Abstract
Phospholipase Cγ2 (PLCγ2) is critical for pre-B-cell receptor (pre-BCR) and BCR signaling. Current studies discovered that PLCγ2-deficient mice had reduced immunoglobulin λ (Igλ) light-chain usage throughout B-cell maturation stages, including transitional type 1 (T1), transitional type 2 (T2), and mature follicular B cells. The reduction of Igλ rearrangement by PLCγ2 deficiency was not due to specifically increased apoptosis or decreased proliferation of mutant Igλ+ B cells, as lack of PLCγ2 exerted a similar effect on apoptosis and proliferation of both Igλ+ and Igκ+ B cells. Moreover, PLCγ2-deficient IgHEL transgenic B cells exhibited an impairment of antigen-induced receptor editing among both the endogenous λ and κ loci in vitro and in vivo. Importantly, PLCγ2 deficiency impaired BCR-induced expression of IRF-4 and IRF-8, the two transcription factors critical for λ and κ light-chain rearrangements. Taken together, these data demonstrate that the PLCγ2 signaling pathway plays a role in activation of light-chain loci and contributes to receptor editing.
During B-cell development, pro-B cells begin the process of immunoglobulin (Ig) heavy (H) gene rearrangement (1, 8, 38, 50). Successful rearrangement of Ig H-chain variable (V), diversity (D), and joining (J) gene segments leads to the formation of the pre-B-cell receptor (pre-BCR), which contains the newly generated H chain in complex with the Vpre-B/λ5 surrogate light (L) chain. Signals from the pre-BCR instruct pre-B cells to expand and halt further H-chain VDJ rearrangement to ensure allelic exclusion (15, 30, 39). Subsequently, the rapidly proliferating pre-B cells exit the cell cycle and undergo rearrangement of Ig L-chain V and J gene segments. The Ig L chain is encoded by the κ and λ loci. Rearrangements within the κ and λ L-chain loci occur independently with a sequential activation of the κ and λ loci (2, 10, 39, 72). In mice, the κ locus comprises 70 to 90 functional Vκ and four functional Jκ gene segments whereas the λ locus contains three functional Vλ and three functional Jλ segments (2, 55). It is believed that the kinetics and efficiency of gene segment rearrangements as well as the number of functional Vκ and Vλ gene segments largely determine the ratio of Igκ+ to Igλ+ mature B cells (2, 10, 14, 72). In mice, the ratio is 95% κ to 5% λ (14, 61). A successfully rearranged L chain in combination with the previously rearranged H chain generates a surface IgM form of the BCR. B cells with a functional BCR quickly progress into immature B cells and emerge from bone marrow (BM) into the spleen (16, 20). In the spleen, signals emanating from the BCR direct immature B cells to mature through transitional B cells of type 1 (T1) and type 2 (T2) stages and thereafter to long-lived follicular B cells (34). Deletion of the BCR not only arrests B-cell development but also causes apoptosis (25, 27).
To establish B-cell tolerance, immature B cells expressing a self-reactive BCR are negatively selected during maturation by several distinct mechanisms, including clonal deletion, clonal anergy, and receptor editing (20, 42, 45, 49, 50, 56). The BCR signaling threshold appears to regulate the negative selection process. Highly self-reactive cells recognizing membrane-bound self antigens are eliminated by clonal deletion (17, 43), whereas self-reactive cells recognizing soluble self antigens become unresponsive to antigens, a state termed anergy (13). Moreover, self-reactive immature B cells can initiate new rearrangements of V genes, mainly at L-chain loci, to change receptor specificity by receptor editing (5, 7, 12, 48, 63). However, the mechanism that regulates the sequential activation of the κ and λ loci for recombination and the initiation of receptor editing in self-reactive B cells is not clear. Elevation of RAG1 and RAG2 mRNA levels has been observed during receptor editing in B cells (21, 36, 37). Although signals from the BCR have been shown to be involved (33, 67), the basis of this RAG up-regulation is not known. IRF-4 and IRF-8 are two members of the interferon regulatory factor (IRF) family of transcription factors whose expression can be induced by BCR engagement (9, 35, 62). Studies have implied important roles for both IRF-4 and IRF-8 in regulating L-chain rearrangements (4, 40, 46, 59). Importantly, lack of both IRF-4 and IRF-8 prevents rearrangements of Ig L-chain but not H-chain genes during B-cell development (31).
