Abstract
Helicobacter pylori infection is associated with altered gastric epithelial cell turnover. To evaluate the role of oxidative stress in cell death, gastric epithelial cells were exposed to various strains of H. pylori, inflammatory cytokines, and hydrogen peroxide in the absence or presence of antioxidant agents. Increased intracellular reactive oxygen species (ROS) were detected using a redox-sensitive fluorescent dye, a cytochrome c reduction assay, and measurements of glutathione. Apoptosis was evaluated by detecting DNA fragmentation and caspase activation. Infection with H. pylori or exposure of epithelial cells to hydrogen peroxide resulted in apoptosis and a dose-dependent increase in ROS generation that was enhanced by pretreatment with inflammatory cytokines. Basal levels of ROS were greater in epithelial cells isolated from gastric mucosal biopsy specimens from H. pylori-infected subjects than in cells from uninfected individuals. H. pylori strains bearing the cag pathogenicity island (PAI) induced higher levels of intracellular oxygen metabolites than isogenic cag PAI-deficient mutants. H. pylori infection and hydrogen peroxide exposure resulted in similar patterns of caspase 3 and 8 activation. Antioxidants inhibited both ROS generation and DNA fragmentation by H. pylori. These results indicate that bacterial factors and the host inflammatory response confer oxidative stress to the gastric epithelium during H. pylori infection that may lead to apoptosis.
Helicobacter pylori infection has been implicated in the pathogenesis of gastritis, peptic ulcer disease, gastric carcinoma, and gastric lymphoma (10, 18), but the mechanisms leading from chronic active gastritis to other disease manifestations remain unclear. Various bacterial factors as well as the host response are believed to contribute to the outcome of infection with H. pylori (25). Strains bearing the cag pathogenicity island (PAI) (15), which includes the cagA gene, have been shown to be associated with increased gastric inflammation (57), increased bacterial load, and both peptic ulcer disease and gastric cancer (11). Increased induction of gastric epithelial cytokines that recruit and activate immune/inflammatory cells is also observed with these strains (17, 41, 55, 67). Although the full functions of genes included in the cag PAI remain unclear, it is known that cag PAI-positive strains activate specific transcription factors and cell signaling pathways (37, 41, 52, 55, 66).
Bacterial and host factors can damage the gastric mucosal barrier and lead to alterations of epithelial cell growth and differentiation. H. pylori infection is associated with increased cellular proliferation in vivo (13, 43) although most in vitro studies demonstrate bacterial inhibition of cell growth (76), suggesting that factors other than H. pylori regulate cell growth in the complex milieu of the infected gastric mucosa. Increased numbers of apoptotic cells are found in the gastric epithelium of infected patients (36, 45, 50, 58), suggesting that induction of apoptosis may be a common method of cell growth regulation for H. pylori (24). In agreement with these findings, we and others have demonstrated that H. pylori induces programmed cell death in cultured gastric epithelial cells, as do proinflammatory cytokines that are released during infection (3, 4, 26, 44, 68, 76). Proliferative (13, 43) and apoptotic rates (24, 36, 50) have both been shown to return to control levels after eradication of infection.
There is increasing evidence that microbial pathogens induce oxidative stress in infected host cells (29, 65, 69), and this may represent an important mechanism leading to epithelial injury in H. pylori infection (70). It is known from other cell systems that oxidative stress regulates cell cycle events via multiple pathways, with net responses that include aberrant proliferation, adaptation cytotoxicity, and cell death (35). Oxidative stress could well play a role in the altered epithelial proliferation, increased apoptosis (16, 34), and increased oxidative DNA damage (7, 16, 27) associated with H. pylori infection. Evidence for this includes increased levels of reactive oxygen species (ROS) measured in the mucosae of infected patients (16, 21, 23). While activated, ROS-releasing phagocytic leukocytes recruited to the gastric mucosa during infection represent one obvious source of oxidative stress (21, 79), other studies demonstrate that H. pylori itself also generates ROS (51) and that ROS accumulate in gastric epithelial cells (5, 6, 73). In addition, proinflammatory cytokines induce ROS in various cell types (47, 48, 61), and the decreased levels of ascorbic acid that are associated with H. pylori infection (64, 71) also contribute to a pro-oxidative environment. H. pylori infection has been shown to increase expression and activity of spermine oxidase, which oxidizes polyamines that are abundant in epithelial cells to release hydrogen peroxide (77), suggesting another mechanism by which H. pylori induces oxidative stress.
To examine oxidative stress that may occur during H. pylori infection, measurements of intracellular ROS were made in cultured and native gastric epithelial cells after exposure to H. pylori or hydrogen peroxide (H2O2), either alone or in combination with cytokines that are increased in infection. Antioxidants were used to evaluate the role of oxidative stress in the induction of apoptosis by these stimuli. To determine the role of the cag PAI in the induction of oxidative stress, cag PAI-bearing H. pylori strains and their isogenic mutants deficient in the cag PAI were compared for their abilities to generate ROS in gastric epithelial cells.
MATERIALS AND METHODS
Cell lines.
Gastric epithelial cell lines Kato III, NCI-N87, and AGS (American Type Culture Collection, Manassas, VA) were grown in standard conditions according to previously published methods (19, 26, 78). Briefly, cells were cultured in flasks containing RPMI 1640 medium supplemented with 10% fetal calf serum at 37°C in a humidified 5% CO2 incubator. To ensure the cells were in comparable stages of growth at the time of stimulation, cultured gastric epithelial cells were seeded at an average density of 1.2 × 106 cells/cm2 24 h before stimulation. Cell viability was assessed by trypan blue exclusion.
Isolation of native epithelial cells.
Using a protocol approved by our institutional review boards, four to six pinch biopsy specimens were collected from the antral gastric mucosae of consenting adult subjects undergoing medically indicated espohagogastroduodenoscopy. Subjects were considered infected with H. pylori if one or more of the following tissue-based diagnostic tests were positive: rapid urease testing, routine histopathology, and immunostaining (42). Biopsy samples were transported to the laboratory in cold, sterile collection medium (calcium- and magnesium-free buffered Hanks’ salt solution with 5% fetal calf serum and penicillin-streptomycin). The tissues were rinsed, gently teased apart, and added to media containing 1 mM dithiothreitol (Sigma Chemical Co., St. Louis, MO) and 1 mM EDTA (Sigma Chemical Co.) (26, 78). After gentle agitation at 37°C for 1 h, the resulting cell suspension was washed and stained with trypan blue to assess cell viability. Only preparations with greater than 80% viability were used for subsequent experiments. Purity was assessed by labeling the cells with fluorescein isothiocyanate-conjugated monoclonal antibodies to an epithelial cell-specific antigen (clone Ber-EP4; Dakopatts A/S, Glostrup, Denmark) and measuring the staining by flow cytometry as previously reported (26, 78).
Bacteria.
H. pylori strains were maintained on blood agar plates under microaerophilic conditions as reported previously (19). Bacteria were cultured overnight in brucella broth supplemented with 10% fetal calf serum before centrifugation at 2,500 × g for 15 min and resuspension in phosphate-buffered saline (PBS). The strains used included CagA+ LC-11, originally isolated from a child with duodenal ulcer disease (19), and two strains bearing the cag PAI, 26695 and 84-183, and their isogenic cag PAI-negative mutants, 8-1 and 2-1, respectively (kindly provided by Doug Berg, Washington University, St. Louis, MO) (1, 41, 75). Formalin-killed bacteria were prepared as previously reported (19, 26) by centrifugation of an overnight culture, suspended in 0.5% formalin, and stored at 4°C. On the day of the experiment, the formalin-killed bacteria were washed three times with PBS and resuspended in PBS to give a concentration comparable to the that of the suspension containing viable bacteria.
Stimulation of epithelial cells.
Various strains of H. pylori were added to epithelial cells at ratios of bacteria to epithelial cells ranging from 1:1 to 1,000:1 to assess strain specificity and dose relationships of epithelial cell responses. Most experiments were conducted using ratios of 300:1. Bacterial numbers were determined as previously reported (26) by measuring absorbance at 530 nm using a spectrophotometer (DU-65; Beckman Instruments, Inc., Fullerton, CA) and comparing the value to a standard curve generated by quantifying viable organisms from aliquots of bacteria at various concentrations that were also assessed for absorbance. Bacterial motility was confirmed by phase-contrast microscopy before experimental use. In some experiments, formalin-killed bacteria, cell-free bacterial culture supernatants, or control culture media were tested. H2O2 (Sigma Chemical Co.) was added at concentrations varying from 50 to 1,000 μM, doses similar to those used in other studies examining the effects of ROS on gastrointestinal epithelial cells (30, 32). Human recombinant tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and interleukin-1β (IL-1β) (R&D Systems, Minneapolis, MN) were used at doses of 10 ng/ml, 100 U/ml, and 10 ng/ml, respectively. We have previously shown that these doses of cytokine stimulate apoptosis (26) and induce IL-8 (19) in gastric epithelial cells. Assays to detect ROS and DNA degradation were performed at various times after stimulation as described below.
