Abstract
Several pathogenic bacteria exploit human carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) for adhesion to and invasion into their host cells. CEACAM isoforms have characteristic expression patterns on epithelial, endothelial, or hematopoietic cells, providing bacteria with distinct sets of receptors on particular tissues. For example, while CEACAM1 and CEACAM6 have a wide tissue distribution, CEACAM3, CEACAM4, and CEACAM8 are uniquely expressed on primary human granulocytes, whereas CEA and CEACAM7 are limited to epithelia. By reconstitution of a CEACAM-deficient cell line with individual CEACAMs, we have analyzed the requirements for CEACAM-mediated internalization of Neisseria gonorrhoeae. Our results point to two mechanistically different uptake pathways triggered by either epithelial CEACAMs (CEACAM1, CEA, and CEACAM6) or the granulocyte-specific CEACAM3. In particular, CEACAM3-mediated uptake critically depends on Src family protein tyrosine kinase (PTK) activity, and CEACAM3 associates with the SH2 domains of several Src PTKs. In contrast, epithelial CEACAMs require the integrity of cholesterol-rich membrane microdomains and are affected by cholesterol depletion, whereas CEACAM3-mediated uptake by transfected cells or the opsonin-independent phagocytosis by human granulocytes is not altered in the presence of cholesterol chelators. These results allow the subdivision of all human CEACAMs known to be utilized as pathogen receptors into functional groups and point to important consequences for bacterial engagement of distinct CEACAM isoforms.
Members of the human carcinoembryonic antigen-related cell adhesion molecule (CEACAM) family serve as cellular receptors for a variety of gram-negative bacterial pathogens. In particular, Neisseria gonorrhoeae, N. meningitidis, Haemophilus influenzae, Moraxella catarrhalis, several Salmonella species, and Escherichia coli have been found to associate with the protein core or carbohydrate structures of these surface glycoproteins (8, 16, 17, 22, 44). In humans, more than 10 CEACAM family members can be expressed by various tissues but are found predominantly on cells of epithelial or hematopoietic origin (12, 20). In an example of convergent evolution, different bacterial species have elaborated distinct surface antigens to engage CEACAMs. For example H. influenzae seems to contact CEACAM family members by the outer membrane protein P5, whereas M. catarrhalis employs the UspA1 antigen (16, 17). The most intensively studied bacterial ligands for CEACAM family members are the neisserial colony opacity-associated (Opa) proteins (13). This multiprotein family is encoded by several genes in the genomes of gonococci (up to 12 copies) and meningococci (up to 4 copies). Their expression is independently controlled by a translation-based mechanism of phase variation (38, 39). Besides a few Opa proteins that have been found to bind heparan sulfate proteoglycans (OpaHSPG) (6, 43), the large majority of the Opa proteins characterized to date associate with members of the human CEACAM family (OpaCEA).
OpaCEA binding to CEACAMs is confined to the N-terminal immunoglobulin variable-like domain of these immunoglobulin superfamily members that is shared by all CEACAMs (3, 21, 31, 45). Nevertheless, OpaCEA proteins studied so far recognize only a restricted set of CEACAM family members, namely, CEACAM1, CEA, CEACAM3 and CEACAM6, and do not recognize CEACAM4, CEACAM7, or CEACAM8 (21, 31). In addition to mediating the close contact between the microorganisms and host cells, CEACAM recruitment by pathogenic bacteria has also been demonstrated to induce the internalization of the bacteria, even in the presence of a bacterial capsule, and to trigger characteristic gene expression events in the infected epithelial cells (4, 27). Receptor-mediated bacterial internalization might allow the colonizing microbes to access intact epithelial layers, as OpaCEA-binding CEACAMs are expressed predominantly on the apical aspect of polarized epithelial cells. This is true for CEACAM1, CEA, and CEACAM6, which are expressed by epithelial cells (epithelial CEACAMs), while CEACAM3 is not found on epithelia. In addition, the engagement of epithelial CEACAMs seems to initiate bacterial transcytosis through intact cell layers, as observed in transwell cell culture models (47).
In striking contrast to the potential benefits CEACAM-binding pathogens might obtain from associating with CEACAMs on epithelial surfaces, CEACAM-binding bacteria are also recognized by cells of the innate immune system, in particular human granulocytes, in an opsonin-independent but CEACAM-dependent manner (8, 14, 44). Importantly, the interaction of granulocytes with CEACAM-binding bacteria results in the elimination of the microorganisms (33, 44). Therefore, the uptake of bacteria by this cell type can be seen as detrimental and as a specific host defense targeted towards CEACAM-binding microbes. Though granulocytes express several CEACAM isoforms (including CEACAM1, CEACAM3, and CEACAM6), efficient uptake and elimination has been attributed to the expression of CEACAM3, a granulocyte-specific member of the CEACAM family (33). Internalization via CEACAM3 depends on a direct and phosphorylation-dependent association of the guanine nucleotide exchange factor Vav with the cytoplasmic domain of the receptor (34). Accordingly, CEACAM3 engagement is linked to stimulation of the small GTPase Rac, a master regulator of phagocytosis and the NADPH oxidase system in phagocytes (10, 33). CEACAM1 and CEACAM6 apparently show only a minor contribution to the opsonin-independent phagocytosis of OpaCEA-expressing bacteria if the microbes are recognized by CEACAM3 (33, 34). However, CEACAM1 and CEACAM6 can mediate uptake into epithelial cells, and bacteria expressing OpaCEA variants that selectively recognize CEACAM1, but not CEACAM3 or CEACAM6, can also trigger an oxidative response in granulocytes (11). These seemingly contradictory results prompted us to investigate the molecular requirements of CEACAM3-mediated phagocytosis versus bacterial uptake via epithelial CEACAMs (CEACAM1, CEA, and CEACAM6).
Here we provide evidence that bacterial internalization via epithelial CEACAMs differs mechanistically from opsonin-independent phagocytosis via CEACAM3. In particular, we find an essential role for Src family protein tyrosine kinases (Src PTKs) in supporting efficient CEACAM3-mediated internalization. In contrast, CEACAM6-initiated uptake is only marginally affected by inhibition of Src kinases. Moreover, depletion of cholesterol-rich membrane microdomains severely attenuates internalization initiated by epithelial CEACAMs but not CEACAM3-initiated uptake. Importantly, interfering with cholesterol-rich lipid rafts in primary human granulocytes does not impair their ability to efficiently phagocytose OpaCEA-expressing N. gonorrhoeae, whereas the inhibition of Src kinases completely abrogates this process. These data provide a mechanistic explanation for the predominant contribution of CEACAM3 in granulocyte phagocytosis of CEACAM-binding pathogens. Furthermore, our results reveal a novel role of cholesterol-rich lipid rafts for bacterial internalization via epithelial CEACAMs.
MATERIALS AND METHODS
Bacteria.
Neisseria gonorrhoeae strain MS11 and its OpaCEA-expressing (Opa52; N309) and nonopaque (N302) variants were kindly provided by Thomas Meyer (Max-Planck-Institut für Infektionsbiologie, Berlin, Germany) and were cultured as described previously (33). For gentamicin protection assays, overnight-grown bacteria were taken from GC agar plates supplemented with vitamins. For analysis of uptake by flow cytometry or immunofluorescence microscopy, Neisseria was grown in brain heart infusion broth supplemented with NaHCO3 and vitamins under constant shaking for at least 3 h. Bacteria were labeled with 0.2 μg/ml 5-(6)-carboxyfluorescein-succinimidylester (CFSE) or 5-(6)-carboxytetramethylrhodamine-succinimidylester (rhodamine) (Molecular Probes, Eugene, OR) in phosphate-buffered saline (PBS) for 20 min at 37°C in the dark and washed three times with PBS prior to use.
