Abstract
Numerous transcription accessory proteins cause alterations in chromatin structure that promote the progression of RNA polymerase II (Pol II) along open reading frames (ORFs). The Saccharomyces cerevisiae Paf1 complex colocalizes with actively transcribing Pol II and orchestrates modifications to the chromatin template during transcription elongation. To better understand the function of the Rtf1 subunit of the Paf1 complex, we created a series of sequential deletions along the length of the protein. Genetic and biochemical assays were performed on these mutants to identify residues required for the various activities of Rtf1. Our results establish that discrete nonoverlapping segments of Rtf1 are necessary for interaction with the ATP-dependent chromatin-remodeling protein Chd1, promoting covalent modification of histones H2B and H3, recruitment to active ORFs, and association with other Paf1 complex subunits. We observed transcription-related defects when regions of Rtf1 that mediate histone modification or association with active genes were deleted, but disruption of the physical association between Rtf1 and other Paf1 complex subunits caused only subtle mutant phenotypes. Together, our results indicate that Rtf1 influences transcription and chromatin structure through several independent functional domains and that Rtf1 may function independently of its association with other members of the Paf1 complex.
The complex organization of eukaryotic chromosomes acts as a significant impediment to gene expression. In this environment, efficient elongation of a transcript by RNA polymerase II (Pol II) requires a multitude of accessory factors to facilitate its movement along chromatin-assembled genes. The Saccharomyces cerevisiae Paf1 complex colocalizes with Pol II during transcription elongation and is required for the normal expression of a subset of genes (22, 34, 35, 37, 55). The Paf1 complex minimally contains five subunits, Paf1, Ctr9, Cdc73, Rtf1, and Leo1, and physically associates with Pol II (22, 27, 58). Consistent with a role in transcription elongation, physical and genetic interactions between components of the Paf1 complex and other Pol II-associated elongation factors, including the Spt4-Spt5 (yDSIF) and Spt16-Pob3 (yFACT) complexes, have been reported (22, 58). Additionally, deletion of genes encoding subunits of the Paf1 complex causes sensitivity to the base analogs 6-azauracil (6-AU) and mycophenolic acid, phenotypes associated with defects in transcription elongation (8, 58).
Proper elongation of a transcript by Pol II requires efficient navigation of a chromatin template. Nucleosomes, the fundamental components of chromatin, form around octamers of the histone proteins H2A, H2B, H3, and H4. Histones are subject to a myriad of posttranslational modifications, including acetylation, methylation, phosphorylation, ubiquitylation, and sumoylation (reviewed in references 17 and 51; 30, 50). Methylation of individual lysine residues within histones can occur in mono-, di-, or trimethyl states (reviewed in reference 51; 63). The regulated placement and removal of these modifications control chromatin structure and influence transcription (24). In several cases, methylated residues on histones have been shown to serve as specific binding sites for effector proteins that further alter chromatin structure (reviewed in reference 25).
The Paf1 complex is required for the modification of specific lysine residues on histones H2B and H3. Monoubiquitylation of histone H2B at lysine 123 (K123) by the ubiquitin-conjugating enzyme Rad6 and the ubiquitin protein ligase Bre1 is eliminated in strains lacking Rtf1 or Paf1 (31, 65). This modification is a prerequisite for the methylation of histone H3 on K4 and K79 by the methyltransferases Set1 and Dot1, respectively (7, 61). Therefore, Rtf1 and Paf1 also are required for histone H3 K4 and K79 methylation (21, 31, 32). Rtf1 is likely the primary component of the Paf1 complex that regulates these modifications, because Rtf1 levels are significantly reduced in strains lacking Paf1, while Paf1 levels remain unchanged in the absence of Rtf1 (28, 36). H2B K123 ubiquitylation and H3 K4 methylation are enriched in the coding regions of active genes (6, 48, 66). Rad6 and Set1 are recruited to open reading frames (ORFs) coincident with gene activation and modify histones during transcription. Rtf1 colocalizes with these histone-modifying enzymes on active genes and is required for their optimal recruitment and activation (21, 32, 66).
Histone ubiquitylation is rapidly reversed by deubiquitylating enzymes, but histone methylation can remain stable for an extended period of time following the cessation of transcription (14, 32). The persistence of histone methylation has been proposed to maintain the chromatin of recently transcribed genes in a state that is more readily accessed by the transcription machinery (32). Consistent with this idea, histone modifications exclude factors that establish heterochromatin from areas of active gene expression (reviewed in reference 45; 46, 47). Heterochromatic regions are transcriptionally silent due to the enrichment of hypomodified histones, which serve as interaction sites for silencing factors that condense chromatin and exclude the transcription machinery. Mutations that globally decrease levels of histone modifications create an abundance of hypomodified histones, interfering with normal heterochromatin formation. Hence, strains lacking Rtf1 or its downstream histone modifications are defective in the silencing of telomere-proximal genes (21, 31).
In addition to the covalent modification of histones, chromatin structure can be altered by chromatin-remodeling proteins that reposition nucleosomes using energy derived from ATP hydrolysis (reviewed in reference 53). Rtf1 physically interacts with the chromatin-remodeling protein Chd1 and promotes its association with active ORFs (55). Interestingly, the human homolog of Chd1 has been shown to associate with histone H3 that is di- or trimethylated on K4 (11, 56). Although conflicting results concerning this interaction in yeast have been reported (11, 38, 56), the intriguing possibility exists that Rtf1 mediates effects on chromatin structure by recruiting Chd1 to ORFs and stabilizing its association by promoting methylation of H3 K4. Furthermore, Chd1 has been identified as a component of the SAGA (Spt-Ada-Gcn5-acetyltransferase) transcriptional coactivator complex (38). SAGA is a multiprotein complex that contains Gcn5, a histone acetyltransferase for lysine residues on histones H2B and H3 (12, 13, 60), and Ubp8, a deubiquitylating enzyme that removes H2B K123 ubiquitylation (9, 14). Together, these studies suggest that the interaction between Rtf1 and Chd1 may coordinate the activities of multiple histone-modifying enzymes.
The involvement of Rtf1 in histone modification, telomeric silencing, and recruitment of Chd1 argues that it influences transcription by altering chromatin structure. Whether these and other activities of Rtf1 are functionally linked or separable is not known. To understand how Rtf1 carries out its various roles during transcription elongation, we have used a combination of genetic and biochemical approaches to characterize a collection of Rtf1 mutants. Our results demonstrate that discrete nonoverlapping segments of Rtf1 are required for interaction with Chd1, regulation of histone modification, association with ORFs, and contact with other components of the Paf1 complex. Additionally, the regions of Rtf1 that mediate its presence on active ORFs or its involvement in histone modification are required for normal transcription and are the most highly conserved parts of the protein. Together, our results indicate that Rtf1 is a multifunctional protein that promotes optimal gene expression by regulating cotranscriptional histone modification.
MATERIALS AND METHODS
Media and yeast strains.
Rich (yeast-extract-peptone-dextrose [YPD]), synthetic complete (SC), synthetic minimal (SD), and 5-fluoroorotic acid (5-FOA) media were prepared as described previously (42). Where indicated, 6-AU was added to SC-Ura media at a final concentration of 50 μg/ml.
S. cerevisiae strains used in these studies are listed in Table 1. All strains, with the exceptions of OKA93 and PJ69-4A, are GAL2+ derivatives of S288C (64). OKA93 is an rtf1Δ derivative of UCC506 (40). Transformation, mating, sporulation, and tetrad dissection were performed according to standard methods (42). Complete disruptions of RTF1 and CHD1 were created by a PCR-based gene replacement method (2). Constructs expressing hemagglutinin-tagged Rtf1Δ1 (HA-Rtf1Δ1), HA-Rtf1Δ3, or HA-Rtf1Δ4 were integrated into the yeast chromosome to replace the endogenous RTF1 locus by a two-step gene replacement method (43). Epitope tagging of Ctr9 at the carboxy terminus with six Myc epitopes and Chd1 at the amino terminus with three HA epitopes was previously described (55, 58).
TABLE 1.