The BCR initiates signaling cascades via the two transmembrane molecules Igα and Igβ and sequential activation of members of three distinct families of cytoplasmic protein tyrosine kinases, including Lyn, Syk, and Btk (22, 24, 64). Subsequent recruitment and tyrosine phosphorylation of the adapter protein, B-cell linker protein (BLNK), and transmembrane protein CD19 facilitate activation of the lipid kinase phosphatidylinositol 3-kinase (22, 26, 44, 51). An important outcome of these events is activation of phospholipase Cγ2 (PLCγ2), which hydrolyzes phosphatidylinositol 4,5-bisphosphate to generate diacylglycerol and inositol 1,4,5-trisphosphate, both of which are required second messengers for cellular responses (53, 54). PLCγ2-deficient mice exhibit impaired early and late B-cell development, and PLCγ2-deficient B cells are unable to respond to antigens, demonstrating that PLCγ2 plays an essential role in B-cell development and function (18, 68-70). Our current studies demonstrate that PLCγ2 plays an important role in activation of the L-chain loci for recombination and receptor editing of self-reactive B cells.
MATERIALS AND METHODS
Mice.
PLCγ2-deficient mice were generated previously (68) and have been backcrossed to the C57BL/6 background. IgHEL transgenic mice (C57BL/6 MD4), which bear rearranged Ig H- and Igκ L-chain genes that encode a hen egg lysozyme (HEL)-specific BCR, and sHEL transgenic mice (C57BL/6 ML5), which express soluble HEL (sHEL), were obtained from the Jackson Laboratory. These transgenic mice were bred with heterozygous PLCγ2+/− mice to ultimately generate wild-type IgHEL, PLCγ2-deficient IgHEL, wild-type IgHEL sHEL, and PLCγ2-deficient IgHEL sHEL mice. Mice used for the experiments were generally 8 to 12 weeks of age except where specifically indicated.
Induction of receptor editing in vitro.
BM cells were isolated from the indicated mice and were depleted of red blood cells by lysis with Gey's solution (0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA). The cells (2 × 106 cells/ml) were cultured in complete medium RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 ng/ml interleukin-7 (IL-7) (R&D Systems) for 5 days. Then, cells were washed extensively with phosphate-buffered saline (PBS) and recultured at 1 × 106 cells/ml in complete medium without IL-7 and in the presence or absence of HEL (500 ng/ml) (lysozyme-biotin-caproyl; lot no. 071k1567; Sigma). After a further 2-day culture, the cells were harvested for analysis of λ chain protein expression and λ chain gene rearrangements.
Splenocytes (2 × 106/ml) from the indicated mice were cultured in complete medium in the presence or absence of freshly added HEL (500 ng/ml). After a 2-day culture, the cells were harvested for analysis of λ chain protein expression and λ chain gene rearrangements.
Detection of λ and κ chain gene rearrangements.
Genomic DNA was isolated from the indicated cells and was quantified by semiquantitative PCR amplification of the β-actin gene (5′ primer, ACTCCTATGTGGGTGACGAG; 3′ primer, CAGGTCCAGACGCAGGATGGC). The genomic DNA then was subjected to semiquantitative PCR analysis of endogenous λ or κ chain gene rearrangement. Primers employed in the PCR were the same as previously described (41, 73). Specifically, Vλ1/2 (AGAAGCTTGTGACTCAGGAATCTGCA) and Jλ1 (CAGGATCCTAGGACAGTCAGTTTGGT) primers were used to amplify λ1 rearrangements, Vλ2 (ACTGGTCTAATCGGTGGTACCAG) and Cλ2 (AGGAAGCTGCTGGCCATGAACTTGTTGC) primers were used to amplify λ2 rearrangements, and Vλx (GAGCTTAAGAAAGATGGAAGCCA) and Cλ2 primers were used to amplify λx rearrangements. The Vκ degenerate primer (GGCTGCAGSTTCAGTGGCAGTGGRTCW) and the reverse primer downstream of the Jκ1 coding region (GTTCTTTGCCTTGGAGAGTGCCAGAATC) were used to amplify V-Jκ1 rearrangement (11, 21). PCRs were performed as previously described (47, 65). Briefly, the PCR was carried out in a 25-μl final volume containing 0.5 μl of deoxynucleoside triphosphate (10 mM), 1 μl of the primers (10 μM), 2.5 μl of 10× PCR buffer, 5 μl 5× Q solution, 2.5 units of Taq enzyme (QIAGEN), and a serial dilution of template (25 to 1 ng). For detection of λ chain gene rearrangement, cycling conditions were 94°C for 4 min followed by 42 cycles of 94°C for 20 s, 60°C for 30 s, and 72°C for 90 s and a final extension step at 72°C for 5 min. For detection of V-Jκ1 rearrangement, cycling conditions were 97°C for 45 s, 70°C for 1 min, and 72°C for 2.5 min for five cycles; followed by 94°C for 45 s, 70°C for 1 min, and 72°C for 2.5 min for another 29 to 32 cycles; and final extension at 72°C for 6 min.