Cell culture.
Human embryonic kidney epithelial 293T cells (293 cells) were cultured in Dulbecco's modified Eagle's medium (DMEM)-10% calf serum. Cells were subcultured every 3 to 4 days and were serum starved in DMEM-0.5% calf serum for 18 h before infection. Primary human granulocytes were isolated from freshly drawn blood essentially as described previously (14). The viability of cells was determined prior to infection by trypan blue staining and in all cases was >95%. Src-, Yes-, and Fyn-deficient mouse embryonic fibroblasts (SYF cells) and Src-reexpressing SYF cells (SYF + Src cells) (19) were obtained from P. Soriano (FHCRC, Seattle, WA) and cultured in DMEM-10% fetal bovine serum (FBS) containing sodium pyruvate and nonessential amino acids at 37°C and 5% CO2.
Recombinant DNA.
Wolfgang Zimmermann (Universitätsklinikum Grosshadern, München, Germany) kindly provided cDNAs of human CEACAM1, CEACAM3, CEACAM4, CEACAM6, CEACAM7, and CEACAM8 in the mammalian expression plasmid pRc/CMV (Invitrogen, Karlsruhe, Germany). Hemagglutinin (HA)-tagged CEACAM3 variants were described previously (33). To generate a red fluorescent protein (RFP) fusion with the C terminus of CEACAM3 (CEACAM3-RFP), wild-type CEACAM3 (CEACAM3 WT) was amplified with primers 5′-ATAGCTAGCGCCACCATGGGGCCCCCCTCAGCCTCTCCCCAC-3′ and 5′-ATAACCGGTGAAGCCACTTCTGCTTTGTGGTCCATCCG-3′ and subcloned via NheI/AgeI into pDS-Red1N1 (Clontech, Palo Alto, CA). A cDNA clone of CEACAM5 (IMAGp998G0911461Q3) was obtained from RZPD (Berlin, Germany), amplified by PCR with primers 5′-TATGGTACCATGGAGTCTCCCTCGGCCCC-3′ and 5′-TATTCTAGACTATATCAGAGCAACCCCC-3′, and cloned into the HindIII/XbaI sites of pCDNA3.1 (Invitrogen). All CEACAM constructs were verified by sequencing. Plasmids encoding C-terminal Src kinase (Csk), kinase inactive Csk (Csk K222M), and Rous sarcoma virus Src (v-Src) were kindly provided by David Schlaepfer (Scripps Research Institute, La Jolla, CA). The expression plasmid encoding yellow fluorescent protein (YFP)-dSH2-Src (18) was provided by Benjamin Geiger (Weizmann Institute, Tel Aviv, Israel).
cDNA clones for different human PTKs of the Src family were obtained from RZPD (Berlin, Germany). The SH2 domains were amplified by PCR from c-Src (IMAGp958B161238q2) with primers 5′-GAAGTTATCAGTCGACCCCTCCGACTCCATCCAG-3′ and 5′-ATGGTCTAGAAAGCTTACAGGCCCTGAGTCTGC-3′, from Fyn (IMAGp998H0211552q3) with primers 5′-GAAGTTATCAGTCGACCCAGTTGACTCTATCCAGGCAG-3′ and 5′-ATGGTCTAGAAAGCTTAGGGGGACATTGTGCCTGG-3′, from Yes (IMAGp998C2411657q3) with primers 5′-GAAGTTATCAGTCGACCCTGCAGATTCCATTCAGG-3′ and 5′-ATGGTCTAGAAAGCTTATGCTAGTCCTTGAGTCTGAGG-3′, from Lck (IMAGp998L159954q3) with primers 5′-GAAGTTATCAGTCGACGCGAACAGCCTGGAGC-3′ and 5′-ATGGTCTAGAAAGCTTAATCCTCCCACCACGGC-3′, and from Hck (IMAGp958H201705q2) with primers 5′-GAAGTTATCAGTCGACGCCCGCGTTGACTCTC-3′ and 5′-ATGGTCTAGAAAGCTTAATCTTTCTCCCAAGGCTTCTGG-3′. All SH2 constructs were cloned into pDNR-Dual via an InFusion cloning kit (Clontech) and subsequently transferred to pGEX4T1-LoxP by Cre/Lox recombination as described previously (1). The SH2 domain of Hck was furthermore transferred by Cre/Lox recombination from pDNR-Dual into pEGFP-N1 loxP (Clontech) to yield GFP-Hck-SH2.
Antibodies and reagents.
Monoclonal antibody (MAb) D14HD11 (cross-reactive with all CEACAMs except CEACAM8) was from GENOVAC (Freiburg, Germany), MAb against CEACAM8 (80H3) was from Immunotech (Marseille, France), MAbs against green fluorescent protein (GFP) (clone JL-8) and against Csk (clone 52) were from BD Biosciences (Palo Alto, CA), MAb against v-Src (clone EC10) was from Upstate Biotechnology (Lake Placid, NY), and MAb against glutathione S-transferase (GST) (clone B-14) was from Santa Cruz Biotechnology (Santa Cruz, CA). MAbs against the HA tag (clone 12CA5) and against c-Src (clone 2-17) were purified from hybridoma cell supernatants. Rabbit antiserum against N. gonorrhoeae (AK92) was generously provided by Thomas Meyer (Max-Planck-Institut für Infektionsbiologie, Berlin, Germany). PP2 and nystatin were purchased from Calbiochem (Schwalbach, Germany), and filipin and methyl-β-cyclodextrin (MβCD) were from Sigma (Taufkirchen, Germany). GST and GST-SH2 domain fusion proteins were expressed in E. coli BL21 and purified according to standard procedures using GSTrap FastFlow (Amersham Biosciences, Freiburg, Germany).
Transfection of cells, cell lysis, and Western blotting.
293 cells were transfected by calcium phosphate precipitation using 3 μg of CEACAM constructs or an empty vector control. For cotransfection, 5 μg of cotransfected constructs together with 3 μg of CEACAM constructs was used, and in all samples total DNA was adjusted to 8 μg using the empty control vector. For transfection of SYF cells, Lipofectamine Plus (Invitrogen, Karlsruhe, Germany) was used according to the manufacturer's instructions. Cells were employed in infection experiments 48 h after the transfection. Cell lysis and Western blotting were performed as described previously (33).
Gentamicin protection assay.
Gentamicin protection assays were conducted as described previously (33). Cells were seeded in gelatin-coated 24-well dishes with 6 × 105 cells/well. A multiplicity of infection (MOI) of 20 bacteria per cell was routinely used, and after 1 hour of infection, extracellular bacteria were killed by a 45-min incubation in 50 μg/ml gentamicin in DMEM. Then, cells were lysed with 1% saponin in PBS for 15 min. The samples were diluted with PBS, and the number of viable bacteria was determined by plating suitable dilutions in duplicate on GC agar.
Flow cytometric determination of granulocyte phagocytosis and bacterial uptake.
Phagocytosis was determined by flow cytometry according to reference 46. Briefly, 1 × 106 granulocytes were infected with 2 × 107 CFSE-labeled bacteria in 1 ml phagocytosis buffer (PB; 1× PBS, 10 mM glucose, 1% heat-inactivated FBS) for 15 min at 37°C. In inhibition experiments, inhibitors were added to the cells 15 min (PP2) or 30 min (filipin, MβCD, nystatin) prior to the infection. Phagocytosis was stopped by the addition of ice-cold PB, and samples were washed, taken up in cold PBS, 2% FBS, 2 mg/ml trypan blue and analyzed on a FACSCalibur (Becton Dickinson). To obtain an estimate of the total amount of phagocytosed bacteria, the percentage of CFSE-positive granulocytes was multiplied by the mean fluorescence of these cells (uptake index). Bacterial uptake by transfected 293 cells was analyzed by flow cytometry in a manner essentially similar to that described previously (30) using an MOI of 20. To measure the ratio of intracellular bacteria to total cell-associated bacteria (cell-adherent as well as intracellular bacteria), cells infected with CFSE-labeled bacteria were analyzed by flow cytometry in the absence of trypan blue (total cell-associated bacteria) as well as in the presence of trypan blue (intracellular bacteria).