S. cerevisiae strains
| Straina | Genotype |
|---|---|
| FY78 | MATahis3Δ200 |
| FY406 | MATa (hta1-htb1)Δ::LEU2 (hta2-htb2)Δ::TRP1 his3Δ200 lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pSAB6 = HTA1-HTB1/CEN/ARS/URA3] |
| PJ69-4A | MATaLYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ trp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ |
| OKA93 | MATα rtf1Δ::kanMX4 TEL-VR::URA3 ura3-52 trp1− |
| KY100 | MATaspt15-122 his4-917δ lys2-173R2 ura3-52 ade8 |
| KY284 | MATα his4-917δ ura3-52 trp1Δ63 |
| KY343 | MATartf1Δ::URA3 his4-917δ lys2-173R2 leu2Δ1 ura3-52 trp1Δ63 |
| KY386 | MATα spt15-122 rtf1Δ::URA3 his4-917δ lys2-173R2 ura3-52 leu2Δ1 ade8 |
| KY404 | MATartf1Δ::LEU2 his4-912δ lys2-128δ leu2Δ1 ura3-52 trp1Δ63 |
| KY432 | MATartf1-1 his4-917δ lys2-173R2 leu2Δ1 ura3-52 trp1Δ63 |
| KY440 | MATα spt15-122 rtf1-1 his4-917δ lys2-173R2 leu2Δ1 ade8 |
| KY452 | MATartf1Δ::URA3 his3Δ200 lys2-173R2 ura3-52 trp1Δ63 |
| KY457 | MATartf1Δ::URA3 leu2Δ1 ura3-52 trp1Δ63 |
| KY619 | MATartf1Δ::ARG4 his4-912δ lys2-173R2 leu2Δ1 trp1Δ63 arg4-12 |
| KY623 | MATα rtf1Δ::LEU2 3XHA-CHD1 his4-912δ lys2-128δ leu2Δ1 ura3-52 trp1Δ63 |
| KY638 | MATα spt15-122 his4-917δ lys2-173R2 ura3-52 |
| KY639 | MATaspt15-122 chd1Δ::URA3 his4-917δ leu2Δ1 ura3-52 trp1Δ63 |
| KY640 | MATahis4-917δ ura3-52 |
| KY641 | MATα chd1Δ::URA3 his4-917δ lys2-173R2 leu2Δ1 ura3-52 trp1Δ63 |
| KY680 | MATα 3XHA-rtf1Δ1 his4-917δ lys2-173R2 leu2Δ1 ura3-52 |
| KY982 | MATartf1Δ::kanMX4 (hta1-htb1)Δ::LEU2 (hta2-htb2)Δ::TRP1 his3Δ200 lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pSAB6 = HTA1-HTB1/CEN/ARS/URA3] |
| KY995 | MATα rtf1Δ::URA3 CTR9-6XMYC::LEU2 his3Δ200 leu2Δ(0 or 1) ura3(Δ0 or 52) trp1Δ63 |
| KY1213 | MATα rtf1Δ::LEU2 3XHA-CHD1 his4-912δ lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pLS20 = untagged RTF1/CEN/ARS/TRP1] |
| KY1214 | MATα rtf1Δ::LEU2 3XHA-CHD1 his4-912δ lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pPC61 = untagged rtf1Δ1/CEN/ARS/TRP1] |
| KY1215 | MATα rtf1Δ::LEU2 3XHA-CHD1 his4-912δ lys2-128δ leu2Δ1 ura3-52 trp1Δ63 [pLS11 = untagged rtf1-1/CEN/ARS/TRP1] |
| KY1216 | MATartf1Δ3 (hta1-htb1)Δ::LEU2 (hta2-htb2)Δ::TRP1 his3Δ200 lys2-128δ leu2Δ1 ura3-52 trp1Δ63 arg4-12 [pSAB6 = HTA1-HTB1/CEN/ARS/URA3] |
| KY1217 | MATartf1Δ4 (hta1-htb1)Δ::LEU2 (hta2-htb2)Δ::TRP1 his3Δ200 lys2-128δ leu2Δ1 ura3-52 trp1Δ63 arg4-12 [pSAB6 = HTA1-HTB1/CEN/ARS/URA3] |
| KY1265 | MATaspt15-122 3XHA-rtf1Δ1 his4-917δ lys2-173R2 ura3-52 ade8 |
FY strains were generated in the laboratory of Fred Winston.
Plasmid construction.
Standard cloning techniques were used to construct all plasmids (2). pLS20 and pLS21-5 are derivatives of pRS314 (54) that express Rtf1 or triple-HA-tagged Rtf1, respectively (59). Site-directed mutagenesis (23) or PCR-based approaches were used to remove segments of the RTF1 coding region from pLS21-5 to create a series of sequential RTF1 internal deletion mutations, which are detailed in Fig. 1. Each rtf1 deletion mutation was confirmed by DNA sequencing. pKR37, which expresses HA-Rtf1Δ1, was digested with NdeI to remove the triple-HA tag and religated to generate pPC61. High-copy-number vectors used to overexpress HA-Rtf1 (pMW6) and HA-Rtf1Δ7 (pMW7) were constructed by subcloning a SalI-SpeI fragment from pLS21-5 or pKR14 (the pLS21-5 derivative carrying HA-rtf1Δ7) into SalI-SpeI-digested pRS426 (54). To create fusions of wild-type Rtf1 and Rtf1 internal deletion mutants to the Gal4 DNA binding domain (GBD), PCR was used to amplify the RTF1 coding region and to introduce an EcoRI site immediately 5′ of RTF1 in each pLS21-5 derivative. An EcoRI/BamHI fragment encompassing RTF1 was ligated to EcoRI/BamHI-digested pGBT9 (4) to create N-terminal fusions of GBD to each Rtf1 derivative. The same strategy was applied to pLS11 to create a GBD fusion to Rtf1-1, an Rtf1 mutant with an amino acid substitution of phenylalanine for leucine at position 11 (59). To create glutathione S-transferase (GST)-Rtf1 fusions, plasmid pJS2 was generated by introducing a BamHI site immediately upstream of RTF1 by site-directed mutagenesis of pKA61, which carries RTF1 on a 3.6-kb insert (59). A plasmid expressing a fusion of GST to full-length Rtf1 (pJS1) was constructed by subcloning a 2.4-kb RTF1-containing BamHI/EcoRI fragment from pJS2 into BamHI/EcoRI-digested pGEX-3X (57). pJS3, which expresses a GST fusion to the most amino-terminal 261 amino acids of Rtf1, was created by digesting pJS2 at the introduced BamHI site and at a natural SmaI site in the RTF1 coding region and subcloning this fragment into BamHI/SmaI-digested pGEX-3X. A plasmid expressing a GST fusion to the most carboxy-terminal 297 amino acids of Rtf1 (pJS1) was constructed by digesting pKA61 at the internal RTF1 SmaI site and at a downstream EcoRI site and cloning this 1.6-kb fragment into SmaI/EcoRI-digested pGEX-3X. pMW4, which expresses a GST fusion to Rtf1 segments 11 through 13, was created by amplifying the 3′ end of the RTF1 coding region from pJS1 to introduce an SmaI site adjacent to nucleotide 1306 of RTF1 (corresponding to the start of amino acid 436). The PCR product was digested with SmaI and EcoRI, and the resulting 1.1-kb fragment was cloned into SmaI/EcoRI-digested pGEX-3X.
FIG. 1.
Rtf1 internal deletion mutants. (A) Schematic representation of amino acids removed by rtf1 internal deletion mutations. Each Rtf1 mutant protein is amino-terminally tagged with a triple-HA epitope. Del., deletion. (B) Immunoblot analysis of wild-type (WT) and mutant Rtf1 protein levels. Extracts were prepared from an rtf1Δ strain (KY404) transformed with CEN/ARS plasmids that express the indicated HA-tagged Rtf1 derivatives. Immunoblots were probed with anti-HA antibody and, as a loading control, anti-L3 antibody. The faster-migrating band observed in most lanes in the anti-HA immunoblot is likely a product of proteolysis, which we frequently observe in extracts prepared by glass bead lysis. This product is less pronounced in extracts prepared by a rapid boiling method (data not shown).
Yeast growth assays.
Strains were grown to saturation at 30°C in appropriate media. Cells were washed twice and were serially diluted in sterile water. Three microliters of each dilution was spotted on the appropriate medium and incubated at 30°C.
Two-hybrid analyses.
pKA202, which expresses a Gal4 activation domain (GAD) fusion to amino acids 863 through 1468 of Chd1, was isolated in a two-hybrid screen using GBD-Rtf1 as the bait (55). Strain PJ69-4A (15), which expresses HIS3, ADE2, and lacZ under the control of galactose-responsive promoters, was transformed with LEU2-marked plasmids expressing GAD alone (pGAD424) (4) or pKA202 and TRP1-marked plasmids corresponding to GBD alone (pGBT9) or GBD fusions to Rtf1, Rtf1-1, or Rtf1 internal deletion mutants. Cell growth was monitored on SC-Leu-Trp and SC-His-Leu-Trp media at 30°C for 3 days.
Sequence alignment.
A BLAST search was conducted using the S. cerevisiae Rtf1 protein sequence (GenBank accession no. NP_011270.1) as the query. Sequences corresponding to Rtf1 homologs in Schizosaccharomyces pombe (NP_595507.1), Caenorhabditis elegans (NP_505473.1), and Homo sapiens (NP_055953.1) were downloaded from the NCBI database. A complete sequence alignment was performed using Clustal X (16), and the resulting alignment was exported into JalView (http://www.jalview.org) to apply grayscale shading at a threshold value of 20% identity. To search for proteins containing a similar sequence, amino acids 62 to 152 of S. cerevisiae Rtf1 were used to query the nonredundant NCBI protein database in four iterations of a Psi-BLAST search.
Immunoblotting analyses.
Transformants of KY404 containing pRS314, pRS424, pMW6, pMW7, pLS20, pLS21-5, or derivatives of pLS21-5 expressing each Rtf1 internal deletion mutant were grown to approximately 4 × 107 cells/ml in SC-Trp medium. Whole-cell extracts were made by glass bead lysis essentially as described previously (52), except that radioimmunoprecipitation assay buffer (50 mM HEPES, pH 7.9, 2 mM EDTA, 0.1% sodium dodecyl sulfate [SDS], 0.1% sodium deoxycholate, 1% Triton X-100, and protease inhibitors) was used. To examine the expression of the Rtf1 internal deletion mutants, 20 μg of extract was run on a 15% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. Membranes were probed with anti-HA (Boehringer Mannheim) or anti-L3 (62) primary antibody at a final concentration of 1:3,000. Sheep anti-mouse horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) were used at 1:5,000 dilutions, and the presence of immunoreactive proteins was visualized by enhanced chemiluminescence detection (Perkin-Elmer). Bulk levels of histone modifications were assayed similarly, except that 30 μg of protein was analyzed using primary antibodies specific for H3 trimethylated (Me3) at K4 (1:2,000 dilution) (Abcam), H3 dimethylated (Me2) at K79 (1:2,500 dilution) (Upstate), or total H3 (1:2,000 dilution) (Upstate). Donkey anti-rabbit horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) were used at 1:5,000 dilutions.