Detection of intracellular oxygen metabolites. (i) Redox-sensitive dye method.
Tubes containing 106 cultured or freshly isolated gastric epithelial cells were loaded with 5 μM dichlorofluorescin diacetate (DCFH2-DA) (Molecular Probes, Eugene, OR) according to standard methods (14, 63, 80). After incubation at 37°C for 15 min, the cells were washed and resuspended before being examined by flow cytometry (FACScan; Becton Dickinson, San Jose, CA). Measurements were made at baseline and at 5-minute intervals after adding the stimulants described above. For experiments to evaluate dose responses and different bacterial strains, this technique was adapted for use in a 96-well plate (2, 62). Briefly, 105 epithelial cells/well were cultured overnight in media with or without cytokines and loaded with 10 μM DCFH2-DA before washing and adding the stimulants described above. Measurements of fluorescence were made using a FluoroCounter (Packard, Downer Grove, IL). DCFH2-DA freely enters the cell and is cleaved by cellular esterases to the nonfluorescent DCFH2, which remains intracellular. Oxidants including hydroxyl radical, hydrogen peroxide, and peroxynitrite, but not superoxide anion, have been shown to oxidize DCFH2 to fluorescent dichlorofluorescein (DCF) (39, 46, 80). Production of these oxidative species has been shown to be proportional to the fluorescence detected in many cell systems using this redox-sensitive dye (14, 63, 80).
(ii) Cytochrome c assay.
Using previously reported methods (5, 73) 105 gastric epithelial cells/well were cultured overnight in 96-well culture plates and washed with PBS before addition of 1 ml of Hanks’ balanced salt solution containing 80 μM cytochrome c (Sigma) to each well. Superoxide dismutase (SOD) (Sigma) was added to the reference wells at a concentration of 40 μg/ml. At various times after stimulation spectrophotometric determinations were carried out at 550 nm. The amount of superoxide anion released was measured by the SOD-inhibitable reduction of cytochrome c and expressed as nmol/1 × 105 cells/unit of time.
(iii) Measurement of GSH levels.
Glutathione (gamma-glutamylcysteinylglycine [GSH]) levels were measured using a commercially available colorimetric assay (OXIS International, Inc., Portland, OR) according to the manufacturer's instructions. Briefly, at various times after stimulation, cells were homogenized and centrifuged at 3,000 × g and supernatants treated with reagents to generate chromophore thiones before measuring absorbance at 400 nm. Diethylmaleate (100 μM; Sigma), which irreversibly depletes intracellular GSH (22), was used as a positive control.
Antioxidants.
Inhibition of the effects of oxidative stress were performed by the addition of the GSH precursor N-acetylcysteine (NAC) to cultures of epithelial cells grown in media alone or stimulated with H2O2 or H. pylori. Ten millimolar NAC was added 1 h before stimulation in all experiments since similar concentrations have been used in other studies of oxidative injury (60), and our initial dose-response experiments demonstrated this to be an optimum dose to inhibit DCF fluorescence after stimulation with 400 μM H2O2.
Other antioxidants used to inhibit ROS generation included sodium azide, diphenylene iodonium (DPI) chloride, and allopurinol at doses previously shown to inhibit oxidant-induced effects in guinea pig gastric epithelial cells (73). GSH, SOD, catalase, hydroxyl radical scavengers, dimethylthiourea (DMTU), and mannitol, as well as iron-chelating agents desferrioxamine (DESF), and diethyltriaminepentaacetic acid (DTPA), were also examined for their ability to inhibit ROS generation. The doses employed were based on those used in previously published studies of ROS generation in gastric epithelial cells (5, 73) and other cell types (49, 60). All antioxidants were obtained from the Sigma Chemical Co.
Assays of apoptosis. (i) Apoptosis ELISA.
Apoptosis was assessed using a sensitive enzyme-linked immunosorbent assay (ELISA) (Boehringer-Mannheim Biochemicals, Indianapolis, IN) that detects endonucleosomes exposed during the DNA fragmentation that occurs in apoptosis but not necrosis. Previous studies demonstrated that this assay yielded results similar to those of other assays of apoptosis but with increased sensitivity since apoptosis could be detected in as few as 102 cells (26). Briefly, absorbance was measured at 405 nm by Multiskan (model MCC/340; Titertek Instruments, Inc., Irvine, CA) and compared with that of a substrate solution as a blank. The apoptotic index was calculated by dividing the absorbance of stimulated cells by the absorbance of control cells. Cells treated with 800 U/ml IFN-γ for 6 h followed by 100 μg/ml anti-Fas antibody (CH 11; Kamiya Biomedical Company, Thousand Oaks, CA) were used as a positive control in some experiments since this treatment was previously shown to induce maximal levels of apoptosis in gastric epithelial cells.
(ii) Caspase activation assays.
Caspase activity was determined using modifications of previously published methods (28, 31) using specific synthetic fluorogenic substrates. Kato III cells (0.5 × 106) stimulated with H. pylori or 400 μM H2O2 were harvested and centrifuged at 1,500 rpm for 5 min. The cell pellets were lysed in 0.1 ml buffer (50 mM HEPES buffer, pH 7.5, 10% sucrose, and 0.1% Triton X-100) for 20 min on ice. After centrifugation at 10,000 × g for 10 min at 4°C, 100 μl of supernatant was transferred to a fresh tube containing 1 μl of 1 M dithiothreitol. After tubes had been placed on ice for 15 min, specific caspase substrates were added to a final concentration of 50 mM, incubated at room temperature for 1 h, and then diluted to 1 ml with PBS, with fluorescence measured using a spectrofluorophotometer (excitation, 400 nm; emission, 505 nm). The substrates used for various caspase activity determinations were as follows: Z-VDVAD-AFC (benzyloxycarbonyl [CBZ]-Val-Asp-Val-Ala-Asp-7-amino-4-trifluoromethyl coumarin [AFC]) for caspase 2, also known as Nedd2; Z-DEVD-AFC (CBZ-Asp-Glu-Val-Asp-AFC) for caspase 3, also known as CPP32; and Z-IETD-AFC (CBZ-Ile-Glu-Thr-Asp-AFC) for caspase 8, also known as Flice (Enzyme Systems Products, Livermore, CA). Each sample was analyzed in duplicate. The AFC fluorescence units versus concentration of AFC were graphed, and the slope was used to convert fluorescence units generated by the enzyme to activity.
To determine the specificity of the responses, the general caspase inhibitor Z-VAD (Bachem, Torrance, CA) as well as specific caspase inhibitors Z-VDVAD-CH2F, Z-DVED-CH2F, and Z-IETD-CH2F (Enzyme Systems Products, Livermore, CA) for caspases 2, 3, and 8, respectively, were used at 20 and 100 μM before stimulation with H. pylori (28, 31).
Statistical analysis.
Results are expressed as the means ± standard errors of the means (SEM). Data were compared by Student's t test (unpaired unless otherwise noted) or analysis of variance, and results were considered significant if P values were less than 0.05.
RESULTS
Induction of intracellular ROS in response to H. pylori.
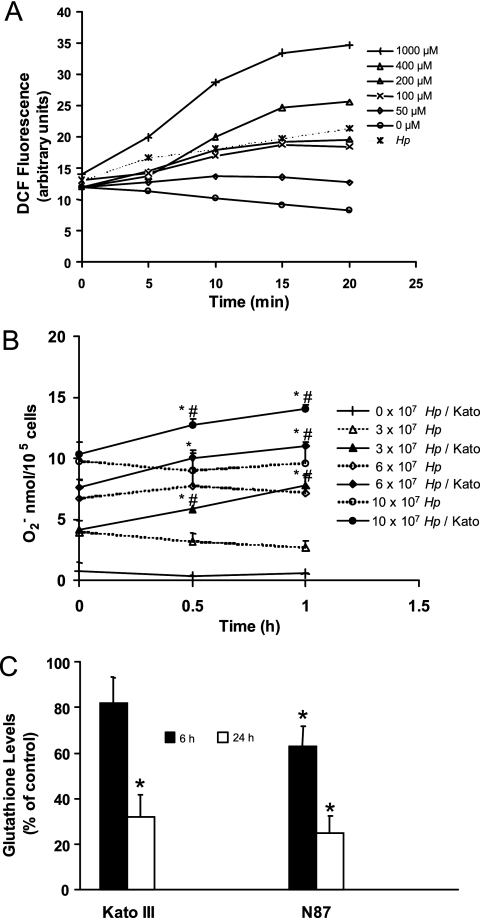
Infection of DCFH2-DA-treated gastric epithelial cell lines with H. pylori was associated with a rapid increase in fluorescence compared to levels of fluorescence measured in uninfected control cells, indicating increased accumulation of intracellular ROS in infected cell lines (Fig. 1A). No increase in DCF fluorescence was detected when DCFH2-DA-treated bacteria were assayed by flow cytometry in the absence of epithelial cells. Since superoxide anion production is not thought to directly induce DCF fluorescence (80), the cytochrome c reduction assay was used to demonstrate a dose-dependent increase in superoxide anion in H. pylori-infected Kato III cells (Fig. 1B). The results of our cytochrome c reduction assay confirm the findings of Nagata et al. (51) and show evidence of superoxide anion generation by bacteria alone, although additional superoxide anion is measured over time when epithelial cells are present with the bacteria (Fig. 1B). As shown in Fig. 1C, GSH levels were decreased in both Kato III cells at 24 h after infection and NCI-N87 cells at 6 and 24 h after infection, providing evidence of a more sustained effect of infection through increased intracellular ROS. Taken together, these data indicate that H. pylori organisms release superoxide anion and demonstrate that additional ROS are generated in host cells through bacterial interaction with epithelial cells.