PCR detection of recombination sequence (RS) rearrangement in the genomic DNA from the indicated cells was performed as previously described (19). Briefly, the primers within the Jκ intron (CTGACTGCAGGTAGCGTGGTCTTCTAG) and downstream of the recombination signal sequence (RSS) (TTGACTGTTTGCTACTTCAGCTCACTG) were used. Cycling conditions were 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min for 35 cycles. Products were separated by electrophoresis in a 1% agarose gel, transferred to a nylon membrane, and hybridized with 32P-labeled 3′ primer. Genomic DNA was quantified by semiquantitative PCR amplification of the β-actin gene.
Flow cytometry.
Single-cell suspensions of spleen and BM cells were treated with Gey's solution to remove red blood cells. Freshly isolated or cultured cells were resuspended in PBS supplemented with 2% fetal bovine serum and then stained with a combination of fluorescence-conjugated antibodies. CyChrome-conjugated anti-B220 (150452) and allophycocyanin-conjugated anti-IgM (17-5790-82) antibodies were purchased from eBioscience. Phycoerythrin (PE)-conjugated anti-IgD (1120-09L), PE-conjugated anti-Igκ (1170-09), and PE-conjugated anti-Igλ (1175-09L) antibodies were purchased from Southern Biotechnology. Fluorescein isothiocyanate (FITC)-conjugated anti-Igλ (553434) antibodies were purchased from BD Biosciences. Samples were applied to a flow cytometer (LSRII; BD Biosciences), and data were collected and analyzed using CellQuest software (BD Biosciences). For all analyses, forward and side scatter gates were set to include viable cells and to exclude dead cells and debris.
TUNEL assay.
Single-cell suspensions of spleen and BM cells were stained with a combination of CyChrome-conjugated anti-B220 and PE-conjugated anti-Igλ or PE-conjugated anti-Igκ antibodies. Then, the cells were washed with PBS, fixed for 2 h in 4% paraformaldehyde in PBS at room temperature, permeabilized with 0.1% Triton X-100 and 0.1% sodium citrate for 2 min on ice, and labeled with FITC-conjugated terminal deoxynucleotidyltransferase dUTP nick-end labeling (TUNEL) enzyme for 1 h in moist darkness at 37°C according to the manufacturer's instructions (In Situ Cell Death Detection Kit; Roche). The degree of TUNEL positivity in the gated B220+ Igλ+ or B220+ Igκ+ subpopulation was analyzed by flow cytometry using CellQuest software.
BrdU incorporation assay.
The in vivo 5-bromo-2′-deoxyuridine (BrdU) staining assay was performed as previously described (71). Briefly, mice were injected intraperitoneally with 1 mg BrdU in 200 μl PBS at 12-h intervals for 5 days. Single-cell suspensions of spleen and BM cells were stained with a combination of CyChrome-conjugated anti-B220 and PE-conjugated anti-Igλ or PE-conjugated anti-Igκ antibodies at 4°C for 30 min. The cells were washed with PBS, resuspended in ice-cold 0.15 M saline, and incubated on ice for 30 min with the addition of 95% ethanol. After washing, the cells were fixed with 1% paraformaldehyde containing 0.01% Tween 20 for 1 h at room temperature. Lastly, the cells were incubated with 50 Kunitz units/ml DNase at 37°C for 10 min, washed with PBS, and stained with FITC-conjugated anti-BrdU antibody (34783; BD Biosciences). The degree of BrdU positivity in the gated B220+ Igλ+ or B220+ Igκ+ subpopulation was analyzed by flow cytometry using CellQuest software.