Analysis of histone H2B K123 monoubiquitylation.
Histone H2B K123 monoubiquitylation was measured using a protocol provided by William Tansey. FY406, KY982, KY1216, and KY1217 were transformed with CEN/ARS/HIS3 plasmids carrying HTA1/HTB1 or HTA1/FLAG-HTB1 (33). URA3-marked HTA1/HTB1 plasmids were eliminated by counterselection on 5-FOA medium. The resulting strains were transformed with 2μ-, URA3-marked plasmids expressing untagged or HIS-tagged ubiquitin under the control of the CUP1 promoter (pUb175 and pUb221, respectively; gifts of Daniel Finley). Transformants were grown to approximately 1 × 107 cells/ml in SC-His-Ura medium, and expression of ubiquitin then was induced with a final concentration of 0.5 mM CuSO4 for 4.5 h. Whole-cell extracts were made by glass bead lysis in buffer A, pH 8.0 (6 M guanidine-HCl, 0.1 M sodium phosphate). Extract (2 mg) was incubated with 250 μl 50% Ni-nitrilotriacetic acid agarose (QIAGEN) at room temperature for 2 h. Affinity-precipitated proteins were washed, separated on a 15% SDS-polyacrylamide gel, and analyzed by immunoblotting using anti-FLAG tag (Sigma) or anti-HIS tag (Invitrogen) antibody at a final concentration of 1:5,000.
Coimmunoprecipitation assays.
To examine the interaction between Rtf1 and Paf1 or Ctr9-Myc, transformants of KY995 carrying pRS314, pLS20, pLS21-5, or mutant derivatives of pLS21-5 were grown in SC-Trp medium to approximately 4 × 107 cells/ml. Whole-cell extracts were made by glass bead lysis in lysis buffer (100 mM sodium acetate, 20 mM HEPES, pH 7.4, 10% glycerol, 2 mM magnesium acetate, 10 mM EDTA, 1 mM dithiothreitol, and protease inhibitors). Extract (500 μg) was incubated at 4°C for 2 h with anti-HA antibody (1:3,000 dilution) (Boehringer Mannheim). Immune complexes were captured by incubation with anti-mouse immunoglobulin G-conjugated agarose (Sigma) for 1 h at 4°C and were washed twice with lysis buffer containing 800 mM sodium acetate. Immunoprecipitates were resolved on a 10% SDS-polyacrylamide gel and analyzed by immunobloting with antibodies specific for the Myc epitope (1:100 dilution) (Covance), Paf1 (1:500 dilution) (a gift of Judith Jaehning), or HA (1:3,000 dilution) (Boehringer Mannheim). Coimmunoprecipitation experiments involving Rtf1 and triple-HA-tagged Chd1 were performed as described previously (55).
GST pull-down assays.
Analysis of the association of Rtf1 with Paf1 and Ctr9-Myc was performed essentially as described previously (3). Log-phase cultures of Escherichia coli DH5α transformants carrying pJS1, pJS3, pJS4, or pMW4 were induced for 2 h with 0.1 mM isopropyl-β-d-thiogalactopyranoside. Cells were sonicated in phosphate-buffered saline (PBS) buffer containing 1 mM EDTA and protease inhibitors. One milliliter of lysate was incubated with 100 μl 50% glutathione-Sepharose (Amersham Biosciences) in PBS for 1 h at 4°C to purify GST fusions. A fraction from each purification was resolved on a 10% SDS-polyacrylamide gel to assess the concentration. The remaining GST fusion-bead complexes were equalized to the lowest concentration by removing a fraction of GST-bound beads and normalizing the overall volume of each sample with an equal amount of unbound glutathione-Sepharose. Whole-cell extract (2 mg), prepared from KY457 by glass bead lysis, was incubated with GST fusion-bead complexes for 30 min at 4°C in binding buffer (50 mM NaCl, 20 mM HEPES, pH 7.5, 10 mM MgCl2, 5 mM EDTA, 10% glycerol, and protease inhibitors). Complexes were washed in binding buffer, bound proteins were resolved on 10% SDS-polyacrylamide gels, and the presence of Paf1 or Ctr9-Myc was analyzed by immunoblotting.
Chromatin immunoprecipitation (ChIP) assays.
Transformants of KY452 or KY995 containing pRS314, pRS424, pLS20, pMW6, pMW7, pLS21-5, or derivatives of pLS21-5 and transformants of KY623 containing pRS314, pLS20, or pPC61 were grown in SC-Trp medium to approximately 1 × 107 cells/ml. Chromatin preparation and treatment were performed essentially as described previously (52). Sonicated chromatin was incubated overnight with anti-HA- or anti-Myc-conjugated agarose (Santa Cruz Biotechnology) to immunoprecipitate HA-Rtf1, HA-Chd1, or Ctr9-Myc. PCR was performed using primers that amplify segments in the 5′ ORFs of PYK1 (+195/381; translation start codon ATG = +1), CLN2 (+126/373; ATG = +1), or TEF2 (+40/291; ATG = +1). Reactions were multiplexed with control primers that amplify an intergenic region of chromosome VIII (coordinates 535129 to 535268). Two dilutions of input and immunoprecipitated DNA were amplified in the presence of [α-32P]dATP (Perkin-Elmer) and Platinum Taq DNA polymerase (Invitrogen). PCR products were separated on 6% native polyacrylamide gels, and signals were visualized and quantitated with a Fujifilm FLA-5100 phosphorimager and MultiGauge software. Signals from input and immunoprecipitated DNA were normalized to the chromosome VIII control signal, and the relative association of Rtf1, Chd1, or Ctr9 at each locus was determined by dividing the average of the two immunoprecipitated samples by the average of the two input samples.
Northern analyses.
Strains were grown to approximately 1 × 107 cells/ml in SD medium supplemented with amino acids necessary for cell growth. RNA isolation and Northern analyses were performed as described previously (59). [α-32P]dATP-labeled DNA hybridization probes were prepared from plasmids containing HIS4 (pFW45) and TUB2 (pYST138) by nick translation (Roche nick translation kit) or random prime labeling (2).
RESULTS
Conserved regions of Rtf1 direct normal transcription.
The known roles for Rtf1 in the recruitment of Chd1 and the modification of histones suggest that Rtf1 affects transcription elongation by altering chromatin structure. However, the mechanism by which Rtf1 functions in these processes is not understood. Although Rtf1 homologs are found in many eukaryotes, the primary amino acid sequences of these proteins contain no recognizable functional domains or motifs. To begin dissecting the functional regions of Rtf1, we constructed a series of sequential internal deletions across the RTF1 coding region and expressed these mutations on CEN/ARS plasmids in a strain containing a complete deletion of RTF1. The internal deletion mutations encode HA epitope-tagged mutant versions of Rtf1 that lack between 23 and 52 amino acids (Fig. 1A). Immunoblot analysis demonstrated that all Rtf1 internal deletion mutants were expressed (Fig. 1B), and serial-dilution tests verified that cell growth was not impaired by any of the mutations on the control medium (Fig. 2, right panel).
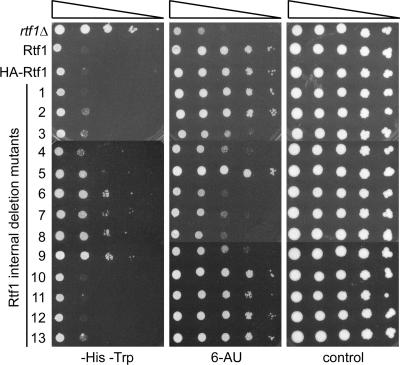
FIG. 2.
Deletions of discrete regions of Rtf1 cause phenotypes associated with transcriptional defects. Tenfold serial dilutions, ranging from 1 × 108 cells/ml to 1 × 104 cells/ml, of an rtf1Δ strain (KY619) expressing the indicated Rtf1 derivatives were spotted on SD-His-Trp medium to examine the Spt− phenotype, SC-Ura-Trp medium containing 50 μg/ml 6-AU to assess 6-AU sensitivity, or SC-Trp medium as a control for growth. Plates were incubated at 30°C for 5 days.
We first utilized the Rtf1 internal deletion mutants to explore which regions of the protein are responsible for the transcription defects observed when Rtf1 is absent from the cell. Deletion of RTF1 causes the suppressor-of-Ty (Spt−) phenotype (59); that is, it suppresses the transcriptional effects of promoter insertion mutations caused by the yeast retrotransposon Ty or its long terminal repeat (δ). In particular, we examined the Spt− phenotype of rtf1 deletion strains that contain his4-912δ, a HIS4 allele in which a Ty δ element is positioned 37 bp upstream of the native TATA box (41). Transcriptional effects at this locus were assayed by growth on medium lacking histidine; growth on this medium indicates an Spt− phenotype. Deletion of region 2, 3, 4, or 13 of Rtf1 caused a weak Spt− phenotype, while deletion of region 5 caused a moderate Spt− phenotype (Fig. 2, left panel). In contrast, a significantly stronger Spt− phenotype was observed when region 6, 7, 8, or 9 was removed. To rule out the possibility that the severe phenotype caused by these deletions arose from mislocalization of the mutant proteins, we performed indirect immunofluorescence assays. These experiments demonstrated that Rtf1 internal deletion mutants 6, 7, 8, and 9 localized to the nucleus (data not shown), where wild-type Rtf1 is known to reside (59).