GST pull-down.
For GST pull-downs, 3 μg of purified GST or GST-fusion proteins attached to glutathione-Sepharose was added to 200 μl of cleared lysates from 293 cells cotransfected with CEACAM constructs or the empty vector (5 μg) and a v-Src-encoding plasmid (1.5 μg) and incubated for 4 h at 4°C. After four washes with modified radioimmunoprecipitation assay buffer (1), precipitates were boiled in 2× sodium dodecyl sulfate sample buffer before sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis.
Immunofluorescence staining.
293 cells transfected with GFP-Hck-SH2 and either CEACAM3 or CEACAM6 were grown on glass coverslips in 24-well plates and infected for 60 min with rhodamine-labeled OpaCEA-expressing N. gonorrhoeae at an MOI of 10. Samples were fixed with 4% paraformaldehyde in PBS and washed three times with PBS prior to incubation in blocking buffer (PBS, 10% fetal calf serum, 0.2% saponin) for 5 minutes. Samples were stained with anti-CEACAM MAb (clone D14HD11; diluted 1:100 in blocking buffer) for 1 h. After three washes and 5 min of incubation in blocking buffer, samples were incubated with Cy5-coupled goat anti-mouse antibodies in blocking buffer for 45 min. Following three washes, samples were embedded in mounting medium (Dako, Glostrup, Denmark).
For differentiating between extra- and intracellular bacteria, cells were infected with CFSE-labeled bacteria, and fixed samples were stained prior to permeabilization with polyclonal rabbit anti-N. gonorrhoeae (1:200) and goat anti-rabbit-Cy5 (1:100) in staining buffer (PBS, 5% fetal calf serum), resulting in CFSE-labeled intracellular and CFSE/Cy5-labeled extracellular bacteria.
Samples were viewed with an LSM510 laser scanning confocal microscope (Zeiss, Oberkochen, Germany) by use of a 63× 1.3 numerical aperture Plan Neofluar oil immersion objective. Fluorescence signals of triply labeled specimens were serially recorded with appropriate excitation and emission filters to avoid bleed-through. Images were digitally processed with Photoshop 6 (Adobe Systems, Mountain View, CA) and merged to yield pseudocolored pictures.
Cholesterol depletion assay.
Granulocytes (2 × 106 cells/ml phagocytosis buffer) were incubated with the indicated concentrations of MβCD for 30 min at 37°C. Cells were either used in phagocytosis assays or washed once with PBS and subsequently resuspended in assay buffer from a cholesterol assay kit (Cayman Chemical, Ann Arbor, MI). The suspension was heated to 95°C to free the remaining cholesterol. Appropriate dilutions (1:5 to 1:50) were subjected to analysis according to the manufacturer's instructions, and the calculation of the cholesterol content was based on a standard curve for purified cholesterol supplied with the kit.
RESULTS
CEACAM family members differentially contribute to the internalization of OpaCEA-expressing gonococci.
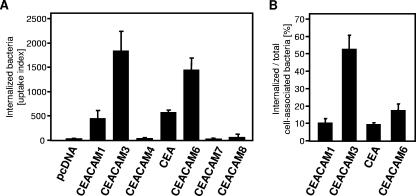
To test the functionality of individual CEACAM family members with regard to the uptake of CEACAM-binding bacteria, the full spectrum of human CEACAMs was individually transfected into human 293 cells, an epithelial cell line devoid of endogenously expressed CEACAMs. Transient transfection resulted in equivalent levels of surface-expressed CEACAMs, with transfection efficiencies ranging between 30 and 40% of the cell population (see Fig. S1 in the supplemental material). Transfected 293 cells were then employed in gentamicin protection assays with OpaCEA-expressing N. gonorrhoeae. Importantly, and as observed by other groups before, only four out of seven CEACAMs mediated adhesion (data not shown) and internalization of the pathogens (Fig. 1A). Clearly, the efficiencies of bacterial uptake as measured by flow cytometry varied between the CEACAM isoforms. Whereas in the case of CEACAM1, CEA, and CEACAM6 only about 10 to 20% of the cell-associated bacteria were internalized, more than 50% of the total cell-associated microbes were found to localize inside CEACAM3-expressing cells within 1 hour after the start of the infection (Fig. 1B). The results obtained with transiently transfected 293 cells are in line with previous observations obtained with stably transfected HeLa or CHO cell lines (24, 31) and suggest that all OpaCEA-binding CEACAMs contribute to bacterial internalization, but they also demonstrate that CEACAM3 is particularly effective in mediating the uptake of OpaCEA gonococci.
FIG. 1.
Internalization of OpaCEA-expressing N. gonorrhoeae by 293 cells expressing various CEACAM isoforms. (A) CEACAM-transfected cells were infected with CFSE-labeled, OpaCEA-expressing N. gonorrhoeae (MOI, 20) for 60 min, and cell-associated CFSE-derived fluorescence was determined in the presence of trypan blue to selectively detect intracellular bacteria. Bars indicate the mean uptake indexes ± standard deviations for three independent experiments done in triplicate. (B) Cells were infected as described for panel A, and cell-associated CFSE-derived fluorescence was measured for identical samples in the absence (total cell-associated bacteria) as well as in the presence (intracellular bacteria) of trypan blue. Values represent the mean percentages ± standard deviations for the internalized bacteria with respect to the total cell-associated bacteria and are derived from three independent experiments.
CEACAM3- but not CEACAM6-dependent phagocytosis of OpaCEA-expressing gonococci is severely blocked by Src family kinase inhibitors.
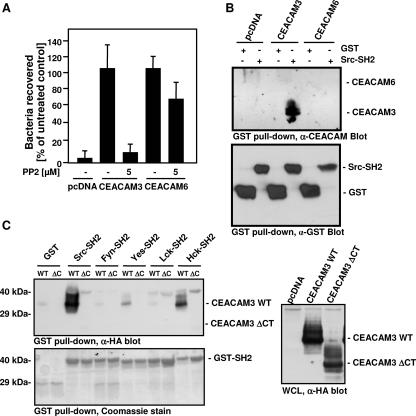
In order to study the mechanisms by which CEACAM3 mediates efficient uptake of OpaCEA-expressing bacteria with regard to those of other OpaCEA-binding CEACAMs, we analyzed the requirements for bacterial internalization by CEACAM3 and compared them to those for CEACAM6, which provided the most efficient uptake within the group of epithelial CEACAMs. Therefore, 293 cells were transfected with cDNA encoding CEACAM3, CEACAM6, or the empty control vector (pcDNA). Prior to infection, cells were treated with or without 5 μM PP2, a selective pharmacological inhibitor of Src PTKs, for 15 min. Cells were infected with bacteria at an MOI of 20 for 1 h, and then the amount of intracellular viable bacteria was analyzed by gentamicin protection assays (Fig. 2A). Both CEACAM family members again displayed internalization of OpaCEA-expressing bacteria, while for control transfected cells, barely any intracellular gonococci were observed (Fig. 2A). Pretreatment of transfected cells with PP2 reduced the internalization of bacteria for both CEACAM molecules. However, the uptake of gonococci was only slightly affected by PP2 in CEACAM6-transfected cells (about 25% reduction), whereas CEACAM3-mediated internalization of gonococci was inhibited by more than 90% by PP2, the inhibitor of Src PTKs (Fig. 2A).