Real-time quantitative RT-PCR analysis.
Total RNA was isolated from the indicated cells, and first-strand cDNA was synthesized from the total RNA with the OneStep reverse transcription-PCR (RT-PCR) kit (210210; QIAGEN) using random primers (HFR704; Gibco) according to the manufacturer's protocols. Real-time PCR was performed to quantitate germ line transcription of Vκ and Vλ using the following primers (23, 57): κ germ line 5′ primer, AGGAGGGTTTTTGTACAGCCAGA; κ germ line 3′ primer, TGGATGGTGGGAAGATGGAT; λ2 germ line 5′ primer, GCTGTGAGAGAACAGGACCA; λ2 germ line 3′ primer, CTCGGGGAAAAGTTGGAAAT. Levels of Vκ and Vλ germ line transcription were normalized to β-actin abundance. The primers used for β-actin were as follows: 5′ primer, CCACAGCTGAGAGGGAAATC, and 3′ primer, CTTCTCCAGGGAGGAAGAGG. Real-time PCR was performed to analyze IRF-4 and IRF-8 gene expression using the following primers: IRF-4 5′ primer, AGATTCCAGGTGACTCTGTG; IRF-4 3′ primer, CTGCCCTGTCAGAGTATTTC; IRF-8 5′ primer, GGGAAGTTTAAAGAGGGAGA; IRF-8 3′ primer, GATCAGCTCCTCAATCTCTG. Levels of IRF-4 and IRF-8 transcript were normalized to CD19 abundance. The primers used for CD19 were as follows: 5′ primer, AATCCACGCATTCAAGTCCAG, and 3′ primer, GAGCCCTCCTCGCTGTCTG. Real-time PCR was carried out in duplicate or triplicate at 50°C for 2 min and then 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min in an iCycler-iQ (Bio-Rad) in 25-μl reaction volumes containing cDNA, primers, and IQ Supermix (170-8862; Bio-Rad). SYBR green was used as the detection reagent. Data were analyzed using standard curves generated for each sample by a series of four consecutive 10-fold dilutions (1 × 101 to 1 × 104) of the cDNA template and using iQ Cycler analyzing software and relative transcription calculated using the threshold cycle method. The specificity of the RT-PCR was controlled by the generation of melting curves, PCR efficiencies were 100% ± 15%, and correlation coefficients were 0.988 to 1.
RESULTS
PLCγ2-deficient mice have reduced Igλ L-chain usage throughout B-cell maturation.
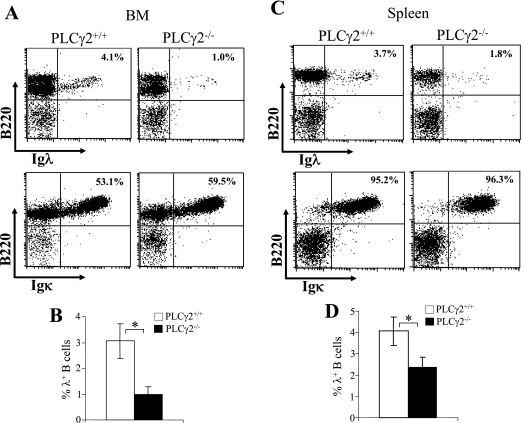
To determine whether PLCγ2 plays a role in L-chain gene rearrangement, we examined the expression of Igλ and Igκ L chains in PLCγ2-deficient mice, which were generated previously (68). First, BM cells were stained with an antibody specific for the Igλ or Igκ L-chain constant regions in conjunction with anti-B220 antibody. The proportion of BM B cells that expressed Igλ L chain was markedly reduced in PLCγ2-deficient mice relative to wild-type mice (Fig. 1A and B). In contrast, the proportion of BM B cells that expressed Igκ L chain was comparable or slightly increased in PLCγ2-deficient mice relative to wild-type mice (Fig. 1A). Next, the expression of Igλ or Igκ L chain in splenic B cells was examined. Again, the fraction of splenic B cells expressing Igλ was markedly reduced in PLCγ2-deficient mice relative to wild-type mice (Fig. 1C and D), whereas the fraction of splenic B cells expressing Igκ was comparable between PLCγ2-deficient and wild-type mice (Fig. 1C). Therefore, PLCγ2 deficiency reduces the subpopulation of B cells that express Igλ.