FIG. 1.
Induction of ROS in gastric epithelial cells after exposure to H. pylori or oxygen metabolites. (A) DCFH2-DA-treated Kato III cells were exposed to H2O2 at concentrations from 0 to 1,000 μM or infected with H. pylori (Hp) at a ratio of bacteria to epithelial cells of 300:1. Intracellular DCF fluorescence was measured by flow cytometry at intervals up to 20 min after stimulation. ROS accumulated in proportion to the concentration of H2O2 added to the cells, and bacteria stimulated the production of ROS in epithelial cells in a time-dependent manner. A representative experiment is shown. (B) Superoxide anion was measured by the cytochrome c reduction assay method at 0, 0.5, and 1 h after various concentrations of H. pylori strain 26695 (equivalent to ratios of bacteria to epithelial cells of 0:1, 300:1, 600:1, and 1,000:1) were added to wells containing media alone (dotted line) or media with Kato III gastric epithelial cells (continuous line). A dose-dependent increase in superoxide anion generation was measured with increasing concentrations of bacteria both with and without epithelial cells. Data represent superoxide anion production expressed as means ± SEM (n = 8 to 12). *, P < 0.05 compared to bacteria alone; #, P < 0.05 compared to Kato III cells alone. (C) Kato III and NCI-N87 gastric epithelial cells were exposed to H. pylori (ratio of bacteria to epithelial cells of 300:1), harvested at 6 or 24 h poststimulation, and assayed for levels of intracellular GSH using a colorimetric assay. Oxidative stress generated by H. pylori results in a sustained decrease in GSH levels. The data are means ± SEM, expressed as percentages of control levels. *, P < 0.05 compared to control (n = 5 to 7).
Bacteria prepared directly from blood agar plates did not consistently induce fluorescence in DCFH2-DA-treated gastric epithelial cells in contrast to preparations made from overnight brucella broth cultures. There was no early effect of cell-free bacterial culture supernatants or formalin-killed H. pylori, but formalin-killed bacteria increased levels of fluorescence in DCFH2-DA-treated Kato III cells at later time points (116% ± 2% of control [mean ± SEM]; n = 3; P < 0.05 compared to control at 30 min). Formalin-killed bacteria also decreased levels of GSH (54.6% ± 9.4% of control [mean ± SEM]; n = 3; P < 0.05 compared to control at 24 h). Together, these results suggest that viable motile bacteria are necessary for early generation of oxidative stress seen in cultured human gastric epithelial cells whereas oxidative stress induced by killed bacteria is more delayed.
ROS induction in native human gastric epithelial cells.
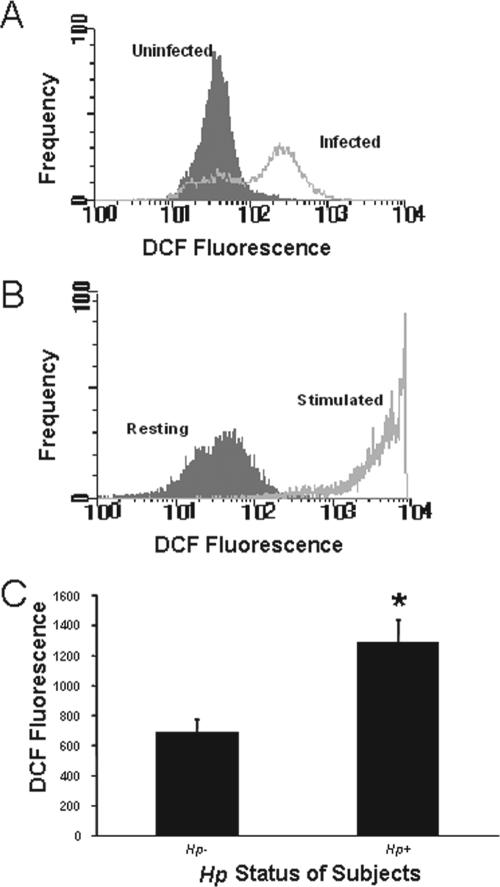
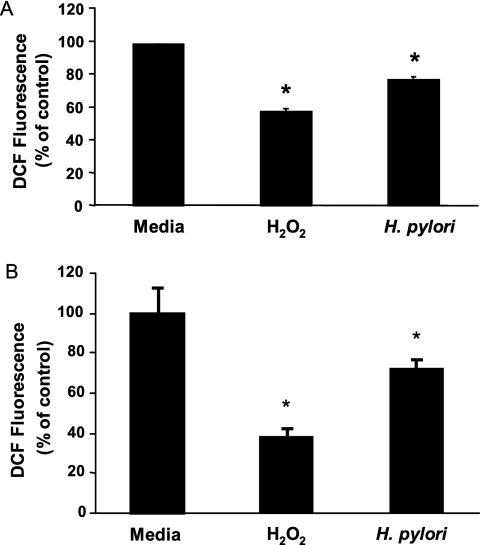
In order to validate the use of gastric epithelial cell lines in studies of human disease pathogenesis, it is important that findings observed in cultured cells be demonstrated in native cells either in situ or in isolated cell preparations. Our data, obtained using an approach that we have employed to demonstrate the expression of various immune adhesion or accessory molecules, including the class II major histocompatibility complex (26) and B7 (78), in native gastric epithelial cells, indicate that intracellular ROS can be detected in freshly isolated gastric epithelial cells. DCF fluorescence levels are increased in cells isolated from H. pylori-infected subjects compared to cells from uninfected individuals (Fig. 2A and B). Moreover, native cells exhibit an accumulation of ROS in response to treatment with exogenous oxidative metabolites similar to that observed in cell lines (Fig. 2C).
FIG. 2.
Detection of ROS in freshly isolated human gastric epithelial cells. Gastric epithelial cells were isolated from gastric biopsy specimens, stained with DCFH2-DA, and analyzed via flow cytometry. The y axes of panels A and B reflect the frequency of events on a linear scale, while the x axes indicate increasing levels of ROS as estimated by the DCF fluorescence on a logarithmic scale. (A) A representative experiment in which ROS levels were measured in cells from a subject infected with H. pylori and from an uninfected subject. (B) In another set of experiments, cells isolated from an uninfected subject were assayed for ROS before (resting) or after (stimulated) exposure to 400 mM H2O2. Treatment with H2O2 markedly increased intracellular ROS. (C) Mean levels of fluorescence ± SEM measured by fluorimeter in DCFH2-DA-treated cells isolated from three uninfected and three infected subjects. *, P < 0.05 compared to results for uninfected subjects. Hp, H. pylori.
Induction of intracellular ROS in response to exogenous oxidative metabolites or inflammatory cytokines.
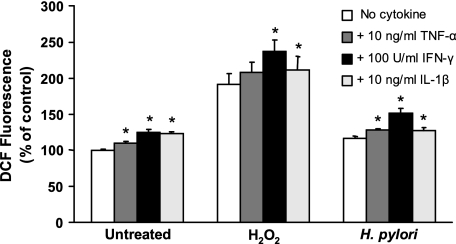
Treatment of Kato III, AGS, and NCI-N87 cells with increasing concentrations of H2O2 resulted in a time- and dose-dependent increase in levels of fluorescence in DCFH2-DA-treated epithelial cells, indicating accumulation of intracellular ROS (Fig. 1A). The patterns of the response were similar in the three cell lines tested. These results confirm that gastric epithelial cells respond to exogenous oxidative metabolites with an increase in intracellular ROS analogous to many other cell types (2, 14, 62, 63, 80). As certain cytokines that are increased during H. pylori infection, including IFN-γ, TNF-α, and IL-1β (53), can induce apoptosis (26, 76) and are reported to induce oxidative stress (47, 48, 61), we examined these cytokines for their ability to generate ROS in gastric epithelial cells. Although TNF-α has been shown to induce a transient surge of ROS in some cell systems (48, 61), no increase in fluorescence was detected by flow cytometry up to 20 min after stimulating DCFH2-DA-treated Kato III or NCI-N87 cells with 10 ng/ml TNF-α or 100 U/ml IFN-γ. However, longer-term (overnight) exposure to these cytokines, as well as 10 ng/ml IL-1β, resulted in increased basal levels of fluorescence and enhanced DCF fluorescence responses to bacteria and H2O2 (Fig. 3). The results demonstrating increased basal levels of ROS after overnight cytokine treatment are consistent with the reduced levels of GSH measured 24 h after treatment with IFN-γ or TNF-α (data not shown). These experiments indicate that factors generated through the host response to infection can also contribute to oxidative stress in the gastric mucosa.