FIG. 2.
Src PTK activity is involved in CEACAM3-mediated uptake of OpaCEA-expressing gonococci. (A) 293 cells were transfected with CEACAM3, CEACAM6, or the empty control vector and employed in a gentamicin protection assay. Cells were pretreated or not with 5 μM PP2 for 15 min before infection with OpaCEA gonococci. The graph shows mean values ± standard deviations from three independent experiments done in triplicate. (B) 293 cells were cotransfected with the empty control vector (pcDNA), CEACAM3, or CEACAM6 together with constitutive active Src (v-Src). Cell lysates were employed in pull-down assays with the isolated Src-SH2 domain fused to GST or GST alone. Precipitates were analyzed by Western blotting with monoclonal anti-CD66 antibody (clone D14HD11; top) and then stripped and reprobed with a monoclonal anti-GST (α-GST) antibody (bottom). (C) 293 cells were cotransfected with the empty control vector (pcDNA), CEACAM3 WT, or CEACAM3 ΔCT (ΔC) together with v-Src. Cell lysates were employed in pull-down assays with the indicated GST fusion proteins or with GST alone. Precipitates were analyzed by Western blotting with monoclonal anti-HA tag antibody (top), and then the membrane was stained with Coomassie brilliant blue to visualize the GST fusion proteins used in the pull-down assay (bottom). The right panel shows the levels of CEACAM3 WT and CEACAM3 ΔCT in the used whole-cell lysates (WCLs) as detected by blotting with anti-HA tag antibody.
In contrast to the glycosylphosphatidylinositol (GPI)-anchored CEACAM6, the most abundant isoform of CEACAM3 possesses an intracellular domain containing two potential tyrosine phosphorylation sites. Indeed, purified Src phosphorylates the recombinant CEACAM3 cytoplasmic domain in vitro (40). On the other hand, previous studies have reported an association between CEACAM6 and active Src family PTKs in human granulocytes (37). As Src family kinases have been shown to bind to tyrosine phosphorylated substrates by means of their SH2 domain (41), we wondered whether CEACAM3 or CEACAM6 might support the binding of the Src SH2 domain. Therefore, lysates of control transfected (pcDNA) and CEACAM3- or CEACAM6-expressing cells were incubated with either GST or a GST fusion of the c-Src SH2 domain, and upon glutathione-agarose-mediated precipitation the samples were analyzed with anti-CEACAM antibodies. Importantly, whereas GST did not interact with either CEACAM3 or CEACAM6, the Src SH2 domain specifically associated with CEACAM3 but not with CEACAM6 (Fig. 2B). As CEACAM3 is expressed in granulocytes that contain distinct Src PTK variants, we tested whether SH2 domains derived from Src PTKs other than c-Src are able to associate with the cytoplasmic domain of CEACAM3. To this end, CEACAM3 or a CEACAM3 mutant lacking the cytoplasmic domain (CEACAM3 ΔCT) was expressed in 293 cells, and the lysates were probed with SH2 domains of various Src PTKs in a GST pull-down format (Fig. 2C). Importantly, both the c-Src- and the Hck-derived SH2 domains strongly associated with the cytoplasmic domain of CEACAM3 (Fig. 2C), demonstrating that not only Src family members present in epithelial cells and fibroblasts (c-Src) but also family members expressed in human granulocytes (Hck) can interact with the phosphorylated cytoplasmic domain of CEACAM3. Together, these data support the view that CEACAM3-mediated internalization might have a requirement for Src family kinase activity more stringent than that for CEACAM6 and that dependence on Src PTK activity might be a characteristic criterion to distinguish between these two routes of uptake.
The isolated Src SH2 domain blocks CEACAM3- but not CEACAM6-mediated internalization of N. gonorrhoeae.
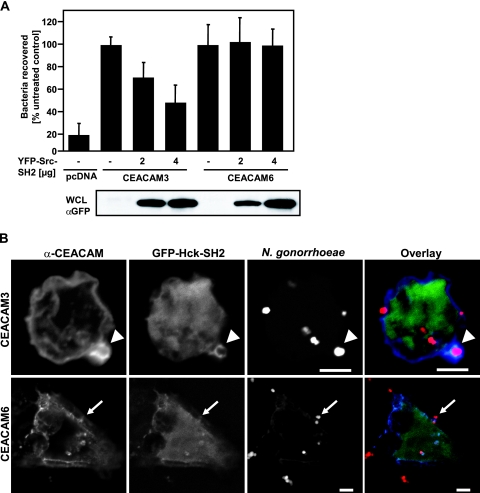
In light of the strong association of the c-Src SH2 domain with CEACAM3, we hypothesized that overexpression of the isolated c-Src SH2 domain should competitively inhibit the access of endogenous c-Src to the CEACAM3 cytoplasmic domain. Therefore, we expressed different amounts of the Src-dSH2 domain fused to YFP in combination with either CEACAM3, CEACAM6, or the empty control vector in 293 cells. The expression of YFP-Src-dSH2 had no effect on CEACAM6-mediated uptake, while CEACAM3-transfected cells showed a dose-dependent decrease in internalization of OpaCEA-expressing bacteria, further corroborating the view that CEACAM3 and CEACAM6 might be differentially connected to Src family kinases (Fig. 3A). In order to verify that SH2 domains of Src family PTKs indeed associated with CEACAM3 in intact cells, we investigated GFP-Hck-SH2-expressing cells by confocal microscopy. One hour after infection, opaque gonococci undergoing internalization showed a marked colocalization with GFP-Hck-SH2 and CEACAM3 (Fig. 3B). This strong association appears to be transient, as the pronounced association was detected in only a fraction of cell-associated bacteria (∼10 to 15%) at different time points (15 to 90 min after infection; data not shown). In contrast, CEACAM6-expressing cells showed colocalization between the receptor and the bacteria; however, only minor amounts of GFP-Hck-SH2 were detected in the vicinity of CEACAM6 (Fig. 3B). Together, these results supported the idea that Src PTKs target phosphorylated tyrosine residues in the cytoplasmic domain of CEACAM3 and that the Src-dSH2 domain functions as a competitive inhibitor of CEACAM3- but not CEACAM6-mediated uptake by interfering with the intracellular signaling events emanating from CEACAM3.
FIG. 3.
The SH2 domain of Hck localizes with CEACAM3 and the isolated Src-SH2 domain blocks CEACAM3- but not CEACAM6-mediated uptake. (A) 293 cells were cotransfected with CEACAM3 or CEACAM6 and the indicated amounts of YFP-Src-dSH2 cDNA. Transfected cells were infected for 60 min with OpaCEA-expressing gonococci and analyzed in gentamicin assays. The bars show mean values ± standard deviations from three independent experiments done in triplicate. Values represent the mean numbers of recovered bacteria relative to that for the control not receiving YFP-Src-dSH2. Expression of YFP-Src-dSH2 in the samples was confirmed by Western blotting of whole-cell lysates (WCL) with anti-GFP (α-GFP) antibody (bottom). (B) 293 cells were cotransfected with CEACAM3 or CEACAM6 and GFP-Hck-SH2 cDNA, infected with rhodamine-labeled OpaCEA-expressing N. gonorrhoeae for 1 h, and then fixed. Samples were stained with monoclonal anti-CEACAM antibody (clone D14HD11) and Cy5-coupled secondary antibodies. The arrowheads point to CEACAM3 colocalizing with gonococci and the Hck-SH2 domain, whereas the small arrows point to CEACAM6 colocalizing with gonococci during uptake. Bars represent 5 μm.