FIG. 1.
Reduction of Igλ chain expression in PLCγ2-deficient B cells. (A) BM cells were isolated from wild-type (PLCγ2+/+) and PLCγ2-deficient (PLCγ2−/−) mice and stained with a combination of anti-B220 and anti-Igλ or anti-Igκ antibodies. Percentages indicate Igλ+ or Igκ+ B cells in the gated live B220+ lymphoid population. Data are representative of 10 mice per genotype. (B) Statistical analysis of the percentages of Igλ+ B cells in the gated live BM B220+ lymphoid population from panel A (n = 10; *, P < 0.01). (C) Splenocytes were isolated from PLCγ2+/+ and PLCγ2−/− mice and stained with a combination of anti-B220 and anti-Igλ or anti-Igκ antibodies. Percentages indicate Igλ+ or Igκ+ B cells in the gated live B220+ lymphoid population. Data are representative of 10 mice per genotype. (D) Statistical analysis of the percentages of Igλ+ B cells in the gated live splenic B220+ lymphoid population from panel C (n = 10; *, P < 0.01).
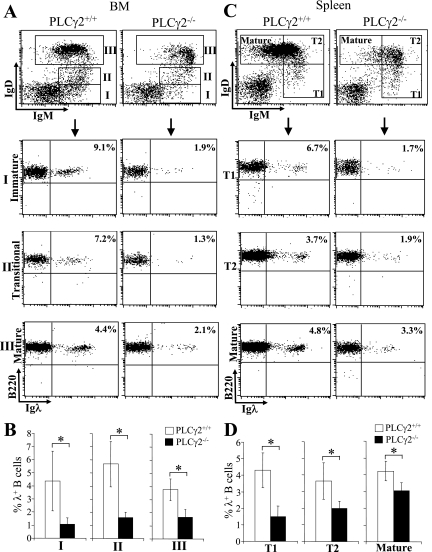
Moreover, we further determined the effect of PLCγ2 deficiency on the expression of Igλ during the whole process of B-cell maturation. Based on the expression of IgM and IgD, B-cell maturation can be divided into several stages (29, 34). Interestingly, the proportions of IgM+ IgD− immature B cells, IgM+ IgDlo transitional B cells, and IgM+ IgDhi mature B cells that expressed Igλ were all reduced in BM cells derived from PLCγ2-deficient mice relative to wild-type mice, with a more severe reduction in Igλ expression observed in B-cell subsets at earlier maturation stages (Fig. 2A and B). Similarly, a reduction of the proportion of cells expressing Igλ was observed in IgMhi IgD− T1, IgMhi IgD+ T2, and IgMlo IgD+ mature splenic B cells from PLCγ2-deficient mice relative to wild-type mice, with the reduction being more severe in the least-mature subpopulations (Fig. 2C and D). Taken together, these data demonstrate that PLCγ2 deficiency results in reduced Igλ usage throughout B-cell maturation.
FIG. 2.
Reduction of Igλ chain expression during B-cell development in PLCγ2-deficient mice. (A) BM cells were isolated from wild-type (PLCγ2+/+) and PLCγ2-deficient (PLCγ2−/−) mice and stained with a combination of anti-B220, anti-IgM, anti-IgD, and anti-Igλ antibodies. Based on expression of IgM and IgD, late BM B cells were defined as IgM+ IgD− immature B-cell (I), IgM+ IgDlow transitional B-cell (II), and IgM+ IgD+ mature B-cell (III) subpopulations (top). The expression of B220 and Igλ in the gated B-cell subpopulations (I, II, and III) was analyzed (bottom). Percentages indicate Igλ+ B cells in the gated B-cell subpopulation. Data are representative of eight mice per genotype. (B) Statistical analysis of the percentages of Igλ+ B cells in the gated B-cell subpopulation from panel A (n = 8; *, P < 0.01). (C) Splenocytes were isolated from wild-type and PLCγ2-deficient mice and stained with a combination of anti-B220, anti-IgM, anti-IgD, and anti-Igλ antibodies. Based on expression of IgM and IgD, splenic B cells were defined as IgMhi IgD− T1 B-cell (I), IgMhi IgD+ T2 B-cell (II), and IgMlo IgD+ mature B-cell (III) subpopulations (top). The expression of B220 and Igλ in the gated B-cell subpopulations (I, II, and III) was analyzed (bottom). Percentages indicate Igλ+ B cells in the gated B-cell subpopulation. Data are representative of eight mice per genotype. (D) Statistical analysis of the percentages of Igλ+ B cells in the gated B-cell subpopulation from panel C (n = 8; *, P < 0.01).