FIG. 3.
Effect of proinflammatory cytokines on ROS generation. Kato III cells were treated overnight with media alone (no cytokine) or media containing 10 ng/ml TNF-α, 100 U/ml IFN-γ, or 10 ng/ml IL-1β before stimulation with media alone (control), 400 μM H2O2, or H. pylori at a ratio of bacteria to epithelial cells of 300:1. Peak increases in ROS levels (measured as increases in DCF fluorescence) are depicted as means ± SEM (n = 3 to 6). All three cytokines increased basal levels of fluorescence and ROS responses to H. pylori, while IFN-γ and IL-1β also increased ROS generation after H2O2 stimulation. *, P < 0.05 compared to cells without cytokine treatment.
Effect of cag PAI on ROS accumulation in gastric epithelial cells.
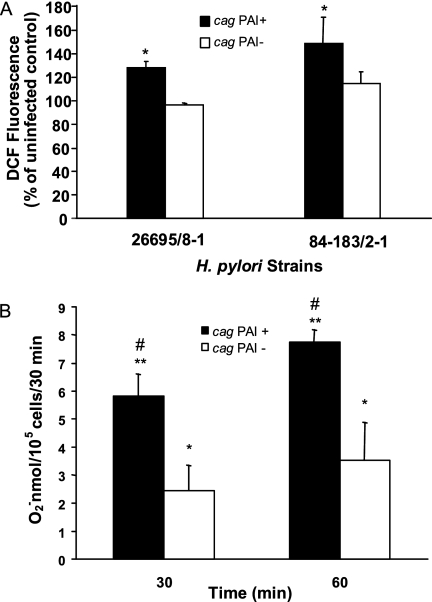
Since H. pylori strains bearing the cag PAI are known to induce more inflammation and are associated with the more significant disease manifestations of chronic H. pylori infection (25), we examined bacteria with and without the cag PAI for their effect on ROS accumulation in Kato III and NCI-N87 cells. As shown in Fig. 4A, both the 26695 and 84-183 strains, which contain the cag PAI, induced intracellular fluorescence while the corresponding cag PAI-deficient isogenic mutants, 8-1 and 2-1, had a more limited effect. A similar difference in superoxide generation was noted at 30 and 60 min after stimulation with strain 26695 and its isogenic mutant, 8-1 (Fig. 4B). These data suggest that bacterial genetic factors may play a role in the generation of oxidative stress. It is not clear why cag PAI-negative strains did not induce DCF fluorescence at levels over those in uninfected control cells while cag PAI-negative strains were capable of inducing superoxide at greater levels than in control cells. This may reflect the different rates at which superoxide is generated compared to other ROS.
FIG. 4.
Induction of ROS by cag PAI-bearing strains in gastric epithelial cells. (A) Kato III cells were infected with cag PAI-positive strains 26695 and 84-183 (solid bars) or their isogenic cag PAI-negative mutants, 8-1 and 2-1, respectively (open bars), at comparable concentrations. Peak increases in DCF fluorescence occurring within 40 min of infection are expressed as percentages of levels in uninfected control cells and depicted as means ± SEM (n = 5 to 7). *, P < 0.05 for PAI+ strains compared to their PAI− counterparts and to control levels. (B) Kato III cells were infected with the cag PAI-positive strain 26695 or its isogenic mutant, 8-1, at comparable concentrations. The amount of superoxide anion released was measured by the cytochrome c assay. Values at 30 and 60 min after infection are depicted as means ± SEM (n = 6, three replicates each). **, P < 0.0001; *, P < 0.05 (compared to control levels for both the PAI+ strain and its PAI− counterpart); #, P < 0.01 for cells infected with the PAI+ strain compared to the PAI− strain at both time points.
Inhibition of oxidative stress in gastric epithelial cells treated with antioxidants.
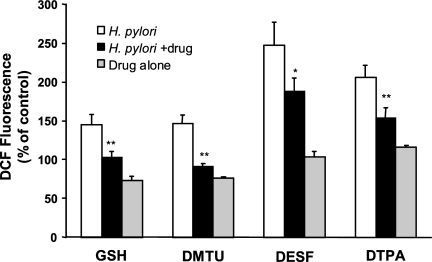
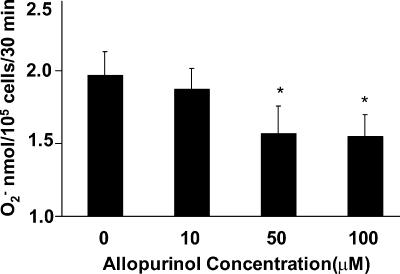
As shown in Fig. 5A, 10 mM NAC significantly reduced DCF fluorescence in Kato III cells after stimulation with H2O2 or H. pylori. Similar inhibitory effects of NAC were seen in epithelial cells isolated from gastric biopsy specimens obtained from uninfected subjects (Fig. 5B) and in NCI-N87 cells (data not shown). Other antioxidants were tested for their ability to inhibit ROS generation after H. pylori infection (Fig. 6 and 7). As shown in Fig. 6, GSH, the hydroxyl scavenger DMTU, and the iron chelators DESF and DTPA each significantly inhibited H. pylori-induced DCF fluorescence. Other antioxidants including catalase, mannitol, and SOD had no inhibitory effect. In order to determine the cellular source of ROS, sodium azide to inhibit mitochondrial electron transport, allopurinol to inhibit xanthine oxidase, and DPI, which inhibits NADPH oxidase, were added to cells before H. pylori stimulation. Only allopurinol had a significant inhibitory effect on superoxide anion generation after infection (Fig. 7). Together, the results of these inhibitor studies suggest that H. pylori infection leads to the formation of several species of ROS within gastric epithelial cells including superoxide anion, hydrogen peroxide, hydroxyl radical, and peroxynitrite.
FIG. 5.
Inhibition of ROS induction by NAC. (A) DCFH2-DA-loaded Kato III cells were treated with 10 mM NAC or media alone 1 h before stimulation with media, 400 μM H2O2, or H. pylori at a ratio of bacteria to epithelial cells of 300:1. Data are depicted as mean levels of maximal DCF fluorescence within 20 min of stimulation in NAC-treated cells expressed as percentages of fluorescence in cells without NAC (means ± SEM; n = 4 to 6 experiments). *, P < 0.05 compared to cells without NAC pretreatment. (B) Identical experiments performed with epithelial cells isolated from subjects without H. pylori infection (n = 3). *, P < 0.05 compared to cells without NAC pretreatment.
FIG. 6.
Effects of antioxidants on H. pylori-induced ROS production in Kato III cells. DCFH2-DA-loaded Kato III cells were treated with various antioxidants or media alone 1 h before stimulation with H. pylori strain 26695 at a ratio of bacteria to epithelial cells of 300:1. Antioxidants tested in these experiments were 10 mM GSH, 50 mM DMTU, 5 mM DESF, and DTPA. Data are depicted as mean levels of DCF fluorescence 30 min after stimulation with H. pylori in untreated cells (H. pylori) or in antioxidant-treated cells (H. pylori + drug), as percentages of values for uninfected, untreated control cells, ± SEM (n = 3 or 4 experiments). The effects of drugs alone are also shown. *, P < 0.05; **, P < 0.01 (compared to H. pylori alone using a paired Student t test).
FIG. 7.
Effects of the xanthine oxidase inhibitor allopurinol on H. pylori-induced superoxide anion in Kato III cells. Cells were incubated with various concentrations of allopurinol (10 to 100 μM) for 1 h before stimulation with H. pylori strain 26695 at a ratio of bacteria to epithelial cells of 300:1. Superoxide anion was assessed by cytochrome c assay 30 min after stimulation. Each experiment was performed in triplicate. Values are means ± SEM of three separate experiments. *, P < 0.05 compared to control cells without allopurinol using a paired Student t test.
Role of ROS in apoptosis induced by H. pylori.
