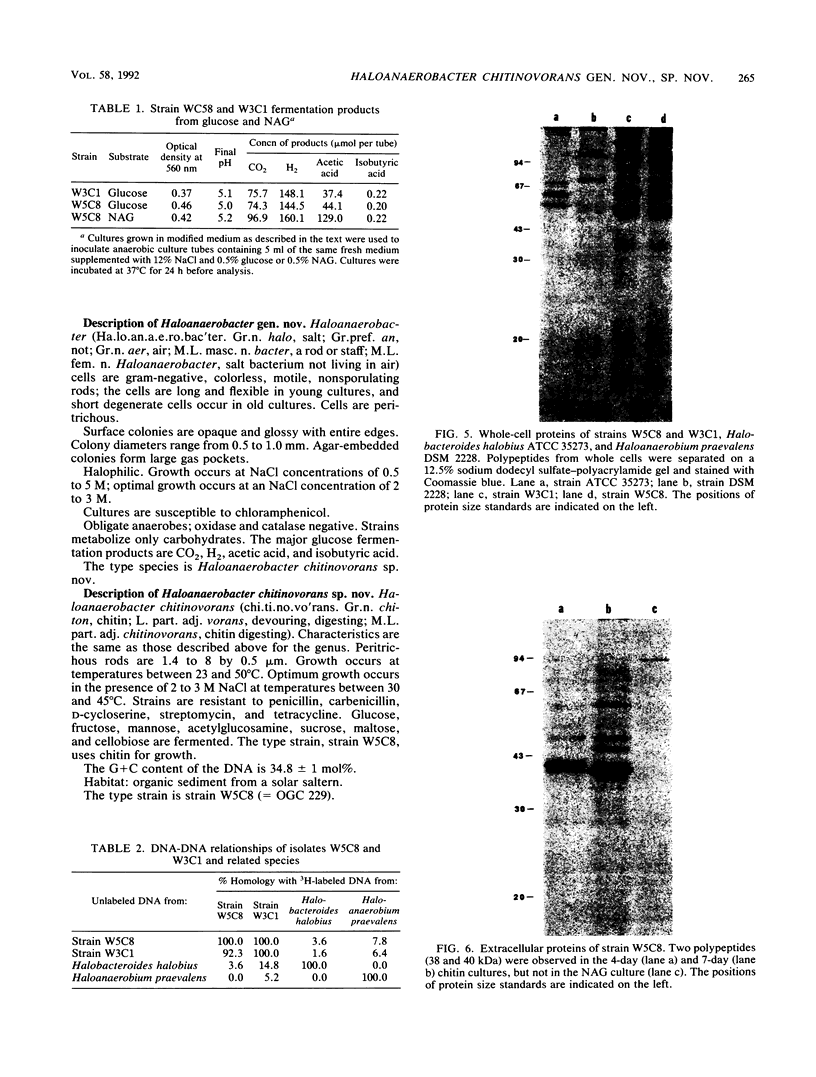
Two halophilic anaerobic bacteria, one of which had chitinolytic activity, were isolated from a solar saltern in southern California. These organisms were long, gram-negative, motile, flexible rods. The biochemical and physiological characteristics of these bacteria were very similar but were different from the characteristics of other haloanaerobic bacteria. Both grew at salt concentrations ranging from 0.5 to 5 M and at temperatures ranging from 23 to 50°C. They were sensitive to chloramphenicol but resistant to penicillin, carbenicillin, d-cycloserine, streptomycin, and tetracycline. An analysis of DNAs and whole-cell proteins showed that they were closely related taxonomically and distinguishable from other halophilic anaerobic bacteria. They exhibited 92.3 to 100% DNA homology as determined by DNA-DNA hybridization. The guanine-plus-cytosine contents of their DNAs were 34.8±1 mol%. The two isolates, strains W5C8 and W3C1, differed from other halophilic anaerobic bacteria sufficiently to support establishment of a new genus and species, Haloanaerobacter chitinovorans. Strain W5C8 exhibited chitinolytic activity and is designated the type strain. Two chitin-induced extracellular proteins with molecular weights of 38 × 103 and 40 × 103 were detected in strain W5C8.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baresi L., Mah R. A., Ward D. M., Kaplan I. R. Methanogenesis from acetate: enrichment studies. Appl Environ Microbiol. 1978 Jul;36(1):186–197. doi: 10.1128/aem.36.1.186-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R. Terminal reactions in the anaerobic digestion of animal waste. Appl Environ Microbiol. 1982 Jan;43(1):57–64. doi: 10.1128/aem.43.1.57-64.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Xun L. Effects of pH, Temperature, and Nutrients on Propionate Degradation by a Methanogenic Enrichment Culture. Appl Environ Microbiol. 1987 Jul;53(7):1589–1592. doi: 10.1128/aem.53.7.1589-1592.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Brenner D. J., Falkow S. Use of a single-strand specific nuclease for analysis of bacterial and plasmid deoxyribonucleic acid homo- and heteroduplexes. J Bacteriol. 1973 Sep;115(3):904–911. doi: 10.1128/jb.115.3.904-911.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUSSAULT H. P. An improved technique for staining red halophilic bacteria. J Bacteriol. 1955 Oct;70(4):484–485. doi: 10.1128/jb.70.4.484-485.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good W. A., Hartman P. A. Properties of the amylase from Halobacterium halobium. J Bacteriol. 1970 Oct;104(1):601–603. doi: 10.1128/jb.104.1.601-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liaw H. J., Srinivasan V. R. Molecular cloning and expression of an Erwinia sp. gene encoding diphenyl ether cleavage in Escherichia coli. Appl Environ Microbiol. 1989 Sep;55(9):2220–2225. doi: 10.1128/aem.55.9.2220-2225.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H. Halophilic amylase from a moderately halophilic Micrococcus. J Bacteriol. 1972 Feb;109(2):570–574. doi: 10.1128/jb.109.2.570-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J. F., Boone D. R. Analytical determination of the buoyant density of DNA in acrylamide gels after preparative CsCl gradient centrifugation. FEBS Lett. 1973 Dec 1;37(2):321–324. doi: 10.1016/0014-5793(73)80487-9. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Westermann P., Ahring B. K., Mah R. A. Acetate production by methanogenic bacteria. Appl Environ Microbiol. 1989 Sep;55(9):2257–2261. doi: 10.1128/aem.55.9.2257-2261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]