Deletion of RTF1 also causes sensitivity to the base analog 6-AU (8), a drug that lowers intracellular nucleotide pools by inhibiting enzymes in the ribonucleotide synthesis pathway (10). Sensitivity to 6-AU is considered an indicator of defects in transcription elongation, because Pol II is believed to become more dependent on accessory factors to overcome elongation impediments under low-nucleotide conditions. Our analysis showed that removal of region 6, 7, or 8 from Rtf1 caused strong sensitivity to 6-AU, while deletion of regions 3, 4, and 9 caused moderate sensitivity (Fig. 2, center panel).
Interestingly, loss of any single region of RTF1 did not cause an effect equal to that of a complete RTF1 disruption for either phenotype tested, suggesting that each internal deletion mutant retains some functionality. This raises the possibility that Rtf1 is composed of distinct functional parts. An alignment of Rtf1 homologs from four species revealed a total of 26 invariant residues, all of which reside in either regions 3 and 4 (8 invariant residues) or 6 to 9 (18 invariant residues) (Fig. 3). These conserved sections of the protein overlap with those that, when deleted, cause the most severe Spt− and 6-AUs phenotypes. Together, these observations suggest that Rtf1 requires two clusters of highly conserved amino acids to direct normal transcription in vivo.
FIG. 3.
Rtf1 homologs contain two clusters of highly conserved residues. An alignment of Rtf1 protein sequences from four different species is shown. Conserved residues are highlighted in gray, with darker shades indicating a greater degree of conservation. Black and gray lines above the S. cerevisiae sequence denote Rtf1 regions 3 and 4 and regions 6 to 9, respectively.
A region near the amino terminus of Rtf1 mediates physical interaction with Chd1.
The observation that the removal of discrete amino acid clusters from Rtf1 caused transcription defects led us to investigate whether these residues are responsible for any of Rtf1's known functions. Rtf1 physically associates with Chd1 and is required for the normal recruitment of Chd1 to ORFs (55). To explore which regions of Rtf1 mediate physical interaction with Chd1, we performed a two-hybrid analysis using GBD fusions to the Rtf1 internal deletion mutants and a GAD fusion to amino acids 863 to 1468 of Chd1 (GAD-Chd1). Interaction between GBD-Rtf1 fusions and GAD-Chd1 was examined in a strain that expresses HIS3, ADE2, and lacZ under the control of galactose-responsive promoters. The expression of GBD-Rtf1 fusions was confirmed by immunoblot analysis and does not affect cell growth on complete medium (Fig. 4A, right panel, and data not shown). We observed significant growth on medium lacking histidine when GAD-Chd1 was expressed in combination with GBD fusions to full-length Rtf1 or to 11 of the 12 Rtf1 internal deletion mutants that were tested. The exception was the GBD fusion to deletion mutant 1, GBD-Rtf1Δ1, which failed to support growth on medium lacking histidine (Fig. 4A, left panel). Similar results were obtained when β-galactosidase activity or growth on medium lacking adenine was measured (data not shown). We confirmed the results of the two-hybrid assays by immunoprecipitating Rtf1Δ1 with an anti-Rtf1 antibody and by demonstrating that coimmunoprecipitation of triple-HA-tagged Chd1 essentially was eliminated (Fig. 4B, lanes 5 to 8). Our results suggest that Rtf1 region 1, defined by amino acids 3 through 30, mediates physical interaction with Chd1.
FIG. 4.
Amino terminus of Rtf1 is required for physical interaction with Chd1. (A) Two-hybrid analysis of the interaction between Rtf1 mutants and Chd1. Tenfold serial dilutions, ranging from 1 × 108 cells/ml to 1 × 104 cells/ml, of the two-hybrid reporter strain PJ69-4A expressing GAD-Chd1 (pKA202) and GBD fusions to wild-type Rtf1 (top row) or the Rtf1 deletion mutants were spotted on SC-His-Leu-Trp medium (−His) to monitor HIS3 activation and SC-Leu-Trp medium to control for growth. Plates were incubated at 30°C for 3 days. A GBD fusion to Rtf1Δ11 was not analyzed for technical reasons. (B) Coimmunoprecipitation of HA-Chd1 in strains expressing Rtf1 region 1 mutants. Extract from strains expressing untagged wild-type Rtf1 (KY1213; lanes 1 to 4), Rtf1Δ1 (KY1214; lanes 5 to 8), or Rtf1-1 (KY1215; lanes 9 to 12) was incubated with anti-Rtf1 antibody or preimmune (Pre-I) serum. Immunoblot analysis was performed to assess the presence of Rtf1 and HA-Chd1 in the immunoprecipitated fraction. Lanes 1, 3, 5, 7, 9, and 11 contain 20 μg of unbound (U) material; lanes 2, 4, 6, 8, 10, and 12 contain the total bound (B) fraction. (C and D) ChIP analysis of Chd1 recruitment to active ORFs in rtf1 mutants. HA-Chd1 and associated DNA were immunoprecipitated (IP) from extracts of a formaldehyde-treated rtf1Δ strain (KY623) that had been transformed with an empty vector or plasmids that express untagged wild-type Rtf1 or Rtf1Δ1. An untagged Chd1 strain (KY452) expressing untagged wild-type Rtf1 (pLS20) was used as a control. Association of Chd1 at the 5′ ORF of PYK1 (C) or TEF2 (D) was assessed by PCR. The means of three independent experiments with standard errors are shown. The signal from strains expressing full-length Rtf1 is set at 1. Rel., relative.
Because we previously observed that Rtf1 is required for normal recruitment of Chd1 to active ORFs (55), we sought to examine the contribution of Rtf1 region 1 to this association. We performed ChIP assays on wild-type, rtf1Δ1, and rtf1Δ strains expressing HA-tagged Chd1. The association of Rtf1 was analyzed at the 5′ ends of two ORFs: PYK1, a highly expressed gene for which association with the Paf1 complex has been detected previously (39), and TEF2, for which we previously demonstrated a role for Rtf1 in the recruitment of Chd1 (55). Our results show that deletion of region 1 of Rtf1 significantly reduced the association of Chd1 at the 5′ ORFs of PYK1 and TEF2, although the effect was not as severe as that observed when RTF1 was completely absent (Fig. 4C and D), suggesting that additional regions of Rtf1 may contribute to stabilizing the interaction of Chd1 with chromatin.
In a previous study, we reported a function for Rtf1 region 1 (59). An Rtf1 mutant with a substitution of phenylalanine for leucine at position 11 (Rtf1-1) was identified as a suppressor of the Spt− phenotype caused by spt15-122, a mutation in the gene encoding the TATA-binding protein (TBP). The TBP mutant encoded by spt15-122, TBP-L205F, exhibits altered DNA binding specificity in vitro and in vivo (1). Amino acid 11 lies within region 1 of Rtf1, which led us to examine whether the Rtf1-1 mutant protein was impaired in its physical interaction with Chd1. Two-hybrid analysis demonstrated that a GBD-Rtf1-1 fusion supported only weak growth on medium lacking histidine when expressed in combination with GAD-Chd1 (data not shown). Coimmunoprecipitation analysis confirmed that the physical association between Rtf1-1 and a triple-HA-tagged version of Chd1 was significantly reduced (Fig. 4B, lanes 9 to 12).
Our observation that the Rtf1-1 mutant protein interacted poorly with Chd1 suggested that suppression of spt15-122 by certain RTF1 mutations was due to a disruption of the Rtf1-Chd1 interaction. To explore this possibility, we examined the effect of mutations that interfere with the Rtf1-Chd1 interaction on the Spt− phenotype of spt15-122. We performed these analyses with a strain carrying his4-917δ, a HIS4 allele in which a Ty δ element is inserted between the native TATA box and the transcription start site (41). We observed that rtf1-1, rtf1Δ1, and chd1Δ strongly suppressed the Spt− phenotype of the spt15-122 mutation, similar to the effect of an rtf1 null allele (Fig. 5A). Northern analysis demonstrated that loss of Chd1 altered his4-917δ transcription in an spt15-122 strain, restoring transcription initiation to the native promoter (Fig. 5B and C). As reported previously, the rtf1Δ and rtf1-1 mutations alone do not suppress the his4-917δ allele (59), and we report here that this allele also is not suppressed by rtf1Δ1 or chd1Δ. Together, our observations suggest that the physical interaction between Rtf1 and Chd1 is mediated by amino acids 3 to 30 of Rtf1. While it does not cause noticeable transcription-related phenotypes in a wild-type strain (Fig. 2), disruption of the physical interaction between Rtf1 and Chd1 may have effects on chromatin structure that become apparent in a TBP mutant strain.
FIG. 5.