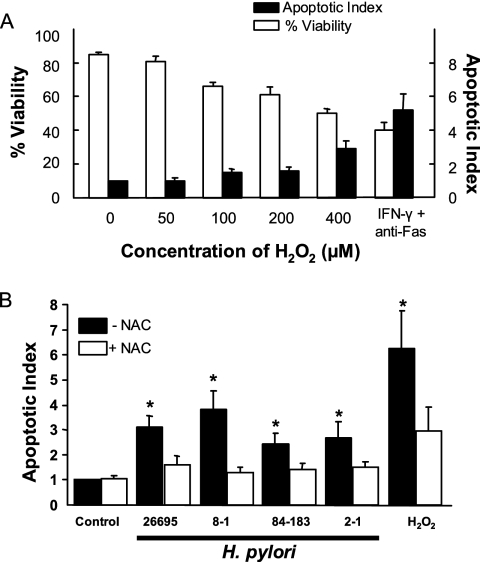
As we have previously shown that H. pylori induces apoptosis in gastric epithelial cells (26), we examined a role for oxidative stress in mediating this response (i) by mimicking the effect of H. pylori with exogenous oxidative metabolites and (ii) by inhibiting the response to bacterial infection with antioxidants. As shown in Fig. 8A, a dose-dependent decrease in cell viability and a dose-dependent increase in apoptosis were observed when NCI-N87 cells were treated with various concentrations of H2O2. The degree of apoptosis induced by 400 μM H2O2 approached that induced by a CD95-activating anti-Fas antibody in IFN-γ-treated cells. This treatment was used as a positive control as it was previously shown to induce maximal apoptosis in gastric epithelial cells. H. pylori infection was also shown to induce apoptosis (Fig. 8B) but to a lesser degree than that induced by 400 μM H2O2. Although we demonstrated greater ROS accumulation in gastric epithelial cells very early during infection with cag PAI-positive strains, this did not correlate with an increased rate of apoptosis measured at 48 h after infection. This discrepancy likely reflects the observation that longer-term effects on ROS, as determined by measuring GSH levels, did not differ significantly at 6 or 24 h of infection between cag PAI-positive and -negative strains (data not shown). Both H. pylori and H2O2 stimulated the activation of caspases 3 and 8, but only H. pylori had a significant effect on caspase 2 activation (Table 1). Inhibition of H. pylori-induced caspase activation by the general caspase inhibitor Z-VAD, as well as by specific caspase inhibitors, demonstrates the specificity of these responses (data not shown). Ten millimolar NAC significantly reduced the degree of apoptosis induced by H. pylori and by H2O2 (Fig. 8B). Together, these data indicate that both exogenous ROS and H. pylori induce apoptosis of gastric epithelial cells, as evidenced by caspase activation and DNA fragmentation, and implicate ROS in the programmed cell death induced by H. pylori infection.
FIG. 8.
Induction of apoptosis in gastric epithelial cells and its inhibition by antioxidants. (A) NCI-N87 cells were exposed to H2O2 at concentrations from 0 to 400 μM with cell viability (expressed as % of control) measured by trypan blue exclusion and apoptosis (shown as apoptotic index), determined by detection of endonucleosomes via ELISA. Cells exposed to 800 U/ml IFN-γ for 6 h followed by 100 μg/ml anti-Fas antibody were used as a positive control. Dose-dependent changes were observed with decreases in cell viability and increases in apoptosis significantly different from those of control cells at doses of 100 to 400 μM H2O2 (P < 0.05). Data shown as means ± SEM (n = 3 to 5). (B) Kato III cells were treated with H. pylori (300 bacteria per epithelial cell), 400 μM H2O2, or media alone (control) for 48 h in the presence or absence of 10 mM NAC. Apoptosis was assessed using an ELISA to detect endonucleosomes exposed by DNA fragmentation. Values depicted are means ± SEM (n = 9 to 15). *, P < 0.05 compared to cells without NAC.
TABLE 1.
H. pylori and H2O2 stimulate caspase activation
| Time (h) | Sp acta of indicated caspase (% of activity in control cells) after stimulation with:
|
|||||
|---|---|---|---|---|---|---|
|
H. pylori (300:1)
|
H2O2 (400 μM)
|
|||||
| Caspase 2 | Caspase 3 | Caspase 8 | Caspase 2 | Caspase 3 | Caspase 8 | |
| 3 | 95 ± 9 | 122 ± 3 | 102 ± 7 | 76 ± 20 | 131 ± 3* | 123 ± 9* |
| 6 | 138 ± 8* | 185 ± 12* | 159 ± 8* | 74 ± 1 | 104 ± 7 | 101 ± 8 |
| 9 | 138 ± 10* | 161 ± 19* | 122 ± 10* | 74 ± 2 | 163 ± 12* | 112 ± 5 |
| 12 | 139 ± 10* | 187 ± 25* | 170 ± 7* | 65 ± 4 | 216 ± 4* | 123 ± 1* |
Caspase activity was determined using specific synthetic fluorogenic substrates in Kato III cells stimulated with H. pylori (ratio of bacteria to epithelial cells of 300:1) or 400 μM H2O2. Each sample was analyzed in duplicate. Values are shown as caspase activities expressed as mean percentages of caspase activity in unstimulated cells ± SEM (*, P < 0.05).
DISCUSSION
Helicobacter pylori infection is a causal factor in various disorders of the gastric epithelium including ulceration, metaplasia, dysplasia, and carcinoma. Alterations of epithelial cell growth and enhanced programmed cell death may play a role in H. pylori disease manifestations (4, 44), but the mechanisms responsible for these changes in the epithelium remain unknown. This study demonstrates that increased levels of reactive oxygen are generated in H. pylori-infected gastric epithelial cells and that this may be one mechanism leading to apoptosis associated with infection. Our results indicate that both bacterial and host factors contribute to the oxidative stress induced by infection. The finding that antioxidants prevent the generation of ROS and inhibit H. pylori-induced programmed cell death has implications for the prevention and treatment of this common and chronic infectious disease.
It is increasingly recognized that microbial pathogens, including the Mycobacterium avium-M. intracellulare complex, bovine viral diarrhea virus, and human immunodeficiency virus, affect host cells via ROS generation (29, 65). Similarly, another species of Helicobacter, Helicobacter hepaticus, has been shown to induce oxidative DNA damage in hepatocytes of infected mice (69). Superoxide anion was also detected in epithelial cell preparations isolated from guinea pig gastric mucosa after experimental H. pylori infection (73). These studies lend support to our results demonstrating that H. pylori infection stimulates the accumulation of intracellular ROS in different human gastric epithelial cell lines (5, 6). Reports demonstrating decreased levels of GSH in H. pylori-infected HMO2 human gastric epithelial cells (9, 54) provide additional evidence that H. pylori serves as a stimulus for the accumulation of ROS within gastric epithelial cells. Furthermore, our results confirm the findings of a previous report describing the accumulation of ROS in another human gastric epithelial cell line, CRL 1739, after infection with H. pylori (5). Importantly, our findings indicating that ROS levels are higher in gastric epithelial cells isolated from infected subjects than in those from uninfected subjects demonstrate that H. pylori-induced oxidative stress is not an artifact of cultured cells or animal models and occurs in naturally infected, native human epithelial cells. Moreover, we have shown that native human gastric epithelial cells respond in a similar fashion to cultured cells with the generation of ROS after experimental H. pylori infection.
The present study confirms a report that H. pylori releases superoxide radicals (51), but since the superoxide anion cannot diffuse across the cell membrane, further studies were needed to identify the source and type of intracellular ROS generated in infected gastric epithelial cells. It was assumed that H. pylori infection must stimulate accumulation of ROS above the levels generated by epithelial cells through normal cellular metabolism. The results of our studies in which various antioxidants were shown to inhibit the generation of ROS by H. pylori suggest that hydrogen peroxide, hydroxyl radical, and peroxynitrite, as well as superoxide, all contribute to increased net levels of ROS in epithelial cells (5, 6, 38). Our findings do not exclude a role for other forms of ROS or reactive nitrogen species. Although the major source of ROS in most cells is mitochondrial electron transport, sodium azide did not inhibit ROS generation by H. pylori in our studies. Similarly, the NADPH oxidase inhibitor DPI was without effect while allopurinol, a xanthine oxidase inhibitor, reduced ROS accumulation by bacterial infection. These findings contrast with the study of guinea pig mucosal cells in which it was shown that H. pylori induced superoxide anion in epithelial cells through an NADPH-oxidase-like system (73). Since there are limitations to using pharmacological inhibitors to determine the type and source of ROS, it is not surprising that such differences exist. Substantial variation in the ability of such agents to inhibit ROS and ROS-mediated events in various cell types is reported in the literature (5, 48, 61, 65, 73), and differences in mechanisms of ROS generation may vary according to the cell type and species of origin (73).