CEACAM3 requires the presence and function of Src family kinases to mediate internalization of OpaCEA-expressing bacteria.
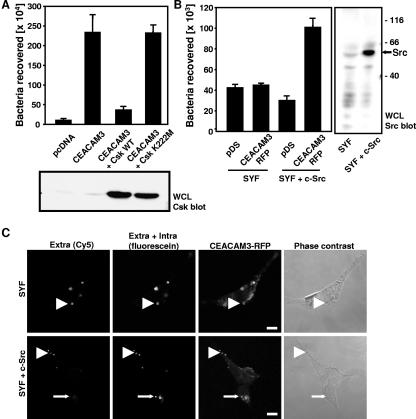
To further corroborate a role for Src family kinases in CEACAM3-mediated internalization in response to OpaCEA gonococci, we overexpressed a negative regulator of Src family tyrosine kinases, C-terminal Src kinase (Csk), in 293 cells. As a negative control, a kinase-inactive variant of Csk (Csk K222M) was employed. Cotransfection of Csk WT together with CEACAM3 resulted in a strong blockage of phagocytosis of OpaCEA bacteria (Fig. 4A). In contrast, expression of Csk K222M at levels comparable to Csk WT levels had no effect on CEACAM3-mediated internalization (Fig. 4A). To finally provide genetic proof that Src PTKs have a critical function in CEACAM3-mediated bacterial internalization, we took advantage of fibroblasts that are deficient in Src, Yes, and Fyn (SYF cells), the three Src family members normally expressed in this cell type (19). SYF cells were transiently transfected with CEACAM3-RFP to monitor transfected cells. In 293 cells, CEACAM3-RFP mediated internalization of OpaCEA gonococci at the same level as unmodified CEACAM3 (data not shown). However, the expression of CEACAM3-RFP in SYF cells did not promote enhanced internalization of OpaCEA bacteria as measured in gentamicin protection assays (Fig. 4B). In contrast to SYF cells, SYF + c-Src cells showed increased uptake of the pathogens upon expression of CEACAM3 (Fig. 4B), demonstrating that Src family PTKs are required for CEACAM3-mediated phagocytosis. To verify the internalization of gonococci by the fibroblasts, infected cultures were differentially labeled for intracellular and extracellular bacteria. Importantly, SYF cells expressing CEACAM3-RFP supported attachment of OpaCEA bacteria and recruitment of the RFP-coupled receptor but not internalization (Fig. 4C). In contrast, CEACAM3-RFP-transfected SYF + c-Src cells showed attached extracellular as well as intracellular gonococci (Fig. 4C). These results support the view that the presence of Src family PTKs is of critical importance for CEACAM3-initiated phagocytosis.
FIG. 4.
Src PTKs are essential for CEACAM3-mediated phagocytosis. (A) 293 cells were transfected with the empty control vector (pcDNA) or CEACAM3 or cotransfected with CEACAM3 and Csk WT or its kinase inactive mutant (Csk K222M). Transfected cells were infected for 60 min with OpaCEA-expressing gonococci and employed in gentamicin protection assays. The graph shows mean values ± standard deviations from two independent experiments done in triplicate. Whole-cell lysates (WCL) of the samples were analyzed for Csk expression by Western blotting with monoclonal anti-Csk antibody (lower panel). (B) SYF mouse fibroblasts and SYF + c-Src cells were transfected with CEACAM3-RFP or with the empty control vector (pDS). Cells were infected for 60 min with OpaCEA-expressing gonococci and employed in gentamicin protection assays. The graph shows mean values ± standard deviations from two independent experiments done in triplicate. Whole-cell lysates of the cells were probed with monoclonal anti-Src antibodies to confirm c-Src expression in SYF + c-Src cells (right panel). (C) SYF cells and SYF + c-Src cells transfected with CEACAM3-RFP were infected as described for panel A with CFSE-labeled bacteria. After fixation, samples were stained for extracellular bacteria (arrowheads) with rabbit anti-gonococcus and goat anti-rabbit Cy5 antibodies [Extra (Cy5)]. CEACAM3-expressing cells were detected by RFP fluorescence. Intracellular bacteria were distinguished by their exclusive CFSE-derived fluorescence (arrows). Bars represent 10 μm.
CEACAM6- but not CEACAM3-mediated internalization is strongly dependent on the integrity of cholesterol-rich membrane microdomains.
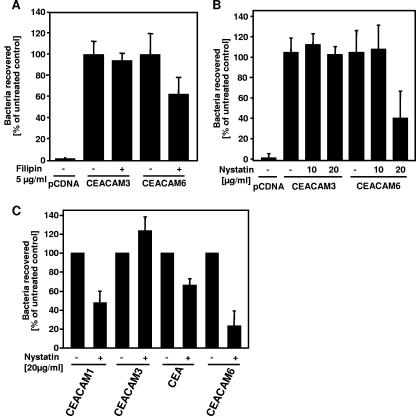
The major difference between CEACAM3 and CEACAM6 is their anchorage to the cell membrane. CEACAM3 is a transmembrane protein with a cytoplasmic tail, while CEACAM6 is linked to the membrane by a GPI anchor. As GPI-linked proteins are regularly found in so-called membrane microdomains or lipid rafts, we investigated if CEACAM6-mediated uptake of gonococci also depends on this kind of membrane organization. Therefore, 293 cells were transfected with CEACAM3, CEACAM6, or empty control vector and applied in gentamicin protection assays. Prior to infection with OpaCEA-expressing gonococci, cells were pretreated with the indicated amounts of the cholesterol chelators filipin (Fig. 5A) and nystatin (Fig. 5B), which destroy the integrity of cholesterol-rich membrane microdomains in the cell membrane. Clearly, only CEACAM6-mediated uptake of bacteria was affected by both agents, while the internalization of bacteria via CEACAM3 was not reduced upon pretreatment with filipin (Fig. 5A) or nystatin (Fig. 5B). These results pointed to a strong association of CEACAM6 with cholesterol-rich membrane microdomains and a functional role of these membrane compartments in CEACAM6-mediated bacterial internalization by 293 cells.
FIG. 5.
Disruption of membrane microdomains inhibits uptake by epithelial CEACAMs. (A and B) 293T cells were transfected with the empty control vector (pcDNA) or cDNA encoding CEACAM3 or CEACAM6. Transfected cells were infected for 60 min with OpaCEA-expressing N. gonorrhoeae (MOI, 20) and applied in gentamicin assays. Prior to infection, cells were pretreated or not with the indicated amounts of nystatin (A) or filipin (B). The bars represent mean values ± standard deviations from three independent experiments done in triplicate. Values are expressed as percentages of the untreated control value. (C) 293T cells expressing the indicated CEACAMs were infected for 60 min with OpaCEA-expressing gonococci and analyzed in gentamicin assays in the presence or absence of 20 μg/ml nystatin. The bars represent mean values ± standard deviations from three independent experiments done in triplicate. Values are expressed as percentages of the untreated control value.
Bacterial uptake by epithelial CEACAMs requires cholesterol-rich membrane microdomains.
As CEACAM6-mediated internalization was blocked by cholesterol-depleting reagents, we wondered whether this uptake mechanism was a general feature of CEACAMs expressed on epithelial cells, such as CEACAM1, CEA, and CEACAM6. Therefore, these receptors were expressed in 293 cells, and the uptake of OpaCEA gonococci was measured after 2 h of infection in the presence or absence of 5 μM nystatin. As observed before, CEACAM3-mediated internalization was not affected under these conditions (Fig. 5C). However, similar to CEACAM6, both CEACAM1-mediated uptake and CEA-mediated uptake were severely impaired (Fig. 5C). Though the absolute number of viable intracellular bacteria was lower for CEACAM1 and for CEA than for CEACAM6 (see also Fig. 1B), the depletion of cholesterol from the membrane reduced the amount of intracellular bacteria by more than 40% (CEA) and up to 75% (CEACAM6) of what was seen for the untreated samples (Fig. 5C). These results demonstrate that bacterial uptake via several CEACAM isoforms found on human epithelia is sensitive to the disruption of membrane microdomains and point to a common uptake mechanism of OpaCEA-expressing Neisseria by CEACAM-expressing epithelial cells.