Mutations that disrupt the Rtf1-Chd1 interaction suppress a TBP mutant. (A) Mutations in RTF1 or CHD1 suppress the Spt− phenotype of an spt15-122 strain. KY343, KY386, KY638, KY639, KY640, and KY641 (top row) or KY100, KY284, KY423, KY440, KY680, and KY1265 (bottom row) were grown on YPD medium and transferred by replica printing to SD complete medium as a control or to SD-His medium to examine the Spt− phenotype. Plates were incubated at 30°C for 3 days. (B) Northern analysis of his4-917δ expression in wild-type (KY640), chd1Δ (KY641), spt15-122 (KY638), and chd1Δ spt15-122 (KY639) strains. Lane 1 contained 1 μg of total RNA from a HIS4+ strain (FY78); lanes 2 through 5 contained 10 μg of total RNA. The filter was analyzed using a HIS4 probe (top panel). TUB2 levels (bottom panel) were analyzed on the same filter as a loading control. (C) Schematic representation of the his4-917δ allele and its transcription. The δ element is represented by the rectangle, and the direction of transcription of the normal Ty message is shown by the arrowhead. Relative positions of the HIS4 upstream activation sequence (UAS), the natural HIS4 TATA element, and the HIS4 coding region are indicated. The diagram is not drawn to scale. Arrows beneath the diagram symbolize the HIS4 transcripts observed in the indicated strains.
Conserved regions of Rtf1 are required for Rtf1-dependent histone modifications and telomeric silencing.
We sought to determine which residues are essential for Rtf1 to direct covalent modification of lysine residues in histones H2B and H3. We began by examining the effects of the Rtf1 internal deletion mutants on histone H3 methylation. Immunoblot analysis demonstrated that Rtf1 regions 3 and 4 are essential for dimethylation and trimethylation of histone H3 K4 and dimethylation of histone H3 K79 (Fig. 6A and data not shown). We also observed a slight reduction of these modifications when region 6, 7, 8, or 9 was eliminated from Rtf1. Interestingly, the regions of Rtf1 that are required for histone methylation correspond to the most highly conserved portions of the protein (Fig. 3).
FIG. 6.
Conserved regions of Rtf1 are essential for Rtf1-dependent histone modifications and telomeric silencing. (A) Immunoblot analysis of Rtf1-dependent histone methylation in strains expressing the Rtf1 internal deletion mutants. Extracts from an rtf1Δ strain (KY404) expressing the indicated Rtf1 derivatives were probed with antibodies specific for H3 K4 Me3, H3 K79 Me2, and total H3. An anti-L3 immunoblot analysis was performed as a loading control. (B) Analysis of H2B K123 ubiquitylation levels in Rtf1 mutants defective for histone methylation. FY406, KY982, KY1216, and KY1217 were transformed with plasmids expressing untagged or HIS-tagged ubiquitin and a plasmid expressing wild-type or FLAG-tagged histone H2B. The HIS-tagged protein fraction from each strain was isolated and analyzed by immunoblotting using an anti-FLAG antibody to detect FLAG-H2B (top panel). An anti-HIS tag immunoblotting also was performed (lower panel) to demonstrate the expression and enrichment of HIS-Ub in the expected strains. (C) Analysis of telomeric silencing in strains expressing Rtf1 internal deletion mutants. Tenfold serial dilutions, ranging from 1 × 108 cells/ml to 1 × 104 cells/ml, of an rtf1Δ strain (OKA93) expressing the indicated Rtf1 derivatives and containing an ectopic URA3 gene integrated proximal to the telomere on the right arm of chromosome V were spotted on SC-Ura-Trp medium containing 5-FOA to assess telomeric silencing defects or were spotted on SC-Trp medium as a control for growth. Plates were incubated at 30°C for 4 days.
Rtf1 also is required for monoubiquitylation of histone H2B K123 (31, 65). Because removal of Rtf1 region 3 or 4 eliminates H3 K4 and K79 methylation, modifications that lie downstream of H2B K123 ubiquitylation, we examined the state of H2B K123 ubiquitylation in strains expressing these Rtf1 mutants. This modification was analyzed in strains expressing HIS-tagged ubiquitin (HIS-Ub) and FLAG-tagged histone H2B (FLAG-H2B). The expression of FLAG-H2B and HIS-Ub was confirmed by immunoblot analysis (Fig. 6B and data not shown). Ubiquitylated proteins from yeast extracts were captured on Ni-nitrilotriacetic acid agarose, separated by SDS-polyacrylamide gel electrophoresis, and analyzed by immunoblotting for the presence of FLAG-H2B. Ubiquitylated H2B was detected in wild-type cells but not in cells in which RTF1 had been deleted or replaced with rtf1Δ3 or rtf1Δ4 (Fig. 6B). The observation that H2B K123 ubiquitylation and H3 K4 and K79 methylation are eliminated by removal of the same residues suggests that the primary role of Rtf1 in histone modification is to direct H2B ubiquitylation.
Rtf1 and its downstream histone modifications are required for normal telomeric silencing (21, 31). To assess which regions of Rtf1 affect telomeric silencing, we analyzed the effects of the Rtf1 internal deletion mutants on the expression of an ectopic copy of URA3 integrated proximal to the telomere on the right arm of chromosome V (TEL-VR::URA3). URA3 expression causes toxicity on media containing 5-FOA. When telomeric silencing is active, transcription from TEL-VR::URA3 is repressed and cell growth is largely unaffected by 5-FOA. However, mutations that compromise telomeric silencing derepress TEL-VR::URA3, resulting in growth inhibition or cell death on 5-FOA-containing media. We found that deletion of Rtf1 region 3, 4, 6, 7, 8, or 9 eliminated cell growth on media containing 5-FOA in a TEL-VR::URA3 strain (Fig. 6C, left panel). Therefore, the regions of Rtf1 that affect telomeric silencing correspond to the same regions that eliminate or reduce Rtf1-dependent histone modifications (Fig. 6A and B).
Association of Rtf1 with active ORFs requires a conserved central region.
Rtf1 occupies active ORFs coincident with Pol II. We performed ChIP assays on strains expressing the Rtf1 internal deletion mutants to investigate which regions of Rtf1 are required for its association with active ORFs. Association of Rtf1 was analyzed at the 5′ ends of PYK1 and CLN2, a gene that requires the Paf1 complex for normal expression (19). Our results show that deletion of segment 6, 7, or 8 reduced association of Rtf1 at the 5′ ORFs of PYK1 and CLN2 to the background levels observed with untagged or rtf1Δ strains (Fig. 7A and B). A strong reduction in association of Rtf1 at these loci also was observed when Rtf1 region 9 was absent. Deletion of Rtf1 regions 3 and 4, which are required for Rtf1-dependent histone modifications, caused an approximately twofold reduction of Rtf1 association with PYK1, but this effect was not observed for CLN2. These observations suggest that a large central region of Rtf1, spanning amino acids 201 to 350, is essential for recruitment of Rtf1 to the two genes tested, and amino acids 351 to 395 contribute significantly to this interaction.
FIG. 7.
Central conserved region of Rtf1 mediates association with active ORFs and influences protein stability. (A and B) ChIP analysis of Rtf1 association with active ORFs. HA-tagged Rtf1 mutants and associated DNA were immunoprecipitated (IP) from extracts of a formaldehyde-treated rtf1Δ strain (KY452) that expressed the indicated Rtf1 derivatives. Association of wild-type or mutant Rtf1 proteins with the 5′ ORF of PYK1 (A) or CLN2 (B) was assessed by PCR. The means of three independent experiments with standard errors are shown. Signal from strains expressing HA-tagged full-length Rtf1 is set at 1. Rel., relative. (C) Effects of Rtf1Δ7 overexpression on protein levels and histone modifications. Twenty micrograms of extract from an rtf1Δ strain (KY404) expressing the indicated Rtf1 derivatives from low-copy-number (CEN/ARS [C/A]) or high-copy-number (2μ) plasmids or empty vectors (rtf1Δ) was subjected to immunoblot analysis with antibodies specific for the HA epitope, H3 K4 Me3, H3 K79 Me2, and total H3. Anti-L3 immunoblotting was performed as a loading control. (D) ChIP analysis to assess the effects of Rtf1Δ7 overexpression on association with active ORFs. The analysis was performed as described for panels A and B on extracts from KY452 transformed with C/A or 2μ plasmids expressing the indicated Rtf1 derivatives. (E) Overexpression of Rtf1Δ7 does not suppress transcription-related phenotypes. Tenfold serial dilutions, ranging from 1 × 108 cells/ml to 1 × 104 cells/ml, of an rtf1Δ strain (KY619) expressing the indicated Rtf1 derivatives from C/A or 2μ plasmids were spotted on SD-His-Trp medium to examine the Spt− phenotype, SC-Ura-Trp medium containing 50 μg/ml 6-AU to assess 6-AU sensitivity, or SC-Trp medium as a control for growth. Plates were incubated at 30°C for 5 days.
Immunoblot analysis of the Rtf1 internal deletion mutants indicated that expression of Rtf1 deletions 6, 7, 8, and 9 is somewhat reduced compared to that of full-length Rtf1 (Fig. 1B), raising the possibility that the effects we observe when regions 6 to 9 are deleted are a consequence of reduced protein levels. To address this possibility, we expressed Rtf1Δ7 from a high-copy-number yeast vector and asked whether increased levels of the mutant protein reduced the severity of its effects. We found that overexpression of Rtf1Δ7 increased its levels to those observed for wild-type Rtf1 when it was expressed from a CEN/ARS vector (Fig. 7C), but it did not increase association of Rtf1Δ7 with the 5′ ORF of PYK1 (Fig. 7D) or suppress the transcription-related or histone modification defects observed with an rtf1Δ7 strain (Fig. 7C and E). These results support the idea that amino acids 201 to 395 are important for recruitment of Rtf1 to active genes and indicate that this association contributes to protein function and stability.
Rtf1 interacts with other Paf1 complex components through its carboxy terminus.