The current study indicates that host factors also contribute to oxidative stress during H. pylori infection. Since activated neutrophils or macrophages are potent sources of ROS, including H2O2 (74), we studied exogenous H2O2 for its effect on gastric epithelial cells. A dose-dependent accumulation of intracellular ROS was observed in both cultured and freshly isolated human gastric epithelial cells. Cytokines that are increased in the gastric mucosae of infected subjects, including TNF-α, IFN-γ, and IL-1β (53), also induced oxidative stress and augmented oxidative responses to both H. pylori and H2O2 in our studies. Although cytokines have been reported to induce ROS in other cell types (47, 48, 61), our results indicate that cytokine-mediated oxidative signaling occurs in gastric epithelial cells.
The role of bacterial genotype has been a focus of recent investigations into H. pylori pathogenesis. H. pylori strains have been classified based on their expression of the cagA gene and the cag PAI (15) as well as the vacA genotype. Strains that are cagA+ have been shown to be associated with increased gastric inflammation, increased bacterial load, and both peptic ulcer disease and gastric cancer (4, 11, 57). Strains bearing the PAI induce higher levels of IL-8 (41) and activate transcription factors NF-κB (37, 66) and AP-1 (activator protein 1) (52, 55). cag PAI status also affects gastric epithelial apoptosis (40) and oxidative DNA damage (20, 56). Our results, which indicate that cag PAI status influences the ability of H. pylori to induce intracellular ROS in gastric epithelial cells, provide further insight into how bacterial genetic factors may play a role in disease pathogenesis. Moreover, since cag PAI-positive strains are associated with greater inflammation, the host response may also contribute to enhanced oxidative stress associated with these strains. The differential induction of ROS shown in the present study may be relevant to the reported associations of the cag PAI and the activation of epithelial cell signaling pathways (41, 52, 55). As the genome sequence of one of the strains used in this study, strain 26695, has been determined (41), the opportunity exists to identify more specific bacterial genes that regulate ROS generation.
We have shown that both H. pylori infection and exogenous ROS treatment induce caspase activation and DNA fragmentation while antioxidant treatment inhibits the induction of apoptosis due to H. pylori infection. Further evidence that oxidative stress may be involved in the alterations of epithelial cell growth in H. pylori infection is found in a study in which decreased epithelial cell apoptosis was observed in gastric tissues from H. pylori-infected patients treated with antioxidant therapy only (45). Although ROS have not been previously shown to play a role in programmed cell death of gastric epithelial cells, ROS have been implicated in apoptosis resulting from various stimuli (12, 34) in other cell types. Of particular relevance to our findings is a recent report of ROS involvement in apoptosis induced in host cells by bovine viral diarrhea virus (65). The present study does not address the mechanisms whereby oxidative stress leads to apoptosis, although ROS have been shown to contribute to p53 (59)-, Fas-Fas ligand (8, 33)-, ceramide (60)-, and TNF-mediated killing (72). It is known that mammalian cells respond to oxidative stress with the initial generation of ROS and the subsequent activation of redox-sensitive signaling pathways which control the transcription of genes that may regulate cell growth, repair, and death processes. Studies to examine redox-dependent pathways leading to epithelial cell death during H. pylori infection are in progress.
In summary, we have demonstrated that H. pylori infection, exogenous oxidative metabolites, and inflammatory cytokines induce the generation of intracellular reactive oxygen species in gastric epithelial cells. These in vitro results are corroborated by the higher levels of ROS measured in native epithelial cells from individuals infected with H. pylori. Our findings suggest that bacterial genotype may be an important determinant of the level of oxidative stress generated by infection. We conclude that oxidative stress may play a role in the increased programmed cell death that occurs during infection, since antioxidant treatment inhibited H. pylori-induced apoptosis. Further studies are necessary to explore how oxidative stress regulates epithelial responses to H. pylori infection, as this will provide new insight into the pathogenesis of H. pylori-associated conditions.
Acknowledgments
We acknowledge the excellent technical assistance of Thuyang N. Nguyen. We are grateful to Doug Berg (Washington University, St. Louis, MO) for providing us with the bacterial strains used in many of these studies.
Support from the National Institutes of Health (RO1 DK51677, RO1 DK50669, R21 AI48173, and R01 DK61769), the John Sealy Memorial Endowment Fund (Development Grant), and a UTMB President's Cabinet Award is also acknowledged.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 11 June 2007.
REFERENCES
- 1.Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Burkanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37-53. [DOI] [PubMed] [Google Scholar]
- 2.Anasagasti, M. J., A. Alvarez, C. Avivi, and F. Vidal-Vanaclocha. 1996. Interleukin-1-mediated H2O2 production by hepatic sinusoidal endothelium in response to B16 melanoma cell adhesion. J. Cell. Physiol. 167:314-323. [DOI] [PubMed] [Google Scholar]
- 3.Ashktorab, H., M. Neapolitano, C. Bomma, C. Allen, A. Ahmed, A. Dubois, T. Naab, and D. T. Smoot. 2002. In vivo and in vitro activation of caspase-8 and -3 associated with Helicobacter pylori infection. Microbes Infect. 4:713-722. [DOI] [PubMed] [Google Scholar]
- 4.Backert, S., T. Schwarz, S. Miehlke, C. Kirsch, C. Sommer, T. Kwok, M. Gerhard, U. B. Goebel, N. Lehn, W. Koenig, and T. F. Meyer. 2004. Functional analysis of the cag pathogenicity island in Helicobacter pylori isolates from patients with gastritis, peptic ulcer, and gastric cancer. Infect. Immun. 72:1043-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagchi, D., G. Bhattacharya, and S. J. Stohs. 1996. Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Radic. Res. 24:439-450. [DOI] [PubMed] [Google Scholar]
- 6.Bagchi, D., T. R. McGinn, X. Ye, M. Bagchi, R. L. Krohn, A. Chatterjee, and S. J. Stohs. 2002. Helicobacter pylori-induced oxidative stress and DNA damage in a primary culture of human gastric mucosal cells. Dig. Dis. Sci. 47:1405-1412. [DOI] [PubMed] [Google Scholar]
- 7.Baik, S.-C., H.-S. Youn, M.-H. Chung, W.-K. Lee, M.-J. Cho, G.-H. Ko, C.-K. Park, H. Kasai, and K.-H. Rhee. 1996. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 56:1279-1282. [PubMed] [Google Scholar]
- 8.Bauer, M. K. A., M. Vogt, M. Los, J. Siegel, S. Weselborg, and K. Schulze-Osthoff. 1998. Role of reactive oxygen intermediates in activation-induced CD95 (APO-1/Fas) ligand expression. J. Biol. Chem. 273:8048-8055. [DOI] [PubMed] [Google Scholar]
- 9.Beil, W., B. Obst, K.-F. Sewing, and S. Wagner. 2000. Helicobacter pylori reduces intracellular glutathione in gastric epithelial cells. Dig. Dis. Sci. 45:1769-1773. [DOI] [PubMed] [Google Scholar]
- 10.Blaser, M. J., and J. Parsonnet. 1994. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J. Clin. Investig. 94:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaser, M. J., G. I. Perez-Perez, H. Kleanthous, T. L. Cover, R. M. Peek, P. H. Chyou, G. N. Stemmermann, and A. Nomura. 1995. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55:2111-2115. [PubMed] [Google Scholar]
- 12.Buttke, T. M., and P. A. Sandstrom. 1994. Oxidative stress as a mediator of apoptosis. Immunol. Today 15:7-10. [DOI] [PubMed] [Google Scholar]
- 13.Cahill, R. J., H. Xia, C. Kilgallen, S. Beattie, H. Hamilton, and C. O'Morain. 1995. Effect of eradication of Helicobacter pylori infection on gastric epithelial cell proliferation. Dig. Dis. Sci. 40:1627-1631. [DOI] [PubMed] [Google Scholar]
- 14.Carter, W. O., P. K. Narayanan, and J. P. Robinson. 1994. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J. Leukoc. Biol. 55:253-258. [DOI] [PubMed] [Google Scholar]
- 15.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clement, M. V., and S. Pervaiz. 1999. Reactive oxygen intermediates regulate cellular response to apoptotic stimuli: an hypothesis. Free Radic. Res. 30:247-252. [DOI] [PubMed] [Google Scholar]
- 17.Crabtree, J. E., S. M. Farmery, I. J. D. Lindley, N. Figura, P. Peichl, and D. S. Tompkins. 1994. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J. Clin. Pathol. 47:945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowe, S. E. 2005. Helicobacter infection, chronic inflammation, and the development of malignancy. Curr. Opin. Gastroenterol. 21:32-38. [PubMed] [Google Scholar]
- 19.Crowe, S. E., L. Alvarez, P. M. Sherman, Y. Jin, M. Dytoc, R. H. Hunt, J. Patel, M. J. Muller, and P. B. Ernst. 1995. Expression of interleukin-8 and CD54 by human gastric epithelium after H. pylori infection in vitro. Gastroenterology 108:65-74. [DOI] [PubMed] [Google Scholar]
- 20.Danese, S., F. Cremonini, A. Armuzzi, M. Candelli, A. Papa, V. Ojetti, A. Pastorelli, S. Di Caro, G. Zannoni, P. De Sole, G. Gasbarrini, and A. Gasbarrini. 2001. Helicobacter pylori CagA-positive strains affect oxygen free radicals generation by gastric mucosa. Scand. J. Gastroenterol. 36:247-250. [DOI] [PubMed] [Google Scholar]
- 21.Davies, G. R., N. J. Simmonds, T. R. J. Stevens, M. T. Sheaff, N. Banatvala, I. F. Laurenson, D. R. Blake, and D. S. Rampton. 1994. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut 35:179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deneke, S. M., and B. L. Fanburg. 1997. Regulation of cellular glutathione. Am. J. Physiol. 257:L163-L173. [DOI] [PubMed] [Google Scholar]
- 23.Drake, I. M., N. P. Mapstone, C. J. Schorah, K. L. M. White, D. M. Chalmers, M. F. Dixon, and A. T. R. Axon. 1998. Reactive oxygen species activity and lipid peroxidation in Helicobacter pylori associated gastritis: relation to gastric mucosal ascorbic acid concentrations and effect of H. pylori eradication. Gut 42:768-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst, P. B., Y. Jin, J. Navarro, V. E. Reyes, and S. E. Crowe. 1994. Overview of the immune response to H. pylori, p. 221-232. In R. H. Hunt (ed.), Helicobacter pylori: basic mechanisms to clinical cure. Kluwer Academic Publishers, Lancaster, United Kingdom.