Internalization of OpaCEA-expressing bacteria by granulocytes is sensitive to Src PTK inhibition but not to cholesterol depletion.
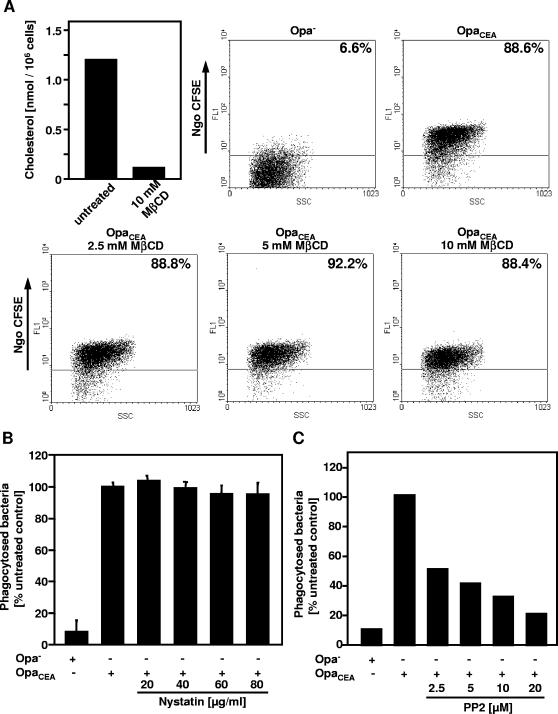
Primary human granulocytes are able to recognize and rapidly internalize OpaCEA-expressing Neisseria gonorrhoeae via CEACAMs in the absence of opsonizing antibodies or complement factors (14, 33, 44). Indeed, within 20 min of interaction, more than 90% of granulocytes had taken up CFSE-labeled, OpaCEA-expressing gonococci in an in vitro phagocytosis assay (see Fig. S2 in the supplemental material). In contrast, nonopaque gonococci were barely internalized under these conditions, demonstrating that the uptake requires the expression of OpaCEA proteins by the microorganisms (see Fig. S2 in the supplemental material). As our investigations revealed a striking difference in the molecular requirements for bacterial internalization via different CEACAM isoforms, we wondered whether this could be used to estimate the contribution of CEACAM3 versus that of other pathogen-binding CEACAMs to the efficient uptake of OpaCEA-expressing gonococci by human granulocytes. Therefore, primary human granulocytes were treated for 30 min with MβCD, a well-established cholesterol-chelating agent. Indeed, pretreatment of granulocytes with 10 mM MβCD resulted in a more than 85% reduction of cellular cholesterol (Fig. 6A). When the same batch of primary cells pretreated with increasing concentrations of MβCD was analyzed for the cells' ability to phagocytose OpaCEA-expressing gonococci in an opsonin-independent manner, no reduction in bacterial uptake was observed (Fig. 6A). Furthermore, pretreatment of granulocytes with nystatin in doses of up to 80 μg/ml did not affect the capacity to phagocytose OpaCEA-expressing N. gonorrhoeae (Fig. 6B). In contrast, treatment of the phagocytes with increasing concentrations of the Src PTK inhibitor PP2 revealed a strong and dose-dependent inhibition of phagocytosis of the gonococci (Fig. 6C). Already at 2.5 μM, PP2 blocked more than 50% of bacterial uptake, making the opsonin-independent phagocytosis by granulocytes as sensitive to inhibition of Src family kinases as internalization by CEACAM3-transfected 293 cells. These observations demonstrated that Src family PTK activity has a major role in the uptake of OpaCEA-expressing N. gonorrhoeae by human granulocytes and suggested that CEACAM3-mediated internalization might be the major route of efficient recognition and elimination of CEACAM-binding pathogens by this cell type. Consistent with a dominant role of CEACAM3, destruction of membrane microdomains and therefore interference with CEACAM1- or CEACAM6-mediated internalization do not affect the efficient opsonin-independent phagocytosis of gonococci by human granulocytes.
FIG. 6.
Inhibition of Src family kinases, but not disruption of membrane microdomains, interferes with phagocytosis of OpaCEA-expressing bacteria by primary human granulocytes. (A) Isolated human granulocytes were treated or not with 10 mM MβCD for 30 min, and then their cholesterol content was determined (left). In addition, the same batch of granulocytes, treated or not with the indicated concentrations of MβCD, was infected for 20 min with CFSE-labeled OpaCEA-expressing (OpaCEA) or nonopaque (Opa−) N. gonorrhoeae (Ngo CFSE). Infected granulocytes were detected by flow cytometry according to their sideward scatter (SSC), and their fluorescence intensity (FL-1) was recorded in the presence of trypan blue. Values represent the percentages of granulocytes containing CFSE-labeled bacteria to the untreated control value. The results are derived from a representative experiment. Similar values were obtained with granulocytes from three (B) Isolated human granulocytes were pretreated prior to infection with the indicated concentrations of nystatin. Infection and analysis was performed as described for panel A. Values represent the percentages of granulocytes containing CFSE-labeled bacteria compared to the untreated control value. The graph shows mean values ± standard deviations from three independent experiments. (C) Granulocytes were pretreated with the indicated concentrations of the Src PTK inhibitor PP2. Infection and analysis was performed as described for panel A. Values represent the percentages of granulocytes containing CFSE-labeled bacteria compared to the untreated control value and are derived from a representative experiment. Similar values were obtained with granulocytes from three different donors.
DISCUSSION
CEACAM recognition is a common feature of several human-adapted bacterial pathogens. Though the adhesins on the bacterial side are highly diverse, they seem to have evolved to bind to the same structure in multiple CEACAM isoforms. In particular, the amino-terminal immunoglobulin variable-like domains of CEACAM1, CEACAM3, CEA, and CEACAM6 can serve as cellular receptors for CEACAM-binding bacteria. Though pathogen-binding CEACAM isoforms are found on distinct human cell types, they are all able to mediate bacterial internalization in vitro. However, recent work has suggested that they might be connected differently to intracellular signaling pathways, potentially affecting the outcome of the pathogen-host cell interaction (24, 33).
Here we provide evidence that bacterial internalization mediated by CEACAM3, a granulocyte-specific member of the family, mechanistically differs from the uptake mediated by epithelial CEACAMs (CEACAM1, CEA, and CEACAM6). In particular, CEACAM3-initiated phagocytosis is strictly dependent on the presence and function of Src PTKs, whereas CEACAM6-mediated uptake does not show this strict requirement. In contrast to what is seen for CEACAM3, pathogen internalization via CEACAM family members expressed on mucosal epithelia (CEACAM1, CEA, and CEACAM6) occurs via cholesterol-rich membrane microdomains and therefore is sensitive to cholesterol-chelating agents such as nystatin or filipin.