Rtf1 is a component of the five-member Paf1 transcription elongation complex. To identify the regions of Rtf1 that are necessary for its association with other Paf1 complex components, the Rtf1 internal deletion mutants were immunoprecipitated with an anti-HA antibody, and coimmunoprecipitation of Paf1 or Myc-tagged Ctr9 was measured by immunoblot analysis (Fig. 8A). We found that Paf1 and Ctr9 coimmunoprecipitated with full-length Rtf1 and Rtf1 internal deletion mutants 1 through 11. The slight decrease in the amounts of Paf1 and Ctr9 coimmunoprecipitated from strains expressing Rtf1 internal deletion mutants 6, 7, 8, and 9 likely is related to the reduced expression of these Rtf1 mutants (Fig. 1). However, when the most carboxy-terminal segments of Rtf1, regions 12 and 13, were deleted, coimmunoprecipitation of Paf1 and Ctr9 was greatly reduced.
FIG. 8.
Carboxy terminus of Rtf1 mediates physical interaction with other Paf1 complex subunits. (A) Coimmunoprecipitation analyses of Rtf1 internal deletion mutants and other Paf1 complex subunits. Extracts from an rtf1Δ strain (KY995) expressing Ctr9-Myc and the indicated Rtf1 derivatives were subjected to immunoprecipitation (IP) with an anti-HA antibody. The precipitated fraction was analyzed by immunoblotting using anti-Myc, anti-Paf1, or anti-HA antibody. (B) Paf1 and Ctr9 bind to the carboxy terminus of Rtf1. GST alone or GST fusions to full-length Rtf1 (GST-Rtf1), the amino-terminal half of Rtf1 (GST N-Rtf1), the carboxy-terminal half of Rtf1 (GST Rtf1-C), or Rtf1 segments 11 through 13 [GST Rtf1(11-13)] were purified from bacteria and incubated with yeast extract from an rtf1Δ strain (KY457) for GST pull-down assays. Bound proteins were detected by immunoblotting using anti-Myc and anti-Paf1 antibodies. (C) ChIP analysis of Ctr9-Myc in Rtf1 internal deletion mutant strains. Myc-tagged Ctr9 and associated DNA were immunoprecipitated from extracts of a formaldehyde-treated rtf1Δ strain (KY995) expressing Ctr9-Myc and the indicated Rtf1 derivatives. Association of Ctr9-Myc with the 5′ ORF of PYK1 was assessed by PCR. The means of three independent experiments with standard errors are shown. Signal from transformants expressing HA-tagged full-length Rtf1 is set at 1. Rel., relative.
To further test the importance of the Rtf1 carboxy terminus in Paf1 complex assembly, we purified bacterially expressed GST fusions to full-length Rtf1, the amino-terminal half of Rtf1, the carboxy-terminal half of Rtf1, or the extreme carboxy terminus of Rtf1 (regions 11 through 13) and examined the ability of these fusions to bind Paf1 or Myc-tagged Ctr9 in yeast lysates. We found that Paf1 and Ctr9 bound GST fusions to full-length Rtf1, the carboxy-terminal half of Rtf1, and the extreme carboxy terminus of Rtf1 (Fig. 8B). However, a GST fusion to the amino-terminal half of Rtf1 showed essentially no interaction with Paf1 or Ctr9. Together, our observations indicate that the extreme carboxy terminus of Rtf1 is both necessary and sufficient to mediate interaction with other components of the Paf1 complex.
A previous report demonstrated that Paf1 complex components fail to associate with active ORFs in a strain completely lacking Rtf1 (28). We therefore decided to use our specific mutations to test whether disruption of the physical interaction between Rtf1 and the Paf1 complex is sufficient to eliminate recruitment of other Paf1 complex components to active ORFs. ChIP analyses were performed on a strain expressing Myc-tagged Ctr9 and a subset of the Rtf1 internal deletion mutants. We found that regions of Rtf1 required for the interaction with Chd1 (region 1) or histone modification (region 3) had only a modest effect on Ctr9 association at the PYK1 gene (Fig. 8C). However, removal of region 6, which is required for the association of Rtf1 with active ORFs, or region 13, which is responsible for the interaction of Rtf1 with other Paf1 complex components, reduced Ctr9 association at PYK1 essentially to the level observed in an rtf1 null strain. Surprisingly, Ctr9 associates with the 5′ ORF of PYK1 at near-wild-type levels in a strain expressing Rtf1Δ12. This observation suggests that a weak physical interaction between Rtf1Δ12 and Ctr9 exists in vivo and that this interaction can be detected once it has been stabilized by formaldehyde cross-linking. These results support the conclusion that Rtf1 is required to tether the Paf1 complex to active ORFs. Additionally, we observed that deletion of region 13 from Rtf1 slightly reduces its occupancy on PYK1 and CLN2 (Fig. 7), suggesting that other Paf1 components play a reciprocal, though minor, role in stabilizing the association of Rtf1 with active ORFs.
DISCUSSION
A large number of accessory factors participate in mRNA synthesis by facilitating the recruitment and modification of Pol II, the cotranscriptional processing of the nascent transcript, the progression of Pol II along ORFs, and transcription termination. Accessory proteins that carry out distinct cotranscriptional events, such as transcript cleavage, can be recruited to discrete segments of a gene during a portion of the transcription cycle (reviewed in reference 5). In contrast, the Paf1 complex associates along the entirety of active ORFs (18, 55), suggesting that it functions throughout transcript elongation. Our results indicate that the Paf1 complex component Rtf1 utilizes several independent mechanisms to orchestrate alterations to the chromatin template during gene expression. Using a collection of sequential rtf1 deletion mutations, we demonstrated that discrete nonoverlapping segments of the protein are required for interaction with Chd1, histone modification, recruitment to active ORFs, and association with the Paf1 complex. In addition, we observed that distinct transcriptional effects resulted from disruption of individual functions of Rtf1 (Fig. 9). Combined with our observation that a complete deletion of RTF1 causes more severe phenotypes than mutations that eliminate only one of its activities (Fig. 2), our data suggest that the functions of Rtf1 are not entirely interdependent.
FIG. 9.
Rtf1 is composed of functionally distinct regions. A schematic diagram of Rtf1 indicating the relative size of each Rtf1 internal deletion (numbered boxes) is shown. Regions of Rtf1 affecting each of the indicated processes or phenotypes are specified by colored lines. The width of the line represents the degree of phenotype or effect that was observed.
Our analyses demonstrated that physical interaction with Chd1 is disrupted by deleting amino acids 3 to 30 of Rtf1 (region 1) or by substituting phenylalanine for leucine at position 11 (Rtf1-1). Although we observed no transcription-related defects when region 1 was deleted in an otherwise wild-type strain, we have shown previously that rtf1-1 and an rtf1 null allele suppress the Spt− phenotype of the spt15-122 mutation (59). We report here that this effect also is caused by rtf1Δ1 and chd1Δ. The similar effects of mutations in RTF1 and CHD1 in an spt15-122 strain suggest that these factors elicit similar effects on chromatin structure. We hypothesize that the interaction between Rtf1 and Chd1 is important for proper chromatin function, particularly when the cell is sensitized to small changes in nucleosome positioning or stability, such as in a TBP mutant strain.
We also demonstrated that amino acids 62 through 152 of Rtf1, which correspond to regions 3 and 4, are required for monoubiquitylation of H2B K123 and methylation of histone H3 K4 and K79. The observation that H2B K123 ubiquitylation and its downstream histone methylation events require the same region of Rtf1 suggests that the primary role of Rtf1 in histone modification is to promote ubiquitylation of H2B. However, analysis of more specific mutations will be required to determine whether it is possible to genetically separate the histone ubiquitylation and methylation functions of Rtf1. A previous study indicated that Rad6 localizes to gene promoters in the absence of Rtf1 but that it remains inactive and fails to associate with active ORFs (66). This observation suggests that Rtf1 is required to stimulate Rad6 activity and recruitment coincident with the onset of transcription elongation. Because no enzymatic functions are predicted from its primary sequence, Rtf1 may activate Rad6 by recruiting additional factors or altering chromatin structure in a way that increases the accessibility of H2B K123. Regions 3 and 4 of Rtf1 contain several highly conserved residues, but a Psi-BLAST search did not identify similar domains in other proteins. This suggests that regions 3 and 4 of Rtf1 stimulate Rad6 activity by a unique conserved mechanism.
Rtf1-dependent histone modifications also were moderately reduced by deletion of regions 6 to 9, which span amino acids 201 to 395. Our analyses show that residues in these regions are important for association of Rtf1 with active ORFs. Interestingly, the association of Rtf1 with chromatin, and presumably with transcribing Pol II, appears to be required for its full stability (Fig. 1). Surprisingly, Rtf1-dependent histone modifications are not eliminated completely when the association between Rtf1 and chromatin is disrupted. This may indicate that Rtf1 promotes histone modification most efficiently when it is associated with active genes but that this interaction is not required absolutely. An additional, and perhaps more likely, explanation is that Rtf1 mutants lacking amino acids in regions 6 to 9 transiently interact with active genes for a sufficient length of time to promote low levels of histone modification or that these mutants associate with coding regions at levels below the limits of detection of the ChIP assay. Because histone methylation is relatively stable, it may accumulate to detectable levels over multiple rounds of transcription.