- 25.Ernst, P. B., D. A. Peura, and S. E. Crowe. 2006. The translation of Helicobacter pylori basic research to patient care. Gastroenterology 130:188-206. [DOI] [PubMed] [Google Scholar]
- 26.Fan, X. J., S. E. Crowe, S. Behar, H. Gunasena, G. Ye, H. Haeberle, N. Van Houten, W. K. Gourley, P. B. Ernst, and V. E. Reyes. 1998. The effect of class II MHC expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for Th1 cell-mediated damage. J. Exp. Med. 187:1659-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farinati, F., R. Cardin, P. Degan, M. Rugge, F. D. Mario, P. Bonvicini, and R. Naccarato. 1998. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut 42:351-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furlong, I. J., R. Ascaso, R. A. Lopez, and M. K. Collins. 1997. Intracellular acidification induces apoptosis by stimulating ICE-like protease activity. J. Cell Sci. 110(Pt. 5):653-661. [DOI] [PubMed] [Google Scholar]
- 29.Giri, D. K., R. T. Mehta, R. G. Kansal, and B. B. Aggarwal. 1998. Mycobacterium avium-intracellulare complex activates nuclear transcription factor-κB in different cell types through reactive oxygen intermediates. J. Immunol. 161:4834-4841. [PubMed] [Google Scholar]
- 30.Grisham, M. B., T. S. Gaginella, C. von Ritter, H. Tamai, R. M. Be, and D. N. Granger. 1990. Effects of neutrophil-derived oxidants on intestinal permeability, electrolyte transport, and epithelial cell viability. Inflammation 14:531-542. [DOI] [PubMed] [Google Scholar]
- 31.Grossmann, J., S. Mohr, E. G. Lapentina, C. Fiocchi, and A. D. Levine. 1998. Sequential and rapid activation of select caspases during apoptosis of normal intestinal epithelial cells. Am. J. Physiol. 274:G1117-G1124. [DOI] [PubMed] [Google Scholar]
- 32.Hocker, M., I. Rosenberg, R. Xavier, R. J. Henihan, B. Wiedenmann, S. Rosewicz, D. K. Podolsky, and T. C. Wang. 1998. Oxidative stress activates the human histidine decarboxylase promoter in AGS gastric cancer cells. J. Biol. Chem. 273:23046-23054. [DOI] [PubMed] [Google Scholar]
- 33.Hug, H., S. Strand, A. Grambihler, J. Galle, V. Hack, W. Stremmel, P. H. Krammer, and P. R. Galle. 1997. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J. Biol. Chem. 272:28191-28193. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson, M. D. 1996. Reactive oxygen species and programmed cell death. Trends Biochem. Sci. 21:83-86. [PubMed] [Google Scholar]
- 35.Janssen, Y. M. W., B. Van Houten, P. J. A. Borm, and B. T. Mossman. 1993. Cell and tissue responses to oxidative damage. Lab. Investig. 69:261-274. [PubMed] [Google Scholar]
- 36.Jones, N. L., P. T. Shannon, E. Cutz, H. Yeger, and P. M. Sherman. 1997. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am. J. Pathol. 151:1695-1703. [PMC free article] [PubMed] [Google Scholar]
- 37.Keates, S., Y. S. Hitti, M. Upton, and C. P. Kelly. 1997. Helicobacter pylori infection activates NF-κB in gastric epithelial cells. Gastroenterology 113:1099-1109. [DOI] [PubMed] [Google Scholar]
- 38.Kim, J. M., J. S. Kim, H. C. Jung, Y. K. Oh, H. Y. Chung, C. H. Lee, and I. S. Song. 2003. Helicobacter pylori infection activates NF-κB signaling pathway to induce iNOS and protect human gastric epithelial cells from apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G1171-G1180. [DOI] [PubMed] [Google Scholar]
- 39.Kooy, N. W., J. A. Royall, and H. Ischiropoulos. 1997. Oxidation of 2′,7′-dichlorofluorescin by peroxynitrite. Free Radic. Res. 27:245-254. [DOI] [PubMed] [Google Scholar]
- 40.Le'Negrate, G., V. Ricci, V. Hofman, B. Mograbi, P. Hofman, and B. Rossi. 2001. Epithelial intestinal cell apoptosis induced by Helicobacter pylori depends on expression of the cag pathogenicity island phenotype. Infect. Immun. 69:5001-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, S. D., D. Kersulyte, I. J. Lindley, B. Neelam, D. E. Berg, and J. E. Crabtree. 1999. Multiple genes in the left half of the cag pathogenicity island of Helicobacter pylori are required for tyrosine kinase-dependent transcription of interleukin-8 in gastric epithelial cells. Infect. Immun. 67:3893-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luthra, G. K., A. R. DiNuzzo, W. K. Gourley, and S. E. Crowe. 1998. Comparison of biopsy and serological methods of diagnosis of Helicobacter pylori infection and the potential role of antibiotics. Am. J. Gastroenterol. 93:1291-1296. [DOI] [PubMed] [Google Scholar]
- 43.Lynch, D. A. F., N. P. Mapstone, A. M. T. Clarke, G. M. Sobala, P. Jackson, L. Morrison, M. F. Dixon, P. Quirke, and A. T. R. Axon. 1995. Cell proliferation in Helicobacter pylori associated gastritis and the effect of eradication therapy. Gut 36:346-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeda, S., H. Yoshida, Y. Mitsuno, Y. Hirata, K. Ogura, Y. Shiratori, and M. Omata. 2002. Analysis of apoptotic and antiapoptotic signalling pathways induced by Helicobacter pylori. Mol. Pathol. 55:286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mannick, E. E., L. E. Bravo, G. Zarama, J. L. Realpe, X.-J. Zhang, B. Ruiz, E. T. H. Fontham, R. Mera, M. J. S. Miller, and P. Correa. 1996. Inducible nitric oxide synthase, nitrotyrosine and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 56:3238-3243. [PubMed] [Google Scholar]
- 46.Marchesi, E., C. Rota, Y. C. Fann, C. F. Chignell, and R. P. Mason. 1999. Photoreduction of the fluorescent dye 2′-7′-dichlorofluorescein: a spin trapping and direct electron spin resonance study with implications for oxidative stress measurements. Free Radic. Biol. Med. 26:148-161. [DOI] [PubMed] [Google Scholar]
- 47.Matsubara, T., and M. Ziff. 1986. Increased superoxide anion release from human endothelial cells in response to cytokines. J. Immunol. 137:3295-3298. [PubMed] [Google Scholar]
- 48.Meier, B., H. H. Radeke, S. Selle, M. Younes, H. Sies, K. Resch, and G. G. Habermehl. 1989. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem. J. 263:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menconi, M. J., N. Unno, M. Smith, D. E. Aguirre, and M. P. Fink. 1998. Nitric oxide donor-induced hyperpermeability of cultured intestinal epithelial monolayers: role of superoxide radical, hydroxyl radical, and peroxynitrite. Biochim. Biophys. Acta 1425:189-203. [DOI] [PubMed] [Google Scholar]
- 50.Moss, S. F., J. Calam, B. Agarwal, S. Wang, and P. G. Holt. 1996. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 38:498-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagata, K., H. Yu, M. Nishikawa, M. Kashiba, A. Nakamura, E. F. Sato, T. Tamura, and M. Inoue. 1998. Helicobacter pylori generates superoxide radicals and modulates nitric oxide metabolism. J. Biol. Chem. 273:14071-14073. [DOI] [PubMed] [Google Scholar]
- 52.Naumann, M., S. Wessler, C. Bartsch, B. Wieland, A. Covacci, R. Haas, and T. F. Meyer. 1999. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J. Biol. Chem. 274:31655-31662. [DOI] [PubMed] [Google Scholar]