The observed mechanistic differences between CEACAM3-mediated uptake and the internalization of opaque N. gonorrhoeae via other CEACAM isoforms appear to have striking functional consequences. This is best exemplified by the dominant role of CEACAM3-mediated opsonin-independent phagocytosis of gonococci by primary human granulocytes. Despite the presence of CEACAM1 and CEACAM6 on the surfaces of these cells, phagocytosis is completely insensitive to cholesterol depletion, suggesting that CEACAM1 or CEACAM6 does not significantly contribute to rapid bacterial internalization by these professional phagocytes. In contrast, phagocytosis is severely impaired by Src PTK inhibition, pointing to CEACAM3 as the relevant family member triggering the opsonin-independent uptake of CEACAM-binding pathogens. Similar conclusions were obtained by using CEACAM3- or CEACAM6-directed MAbs to interfere with the uptake process (33).
It is interesting that CEA and CEACAM6 are anchored to the membrane via GPI anchors, a posttranslational modification that is known to direct membrane proteins to cholesterol- and sphingolipid-enriched membrane microdomains (lipid rafts) (36). Furthermore, CEACAM1 has been detected in a Triton-insoluble membrane fraction in rat endothelial cells, suggesting that this CEACAM isoform also partitions into lipid rafts (28). In mice, the CEACAM1 homologue serves as the cellular receptor for the murine hepatitis virus (MHV). Though MHV accesses cells by fusion of the virus envelope with the host cell membrane and not by endocytosis, MHV infectivity is increased by media enriched in cholesterol (42). These data are in line with our results and suggest that CEACAM1, CEA, and CEACAM6, or at least significant fractions of these receptor molecules, localize to lipid rafts.
Our experiments reveal that the particular membrane distribution of epithelial CEACAMs has functional consequences for the internalization of bacteria, as uptake is strictly dependent on the integrity of cholesterol-rich membrane microdomains. Such cholesterol- and sphingolipid-enriched membrane microdomains are often termed lipid rafts and can be found in most cell types. Lipid rafts contribute to a variety of cellular processes, including signal transduction (36) and vesicle trafficking (15). Interestingly, a number of pathogens exploit the endocytic properties of lipid rafts to enter host cells (9, 23, 35). Examples of lipid raft-mediated bacterial internalization include FimH-expressing Escherichia coli and gram-positive group A Streptococcus pyogenes, which enter mammalian cells via caveola-like structures (2, 32). An important aspect of membrane microdomain-mediated uptake might be the further maturation of the endocytic vesicle. Indeed, endocytic vesicles resulting from membrane microdomain-initiated uptake seem to avoid acidic lysosomes and display only limited degradation of their content (23). Furthermore, lipid rafts could contribute to the apical-to-basolateral transport in polarized epithelia, a process that would promote the transcytosis of CEACAM-binding bacteria through intact epithelial layers. Importantly, such an OpaCEA-CEACAM-dependent transcytosis has been observed in in vitro transcytosis studies with N. gonorrhoeae utilizing polarized epithelial monolayers (47). Therefore, the intracellular trafficking of the Neisseria-containing compartment arising from endocytosis by epithelial CEACAMs warrants further investigation.
The strict requirement for Src PTK activity during CEACAM3-mediated bacterial internalization is not surprising, as the cytoplasmic domain of this granulocyte receptor contains an immunoreceptor tyrosine-based activation motif (ITAM)-like sequence that is phosphorylated in vitro by recombinant Src (5). Also in response to bacterial binding, CEACAM3 is phosphorylated at two tyrosine residues within the ITAM-like sequence in a Src PTK-dependent manner, and mutation of these residues impairs CEACAM3-mediated pathogen uptake (7, 25, 33). Our results demonstrate that genetic or functional ablation of Src PTKs abrogates CEACAM3-mediated uptake, further supporting the concept that no other cellular PTKs can compensate for Src family kinase function during CEACAM3-initiated bacterial internalization. Importantly, in addition to what we show for c-Src, which is only weakly expressed in human granulocytes and which is not found in association with CEACAMs in this cell type (37), we demonstrate that CEACAM3 also directly binds to the SH2 domain of Hck, a Src PTK family member present in granulocytes (48). In contrast to CEACAM3, CEACAM6 does not strictly rely on Src PTK activity, as pharmacologic inhibition of Src or overexpression of the isolated Src SH2 domain does not interfere with the uptake of bacteria in the case of CEACAM6. This is surprising, as previous reports have detected an association between CEACAM6 and active Src family PTKs in human granulocytes (37). How such an association is accomplished is not known, but the GPI anchor of CEACAM6 would allow only an indirect connection with cytoplasmic tyrosine kinases. Accordingly, pull-downs with the Src SH2 domain failed to detect a direct interaction between CEACAM6 and Src PTKs. Therefore, the strong dependence on Src PTK activity of the opsonin-independent uptake of CEACAM-binding bacteria by human granulocytes most likely reflects the major role of CEACAM3 in this process. The question of whether Src PTK activity also contributes to the regulation of bactericidal activities by the granulocytes after CEACAM3-mediated uptake, such as azurophil or specific granule delivery to phagosomes (26, 29), needs to be explored further.
Together, our results support the concept that there are mechanistic differences in uptake mediated by pathogen-binding human CEACAMs (7, 24, 33). Furthermore, they provide a first functional insight that membrane microdomains play a key role in internalization via epithelial CEACAMs. The distinct cellular connections of CEACAMs might help to explain why several human-specific gram-negative pathogens have evolved CEACAM-binding adhesins to exploit epithelial CEACAMs, while the same surface molecules of the bacteria can be utilized as a determinant for selective recognition and elimination by innate immune cells.
Supplementary Material
Acknowledgments
We thank B. Geiger for the Src-dSH2-YFP construct, T. F. Meyer for the Neisseria strains used in this study, D. D. Schlaepfer for Csk and v-Src cDNAs, P. Soriano for the SYF cells, W. Zimmermann for CEACAM cDNAs, and D. Deininger for expert technical assistance.