Our telomeric silencing assays demonstrated that all rtf1 mutations that decreased histone modifications also interfered with telomeric silencing, confirming prior reports that tightly linked these processes (20, 21, 31, 61). Interestingly, the absence of Rtf1 region 6, 7, 8, or 9 caused only a moderate decrease in Rtf1-dependent histone methylation but dramatically affected telomeric silencing. This observation suggests that even modest effects on histone modifications can disrupt telomeric silencing. Alternatively, these results may simply reflect the sensitivity of the TEL-VR::URA3 reporter. A study on the Set1-containing COMPASS complex that used a telomeric URA3 reporter system also reported strong effects on telomeric silencing by mutations that only mildly affect histone modification levels (29).
All amino acids that are invariant across the four Rtf1 homologs examined in Fig. 3 reside in either regions 3 and 4, which are required for histone modification, or in regions 6 to 9, which regulate localization of Rtf1 to active ORFs. We also observe that the most severe transcription-related phenotypes result from deletion of these regions of the protein, indicating that directing cotranscriptional modification of histones is a critical means by which Rtf1 exerts its effects on transcription. Deletion of sequences required for either histone methylation or ORF association causes similar levels of 6-AU sensitivity, but disruption of the association of Rtf1 with ORFs causes a more severe Spt− phenotype. This observation suggests that the association of Rtf1 with active genes is required for a function in addition to H2B K123 ubiquitylation or H3 K4 and K79 methylation. Another indication that the 6-AU and Spt− phenotypes measure, at least partly, distinct functions of Rtf1 comes from our observation that Rtf1Δ5 causes a moderate Spt− phenotype but no sensitivity to 6-AU (Fig. 2).
Finally, we demonstrated that the carboxy terminus of Rtf1, defined by region 13, is necessary and sufficient for the interaction between Rtf1 and other Paf1 complex subunits. It is surprising that Rtf1Δ13 apparently has wild-type stability, because previous studies revealed a 10- to 20-fold reduction in Rtf1 levels in crude extracts from paf1Δ and ctr9Δ strains (28, 36). Our results suggest that a stable physical interaction with the Paf1 complex is not required for Rtf1 stability or that residues within Rtf1 that confer instability are deleted from the Rtf1Δ13 mutant protein. Consistent with findings of a prior study, we show that interaction with Rtf1 mediates recruitment of Paf1 and Ctr9 to active ORFs (28). Remarkably, disruption of the physical interaction between Rtf1 and the Paf1 complex causes only a mild Spt− phenotype (Fig. 2), and the known biochemical functions of Rtf1 remain largely intact. This observation suggests that Rtf1 retains function independent of stable association with the Paf1 complex. In agreement with this idea, Rtf1 does not copurify with the human Paf1 complex (44, 67, 68), and we note that the carboxy terminus is poorly conserved (Fig. 3). Human Rtf1 may interact only with other Paf1 complex subunits while associated with chromatin, or the human Paf1 complex may execute some functions independently of ORF association, as previously suggested by studies with yeast (28). Alternatively, Cdc73, which also promotes association of Paf1 and Ctr9 with active genes in yeast (28), may be the sole Paf1 complex subunit required for this function in humans.
The conservation of Rtf1 and the proteins that mediate its dependent histone modifications indicates that these factors perform a valuable function. In accordance with this idea, mutations in human homologs of Paf1, Cdc73, and Set1 are involved in the etiology of various cancers (26, 44, 49). Although Rtf1 performs various transcription-associated functions, the mechanisms and consequences of these functions remain unknown. Here, we show that the multiple activities of Rtf1 are functionally separable, that Rtf1 affects transcription by directing the cotranscriptional modification of histones, and that Rtf1 may have functions independent of the Paf1 complex. In addition, the separation-of-function mutations we have identified represent valuable tools for dissecting the contributions of the individual functions of Rtf1 to proper gene expression.
Acknowledgments
We are grateful to Patrick Costa for experimental contributions to Fig. 4 and 5, Jakub Svoboda and Rajna Simic for technical assistance, Judith Jaehning for the antibody directed against Paf1, and Fred Winston, Grant Hartzog, Daniel Finley, Rick Young, and Kevin Struhl for the gifts of plasmids, PCR primer information, and strains. We also thank Joe Martens and members of the Arndt laboratory for valuable discussions and critical reading of the manuscript.
This work was supported by NIH grant GM52593 to K.M.A.
Footnotes
Published ahead of print on 18 June 2007.
REFERENCES
- 1.Arndt, K. M., S. L. Ricupero, D. M. Eisenmann, and F. Winston. 1992. Biochemical and genetic characterization of a yeast TFIID mutant that alters transcription in vivo and DNA binding in vitro. Mol. Cell. Biol. 12:2372-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1988. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, NY.
- 3.Barlev, N. A., R. Candau, L. Wang, P. Darpino, N. Silverman, and S. L. Berger. 1995. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J. Biol. Chem. 270:19337-19344. [DOI] [PubMed] [Google Scholar]
- 4.Bartel, P. L., C.-T. Chien, R. Sternglanz, and S. Fields. 1993. Cellular interactions in development: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 5.Bentley, D. L. 2005. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr. Opin. Cell Biol. 17:251-256. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein, B. E., E. L. Humphrey, R. L. Erlich, R. Schneider, P. Bouman, J. S. Liu, T. Kouzarides, and S. L. Schreiber. 2002. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc. Natl. Acad. Sci. USA 99:8695-8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briggs, S. D., T. Xiao, Z. W. Sun, J. A. Caldwell, J. Shabanowitz, D. F. Hunt, C. D. Allis, and B. D. Strahl. 2002. Gene silencing: trans-histone regulatory pathway in chromatin. Nature 418:498. [DOI] [PubMed] [Google Scholar]
- 8.Costa, P. J., and K. M. Arndt. 2000. Synthetic lethal interactions suggest a role for the Saccharomyces cerevisiae Rtf1 protein in transcription elongation. Genetics 156:535-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel, J. A., M. S. Torok, Z. W. Sun, D. Schieltz, C. D. Allis, J. R. Yates III, and P. A. Grant. 2004. Deubiquitination of histone H2B by a yeast acetyltransferase complex regulates transcription. J. Biol. Chem. 279:1867-1871. [DOI] [PubMed] [Google Scholar]
- 10.Exinger, F., and F. Lacroute. 1992. 6-Azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr. Genet. 22:9-11. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan, J. F., L. Z. Mi, M. Chruszcz, M. Cymborowski, K. L. Clines, Y. Kim, W. Minor, F. Rastinejad, and S. Khorasanizadeh. 2005. Double chromodomains cooperate to recognize the methylated histone H3 tail. Nature 438:1181-1185. [DOI] [PubMed] [Google Scholar]
- 12.Grant, P. A., L. Duggan, J. Cote, S. M. Roberts, J. E. Brownell, R. Candau, R. Ohba, T. Owen-Hughes, C. D. Allis, F. Winston, S. L. Berger, and J. L. Workman. 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11:1640-1650. [DOI] [PubMed] [Google Scholar]
- 13.Grant, P. A., A. Eberharter, S. John, R. G. Cook, B. M. Turner, and J. L. Workman. 1999. Expanded lysine acetylation specificity of Gcn5 in native complexes. J. Biol. Chem. 274:5895-5900. [DOI] [PubMed] [Google Scholar]
- 14.Henry, K. W., A. Wyce, W. S. Lo, L. J. Duggan, N. C. Emre, C. F. Kao, L. Pillus, A. Shilatifard, M. A. Osley, and S. L. Berger. 2003. Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev. 17:2648-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 17.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 18.Kim, M., S. H. Ahn, N. J. Krogan, J. F. Greenblatt, and S. Buratowski. 2004. Transitions in RNA polymerase II elongation complexes at the 3′ ends of genes. EMBO J. 23:354-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch, C., P. Wollmann, M. Dahl, and F. Lottspeich. 1999. A role for Ctr9p and Paf1p in the regulation G1 cyclin expression in yeast. Nucleic Acids Res. 27:2126-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krogan, N. J., J. Dover, S. Khorrami, J. F. Greenblatt, J. Schneider, M. Johnston, and A. Shilatifard. 2002. COMPASS, a histone H3 (lysine 4) methyltransferase required for telomeric silencing of gene expression. J. Biol. Chem. 277:10753-10755. [DOI] [PubMed] [Google Scholar]
- 21.Krogan, N. J., J. Dover, A. Wood, J. Schneider, J. Heidt, M. A. Boateng, K. Dean, O. W. Ryan, A. Golshani, M. Johnston, J. F. Greenblatt, and A. Shilatifard. 2003. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol. Cell 11:721-729. [DOI] [PubMed] [Google Scholar]
- 22.Krogan, N. J., M. Kim, S. H. Ahn, G. Zhong, M. S. Kobor, G. Cagney, A. Emili, A. Shilatifard, S. Buratowski, and J. F. Greenblatt. 2002. RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol. Cell. Biol. 22:6979-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunkel, T. A., J. D. Roberts, and R. A. Zakour. 1987. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 154:367-382. [DOI] [PubMed] [Google Scholar]