- 53.Noach, L. A., N. B. Bosma, J. Jansen, F. J. Hoek, S. J. van-Deventer, and G. N. Tytgat. 1994. Mucosal tumor necrosis factor-alpha, interleukin-1 beta and interleukin-8 production in patients with Helicobacter pylori. Scand. J. Gastroenterol. 29:425-429. [DOI] [PubMed] [Google Scholar]
- 54.Obst, B., S. Wagner, K. F. Sewing, and W. Beil. 2000. Helicobacter pylori causes DNA damage in gastric epithelial cells. Carcinogenesis 21:1111-1115. [PubMed] [Google Scholar]
- 55.O'Hara, A. M., A. Bhattacharyya, R. C. Mifflin, M. F. Smith, K. A. Ryan, K. G. Scott, M. Naganuma, A. Casola, T. Izumi, S. Mitra, P. B. Ernst, and S. E. Crowe. 2006. Interleukin-8 induction by Helicobacter pylori in gastric epithelial cells is dependent on apurinic/apyrimidinic endonuclease-1/redox factor-1. J. Immunol. 177:7990-7999. [DOI] [PubMed] [Google Scholar]
- 56.Papa, A., S. Danese, A. Sgambato, R. Ardito, G. Zannoni, A. Rinelli, F. M. Vecchio, N. Gentiloni-Silveri, A. Cittadini, G. Gasbarrini, and A. Gasbarrini. 2002. Role of Helicobacter pylori CagA+ infection in determining oxidative DNA damage in gastric mucosa. Scand. J. Gastroenterol. 37:409-413. [DOI] [PubMed] [Google Scholar]
- 57.Peek, R. M., Jr., G. G. Miller, K. T. Tham, G. I. Perez-Perez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 71:760-770. [PubMed] [Google Scholar]
- 58.Peek, R. M., Jr., S. F. Moss, K. Y. Tham, G. I. Perez-Perez, S. Wang, G. G. Miller, J. C. Atherton, P. R. Holt, and M. J. Blaser. 1997. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl. Cancer Inst. 89:863-868. [DOI] [PubMed] [Google Scholar]
- 59.Polyak, K., Y. Xia, J. L. Zweier, K. W. Kinzler, and B. Vogelstein. 1997. A model of p53-induced apoptosis. Nature 389:300-305. [DOI] [PubMed] [Google Scholar]
- 60.Quillet-Mary, A., J. P. Jaffrezou, V. Mansat, C. Bordier, J. Naval, and G. Laurent. 1997. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J. Biol. Chem. 272:21388-21395. [DOI] [PubMed] [Google Scholar]
- 61.Radeke, H. H., B. Meier, N. Topley, J. Floge, G. G. Habermehl, and K. Resch. 1990. Interleukin 1-α and tumor necrosis factor-α induce oxygen radical production in mesangial cells. Kidney Int. 37:767-775. [DOI] [PubMed] [Google Scholar]
- 62.Rosenkranz, A. R., S. Schmaldienst, K. M. Stuhlmeier, W. Chen, W. Knapp, and G. J. Zlabinger. 1992. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J. Immunol. Methods 156:39-45. [DOI] [PubMed] [Google Scholar]
- 63.Royall, J. A., and H. Ischiropoulos. 1993. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 302:348-355. [DOI] [PubMed] [Google Scholar]
- 64.Ruiz, B., J. C. Rood, E. T. H. Fontham, G. T. Malcom, F. M. Hunter, M. Sobhan, W. D. Johnson, and P. Correa. 1994. Vitamin C concentration in gastric juice before and after anti-Helicobacter pylori treatment. Am. J. Gastroenterol. 89:533-539. [PubMed] [Google Scholar]
- 65.Schweizer, M., and E. Peterhans. 1999. Oxidative stress in cells infected with bovine viral diarrhoea virus: a crucial step in the induction of apoptosis. J. Gen. Virol. 80:1147-1155. [DOI] [PubMed] [Google Scholar]
- 66.Sharma, S. A., M. K. Tummuru, M. J. Blaser, and L. D. Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-κB in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]
- 67.Sharma, S. A., M. K. R. Tummuru, G. G. Miller, and M. J. Blaser. 1995. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect. Immun. 63:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shibayama, K., Y. Doi, N. Shibata, T. Yagi, T. Nada, Y. Iinuma, and Y. Arakawa. 2001. Apoptotic signaling pathway activated by Helicobacter pylori infection and increase of apoptosis-inducing activity under serum-starved conditions. Infect. Immun. 69:3181-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sipowicz, M. A., P. Chomarat, B. A. Diwan, M. A. Anver, Y. C. Awasthi, J. M. Ward, J. M. Rice, K. S. Kasprzak, C. P. Wild, and L. M. Anderson. 1997. Increased oxidative DNA damage and hepatocyte overexpression of specific cytochrome p450 isoforms in hepatitis of mice infected with Helicobacter hepaticus. Am. J. Pathol. 151:933-941. [PMC free article] [PubMed] [Google Scholar]
- 70.Smoot, D. T., T. B. Elliott, H. W. Verspaget, D. Jones, C. R. Allen, K. G. Vernon, T. Bremner, L. C. Kidd, K. S. Kim, J. D. Groupman, and H. Ashktorab. 2000. Influence of Helicobacter pylori on reactive oxygen-induced gastric epithelial cell injury. Carcinogenesis 21:2091-2095. [DOI] [PubMed] [Google Scholar]
- 71.Sobalo, G. M., C. J. Schorah, S. Shires, D. A. F. Lynch, B. Gallacher, M. F. Dixon, and A. T. R. Axon. 1993. Effect of eradication of Helicobacter pylori on gastric juice ascorbic acid concentrations. Gut 34:1038-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suematsu, N., H. Tsutsui, J. Wen, D. Kang, M. Ikeuchi, T. Ide, S. Hayashidani, T. Shiomi, T. Kubota, N. Hamasaki, and A. Takeshita. 2003. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 107:1418-1423. [DOI] [PubMed] [Google Scholar]
- 73.Teshima, S., K. Rokutan, T. Nikawa, and K. Kishi. 1998. Guinea pig gastric mucosal cells produce abundant superoxide anion through an NADPH oxidase-like system. Gastroenterology 115:1186-1196. [DOI] [PubMed] [Google Scholar]
- 74.Thelen, M., B. Dewald, and M. Baggiolini. 1993. Neutrophil signal transduction and activation of the respiratory burst. Physiol. Rev. 73:797-821. [DOI] [PubMed] [Google Scholar]
- 75.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzgerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 76.Wagner, S., W. Beil, J. Westermann, R. P. Logan, C. T. Bock, C. Trautwein, J. S. Bleck, and M. P. Manns. 1997. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology 113:1836-1847. [DOI] [PubMed] [Google Scholar]
- 77.Xu, H., R. Chaturvedi, Y. Cheng, F. I. Bussiere, M. Asim, M. D. Yao, D. Potosky, S. J. Meltzer, J. G. Rhee, S. S. Kim, S. F. Moss, A. Hacker, Y. Wang, R. A. Casero, Jr., and K. T. Wilson. 2004. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 64:8521-8525. [DOI] [PubMed] [Google Scholar]
- 78.Ye, G., C. Barrera, X. J. Fan, W. K. Gourley, S. E. Crowe, P. B. Ernst, and V. E. Reyes. 1997. Expression of B7-1 and B7-2 costimulatory molecules by human gastric epithelial cells. Potential role in CD4+ T cell activation during Helicobacter pylori infection. J. Clin. Investig. 99:1628-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, Q. B., J. B. Dawodu, A. Husain, G. Etolhi, C. G. Gemmell, and R. I. Russell. 1997. Association of antral mucosal levels of interleukin 8 and reactive oxygen radicals in patients infected with Helicobacter pylori. Clin. Sci. 92:69-73. [DOI] [PubMed] [Google Scholar]
- 80.Zhu, H., G. L. Bannenberg, P. Moldeus, and H. G. Shertzer. 1994. Oxidation pathways for the intracellular probe 2′,7′-dichlorofluorescin. Arch. Toxicol. 68:582-587. [DOI] [PubMed] [Google Scholar]