This study was supported by funds from the DFG (Ha2568/3-2) to C.R.H.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 21 May 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Agerer, F., A. Michel, K. Ohlsen, and C. R. Hauck. 2003. Integrin-mediated invasion of Staphylococcus aureus into human cells requires Src family protein tyrosine kinases. J. Biol. Chem. 278:42524-42531. [DOI] [PubMed] [Google Scholar]
- 2.Baorto, D. M., Z. Gao, R. Malaviya, M. L. Dustin, A. van der Merwe, D. M. Lublin, and S. N. Abraham. 1997. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389:636-639. [DOI] [PubMed] [Google Scholar]
- 3.Bos, M. P., D. Hogan, and R. J. Belland. 1999. Homologue scanning mutagenesis reveals CD66 receptor residues required for neisserial Opa protein binding. J. Exp. Med. 190:331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley, C. J., N. J. Griffiths, H. A. Rowe, R. S. Heyderman, and M. Virji. 2005. Critical determinants of the interactions of capsule-expressing Neisseria meningitidis with host cells: the role of receptor density in increased cellular targeting via the outer membrane Opa proteins. Cell. Microbiol. 7:1490-1503. [DOI] [PubMed] [Google Scholar]
- 5.Brummer, J., M. Neumaier, C. Gopfert, and C. Wagener. 1995. Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene 11:1649-1655. [PubMed] [Google Scholar]
- 6.Chen, T., R. J. Belland, J. Wilson, and J. Swanson. 1995. Adherence of pilus− Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. J. Exp. Med. 182:511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, T., S. Bolland, I. Chen, J. Parker, M. Pantelic, F. Grunert, and W. Zimmermann. 2001. The CGM1a (CEACAM3/CD66d)-mediated phagocytic pathway of Neisseria gonorrhoeae expressing opacity proteins is also the pathway to cell death. J. Biol. Chem. 276:17413-17419. [DOI] [PubMed] [Google Scholar]
- 8.Chen, T., and E. C. Gotschlich. 1996. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc. Natl. Acad. Sci. USA 93:14851-14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan, M. J., J. S. Shin, and S. N. Abraham. 2002. Microbial entry through caveolae: variations on a theme. Cell. Microbiol. 4:783-791. [DOI] [PubMed] [Google Scholar]
- 10.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 11.Gray-Owen, S. D., D. R. Lorenzen, A. Haude, T. F. Meyer, and C. Dehio. 1997. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol. Microbiol. 26:971-980. [DOI] [PubMed] [Google Scholar]
- 12.Hammarstrom, S. 1999. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 9:67-81. [DOI] [PubMed] [Google Scholar]
- 13.Hauck, C. R., and T. F. Meyer. 2003. ‘Small’ talk: Opa proteins as mediators of Neisseria-host-cell communication. Curr. Opin. Microbiol. 6:43-49. [DOI] [PubMed] [Google Scholar]
- 14.Hauck, C. R., T. F. Meyer, F. Lang, and E. Gulbins. 1998. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 17:443-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms, J. B., and C. Zurzolo. 2004. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic 5:247-254. [DOI] [PubMed] [Google Scholar]
- 16.Hill, D. J., M. A. Toleman, D. J. Evans, S. Villullas, L. Van Alphen, and M. Virji. 2001. The variable P5 proteins of typeable and non-typeable Haemophilus influenzae target human CEACAM1. Mol. Microbiol. 39:850-862. [DOI] [PubMed] [Google Scholar]
- 17.Hill, D. J., and M. Virji. 2003. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol. Microbiol. 48:117-129. [DOI] [PubMed] [Google Scholar]
- 18.Kirchner, J., Z. Kam, G. Tzur, A. D. Bershadsky, and B. Geiger. 2003. Live-cell monitoring of tyrosine phosphorylation in focal adhesions following microtubule disruption. J. Cell Sci. 116:975-986. [DOI] [PubMed] [Google Scholar]
- 19.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuespert, K., S. Pils, and C. R. Hauck. 2006. CEACAMs—their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 18:565-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuespert, K., S. Weibel, and C. R. Hauck. 2007. Profiling of bacterial adhesin-host receptor recognition by soluble immunoglobulin superfamily domains. J. Microbiol. Methods 68:478-485. [DOI] [PubMed] [Google Scholar]
- 22.Leusch, H. G., Z. Drzeniek, Z. Markos-Puztai, and C. Wagener. 1991. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic glycosides of mannose. Infect. Immun. 59:2051-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manes, S., G. del Real, and A. C. Martinez. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557-568. [DOI] [PubMed] [Google Scholar]
- 24.McCaw, S. E., E. H. Liao, and S. D. Gray-Owen. 2004. Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect. Immun. 72:2742-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCaw, S. E., J. Schneider, E. H. Liao, W. Zimmermann, and S. D. Gray-Owen. 2003. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol. Microbiol. 49:623-637. [DOI] [PubMed] [Google Scholar]
- 26.Möhn, H., V. Le Cabec, S. Fischer, and I. Maridonneau-Parini. 1995. The src-family protein-tyrosine kinase p59-hck is located on the secretory granules in human neutrophils and translocates towards the phagosome during cell activation. Biochem. J. 309:657-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muenzner, P., M. Rohde, S. Kneitz, and C. R. Hauck. 2005. CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J. Cell Biol. 170:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller, M. M., B. B. Singer, E. Klaile, B. Obrink, and L. Lucka. 2005. Transmembrane CEACAM1 affects integrin-dependent signaling and regulates extracellular matrix protein-specific morphology and migration of endothelial cells. Blood 105:3925-3934. [DOI] [PubMed] [Google Scholar]
- 29.N′Diaye, E. N., X. Darzacq, C. Astarie-Dequeker, M. Daffe, J. Calafat, and I. Maridonneau-Parini. 1998. Fusion of azurophil granules with phagosomes and activation of the tyrosine kinase Hck are specifically inhibited during phagocytosis of mycobacteria by human neutrophils. J. Immunol. 161:4983-4991. [PubMed] [Google Scholar]
- 30.Pils, S., T. Schmitter, F. Neske, and C. R. Hauck. 2006. Quantification of bacterial invasion into adherent cells by flow cytometry. J. Microbiol. Methods 65:301-310. [DOI] [PubMed] [Google Scholar]
- 31.Popp, A., C. Dehio, F. Grunert, T. F. Meyer, and S. D. Gray-Owen. 1999. Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell. Microbiol. 1:169-181. [DOI] [PubMed] [Google Scholar]
- 32.Rohde, M., E. Muller, G. S. Chhatwal, and S. R. Talay. 2003. Host cell caveolae act as an entry-port for group A streptococci. Cell. Microbiol. 5:323-342. [DOI] [PubMed] [Google Scholar]
- 33.Schmitter, T., F. Agerer, L. Peterson, P. Muenzner, and C. R. Hauck. 2004. Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J. Exp. Med. 199:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitter, T., S. Pils, V. Sakk, R. Frank, K. D. Fischer, and C. R. Hauck. 2007. The granulocyte receptor CEACAM3 directly associates with Vav to promote phagocytosis of human pathogens. J. Immunol. 178:3797-3805. [DOI] [PubMed] [Google Scholar]
- 35.Shin, J. S., and S. N. Abraham. 2001. Caveolae as portals of entry for microbes. Microbes Infect. 3:755-761. [DOI] [PubMed] [Google Scholar]
- 36.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 37.Skubitz, K. M., K. D. Campbell, K. Ahmed, and A. P. Skubitz. 1995. CD66 family members are associated with tyrosine kinase activity in human neutrophils. J. Immunol. 155:5382-5390. [PubMed] [Google Scholar]
- 38.Stern, A., M. Brown, P. Nickel, and T. F. Meyer. 1986. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell 47:61-71. [DOI] [PubMed] [Google Scholar]
- 39.Stern, A., and T. F. Meyer. 1987. Common mechanism controlling phase and antigenic variation in pathogenic neisseriae. Mol. Microbiol. 1:5-12. [DOI] [PubMed] [Google Scholar]
- 40.Streichert, T., A. Ebrahimnejad, S. Ganzer, R. Flayeh, C. Wagener, and J. Brummer. 2001. The microbial receptor CEACAM3 is linked to the calprotectin complex in granulocytes. Biochem. Biophys. Res. Commun. 289:191-197. [DOI] [PubMed] [Google Scholar]
- 41.Thomas, S. M., and J. Brugge. 1997. Cellular functions regulated by src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 42.Thorp, E. B., and T. M. Gallagher. 2004. Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J. Virol. 78:2682-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Putten, J. P., and S. M. Paul. 1995. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 14:2144-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Virji, M., K. Makepeace, D. J. P. Ferguson, and S. M. Watt. 1996. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic Neisseriae. Mol. Microbiol. 22:941-950. [DOI] [PubMed] [Google Scholar]
- 45.Virji, M., S. M. Watt, S. Barker, K. Makepeace, and R. Doyonnas. 1996. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol. Microbiol. 22:929-939. [DOI] [PubMed] [Google Scholar]
- 46.Voyich, J. M., and F. R. DeLeo. 2002. Host-pathogen interactions: leukocyte phagocytosis and associated sequelae. Methods Cell Sci. 24:79-90. [DOI] [PubMed] [Google Scholar]
- 47.Wang, J., S. D. Gray-Owen, A. Knorre, T. F. Meyer, and C. Dehio. 1998. Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarized T84 epithelial cell monolayers. Mol. Microbiol. 30:657-671. [DOI] [PubMed] [Google Scholar]
- 48.Xu, Y., J. W. Potter, and C. L. Willman. 1996. The function of src family tyrosine kinases in hematopoietic cells. Leukemia Res. 20:229-234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.