- 24.Lieb, J. D., and N. D. Clarke. 2005. Control of transcription through intragenic patterns of nucleosome composition. Cell 123:1187-1190. [DOI] [PubMed] [Google Scholar]
- 25.Martin, C., and Y. Zhang. 2005. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 6:838-849. [DOI] [PubMed] [Google Scholar]
- 26.Moniaux, N., C. Nemos, B. M. Schmied, S. C. Chauhan, S. Deb, K. Morikane, A. Choudhury, M. Vanlith, M. Sutherlin, J. M. Sikela, M. A. Hollingsworth, and S. K. Batra. 2006. The human homologue of the RNA polymerase II-associated factor 1 (hPaf1), localized on the 19q13 amplicon, is associated with tumorigenesis. Oncogene 25:3247-3257. [DOI] [PubMed] [Google Scholar]
- 27.Mueller, C. L., and J. A. Jaehning. 2002. Ctr9, Rtf1, and Leo1 are components of the Paf1/RNA polymerase II complex. Mol. Cell. Biol. 22:1971-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller, C. L., S. E. Porter, M. G. Hoffman, and J. A. Jaehning. 2004. The Paf1 complex has functions independent of actively transcribing RNA polymerase II. Mol. Cell 14:447-456. [DOI] [PubMed] [Google Scholar]
- 29.Mueller, J. E., M. Canze, and M. Bryk. 2006. The requirements for COMPASS and Paf1 in transcriptional silencing and methylation of histone H3 in Saccharomyces cerevisiae. Genetics 173:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan, D., K. Ingvarsdottir, D. E. Sterner, G. R. Bylebyl, M. Dokmanovic, J. A. Dorsey, K. A. Whelan, M. Krsmanovic, W. S. Lane, P. B. Meluh, E. S. Johnson, and S. L. Berger. 2006. Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev. 20:966-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng, H. H., S. Dole, and K. Struhl. 2003. The Rtf1 component of the Paf1 transcriptional elongation complex is required for ubiquitination of histone H2B. J. Biol. Chem. 278:33625-33628. [DOI] [PubMed] [Google Scholar]
- 32.Ng, H. H., F. Robert, R. A. Young, and K. Struhl. 2003. Targeted recruitment of Set1 histone methyltransferase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11:709-719. [DOI] [PubMed] [Google Scholar]
- 33.Ng, H. H., R. M. Xu, Y. Zhang, and K. Struhl. 2002. Ubiquitination of histone H2B by Rad6 is required for efficient Dot1-mediated methylation of histone H3 lysine 79. J. Biol. Chem. 277:34655-34657. [DOI] [PubMed] [Google Scholar]
- 34.Penheiter, K. L., T. M. Washburn, S. E. Porter, M. G. Hoffman, and J. A. Jaehning. 2005. A posttranscriptional role for the yeast Paf1-RNA polymerase II complex is revealed by identification of primary targets. Mol. Cell 20:213-223. [DOI] [PubMed] [Google Scholar]
- 35.Pokholok, D. K., N. M. Hannett, and R. A. Young. 2002. Exchange of RNA polymerase II initiation and elongation factors during gene expression in vivo. Mol. Cell 9:799-809. [DOI] [PubMed] [Google Scholar]
- 36.Porter, S. E., K. L. Penheiter, and J. A. Jaehning. 2005. Separation of the Saccharomyces cerevisiae Paf1 complex from RNA polymerase II results in changes in its subnuclear localization. Eukaryot. Cell 4:209-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter, S. E., T. M. Washburn, M. Chang, and J. A. Jaehning. 2002. The yeast Paf1-RNA polymerase II complex is required for full expression of a subset of cell cycle-regulated genes. Eukaryot. Cell 1:830-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pray-Grant, M. G., J. A. Daniel, D. Schieltz, J. R. Yates III, and P. A. Grant. 2005. Chd1 chromodomain links histone H3 methylation with SAGA- and SLIK-dependent acetylation. Nature 433:434-438. [DOI] [PubMed] [Google Scholar]
- 39.Qiu, H., C. Hu, C. M. Wong, and A. G. Hinnebusch. 2006. The Spt4p subunit of yeast DSIF stimulates association of the Paf1 complex with elongating RNA polymerase II. Mol. Cell. Biol. 26:3135-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renauld, H., O. M. Aparicio, P. D. Zierath, B. L. Billington, S. K. Chhablani, and D. E. Gottschling. 1993. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev. 7:1133-1145. [DOI] [PubMed] [Google Scholar]
- 41.Roeder, G. S., and G. R. Fink. 1982. Movement of yeast transposable elements by gene conversion. Proc. Natl. Acad. Sci. USA 79:5621-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose, M. D., F. Winston, and P. Hieter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 43.Rothstein, R. 1991. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 194:281-301. [DOI] [PubMed] [Google Scholar]
- 44.Rozenblatt-Rosen, O., C. M. Hughes, S. J. Nannepaga, K. S. Shanmugam, T. D. Copeland, T. Guszczynski, J. H. Resau, and M. Meyerson. 2005. The parafibromin tumor suppressor protein is part of a human Paf1 complex. Mol. Cell. Biol. 25:612-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusche, L. N., A. L. Kirchmaier, and J. Rine. 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72:481-516. [DOI] [PubMed] [Google Scholar]
- 46.San-Segundo, P. A., and G. S. Roeder. 2000. Role for the silencing protein Dot1 in meiotic checkpoint control. Mol. Biol. Cell 11:3601-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos-Rosa, H., A. J. Bannister, P. M. Dehe, V. Geli, and T. Kouzarides. 2004. Methylation of H3 lysine 4 at euchromatin promotes Sir3p association with heterochromatin. J. Biol. Chem. 279:47506-47512. [DOI] [PubMed] [Google Scholar]
- 48.Santos-Rosa, H., R. Schneider, A. J. Bannister, J. Sherriff, B. E. Bernstein, N. C. Emre, S. L. Schreiber, J. Mellor, and T. Kouzarides. 2002. Active genes are tri-methylated at K4 of histone H3. Nature 419:407-411. [DOI] [PubMed] [Google Scholar]
- 49.Schneider, R., A. J. Bannister, and T. Kouzarides. 2002. Unsafe SETs: histone lysine methyltransferases and cancer. Trends Biochem. Sci. 27:396-402. [DOI] [PubMed] [Google Scholar]
- 50.Shiio, Y., and R. N. Eisenman. 2003. Histone sumoylation is associated with transcriptional repression. Proc. Natl. Acad. Sci. USA 100:13225-13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shilatifard, A. 2006. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu. Rev. Biochem. 75:243-269. [DOI] [PubMed] [Google Scholar]
- 52.Shirra, M. K., S. E. Rogers, D. E. Alexander, and K. M. Arndt. 2005. The Snf1 protein kinase and Sit4 protein phosphatase have opposing functions in regulating TATA-binding protein association with the Saccharomyces cerevisiae INO1 promoter. Genetics 169:1957-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sif, S. 2004. ATP-dependent nucleosome remodeling complexes: enzymes tailored to deal with chromatin. J. Cell. Biochem. 91:1087-1098. [DOI] [PubMed] [Google Scholar]
- 54.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simic, R., D. L. Lindstrom, H. G. Tran, K. L. Roinick, P. J. Costa, A. D. Johnson, G. A. Hartzog, and K. M. Arndt. 2003. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J. 22:1846-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sims, R. J., III, C. F. Chen, H. Santos-Rosa, T. Kouzarides, S. S. Patel, and D. Reinberg. 2005. Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J. Biol. Chem. 280:41789-41792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 58.Squazzo, S. L., P. J. Costa, D. L. Lindstrom, K. E. Kumer, R. Simic, J. L. Jennings, A. J. Link, K. M. Arndt, and G. A. Hartzog. 2002. The Paf1 complex physically and functionally associates with transcription elongation factors in vivo. EMBO J. 21:1764-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stolinski, L. A., D. M. Eisenmann, and K. M. Arndt. 1997. Identification of RTF1, a novel gene important for TATA site selection by TATA box-binding protein in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:4490-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suka, N., Y. Suka, A. A. Carmen, J. Wu, and M. Grunstein. 2001. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol. Cell 8:473-479. [DOI] [PubMed] [Google Scholar]
- 61.Sun, Z. W., and C. D. Allis. 2002. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418:104-108. [DOI] [PubMed] [Google Scholar]
- 62.Vilardell, J., and J. R. Warner. 1997. Ribosomal protein L32 of Saccharomyces cerevisiae influences both the splicing of its own transcript and the processing of rRNA. Mol. Cell. Biol. 17:1959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waterborg, J. H. 1993. Dynamic methylation of alfalfa histone H3. J. Biol. Chem. 268:4918-4921. [PubMed] [Google Scholar]
- 64.Winston, F., C. Dollard, and S. L. Ricupero-Hovasse. 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53-55. [DOI] [PubMed] [Google Scholar]
- 65.Wood, A., J. Schneider, J. Dover, M. Johnston, and A. Shilatifard. 2003. The Paf1 complex is essential for histone monoubiquitination by the Rad6-Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J. Biol. Chem. 278:34739-34742. [DOI] [PubMed] [Google Scholar]
- 66.Xiao, T., C. F. Kao, N. J. Krogan, Z. W. Sun, J. F. Greenblatt, M. A. Osley, and B. D. Strahl. 2005. Histone H2B ubiquitylation is associated with elongating RNA polymerase II. Mol. Cell. Biol. 25:637-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yart, A., M. Gstaiger, C. Wirbelauer, M. Pecnik, D. Anastasiou, D. Hess, and W. Krek. 2005. The HRPT2 tumor suppressor gene product parafibromin associates with human PAF1 and RNA polymerase II. Mol. Cell. Biol. 25:5052-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu, B., S. S. Mandal, A. D. Pham, Y. Zheng, H. Erdjument-Bromage, S. K. Batra, P. Tempst, and D. Reinberg. 2005. The human PAF complex coordinates transcription with events downstream of RNA synthesis. Genes Dev. 19:1668-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]