Reduced Igλ usage as a consequence of PLCγ2 deficiency is not due to increased apoptosis or decreased proliferation of PLCγ2-deficient Igλ+ relative to Igκ+ B cells.
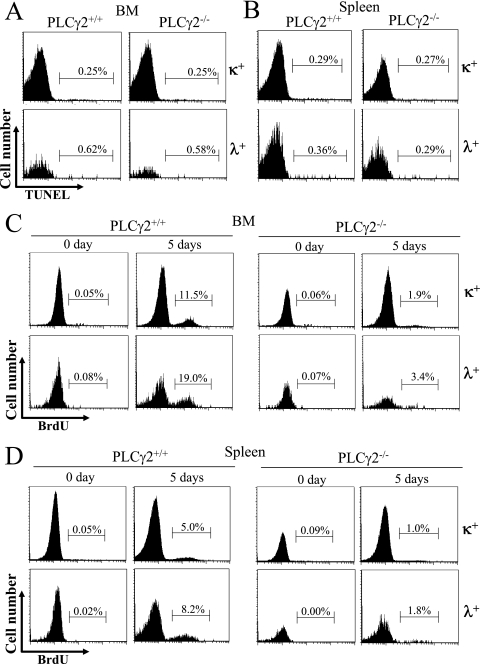
A possible explanation for the decreased frequency of Igλ+ B cells in PLCγ2-deficient mice relative to wild-type mice is that PLCγ2 deficiency specifically increases apoptosis or decreases proliferation of Igλ+ relative to Igκ+ B cells. To address this issue, we first used TUNEL staining to quantify the degree of apoptosis in Igλ+ and Igκ+ B cells from wild-type and PLCγ2-deficient mice. In freshly isolated BM, wild-type and PLCγ2-deficient Igκ+ B cells exhibited comparable fractions of TUNEL-positive cells (Fig. 3A). Similarly, PLCγ2-deficient and wild-type BM Igλ+ B cells also displayed comparable fractions of TUNEL-positive cells (Fig. 3A). Moreover, Igκ+ and Igλ+ B cells in the freshly harvested spleens of wild-type and PLCγ2-deficient mice had comparable fractions of TUNEL-positive cells (Fig. 3B). Therefore, PLCγ2 deficiency does not specifically increase apoptosis of Igλ+ B cells.
FIG. 3.
Comparable rates of apoptosis and proliferation of Igκ+ and Igλ+ B cells in wild-type and PLCγ2-deficient mice. Freshly isolated BM cells (A) and splenocytes (B) from wild-type (PLCγ2+/+) and PLCγ2-deficient (PLCγ2−/−) mice were stained with a combination of anti-B220 and anti-Igλ or anti-Igκ antibodies. Apoptotic cells were identified by TUNEL staining, and percentages indicate TUNEL-positive cells in the gated B220+ Igκ+ and B220+ Igλ+ BM or splenic B-cell populations. BM cells (C) and splenocytes (D) from wild-type (PLCγ2+/+) and PLCγ2-deficient (PLCγ2−/−) mice that were injected with BrdU every 12 h for 5 days (5 days) were stained with a combination of anti-BrdU, anti-B220, and anti-Igλ or anti-Igκ antibodies. Mice that were not injected with BrdU served as the baseline control (0 day). The degree of BrdU-positive proliferating cells in the gated B220+ Igκ+ or B220+ Igλ+ BM or splenic B-cell populations was determined by flow cytometry, and percentages indicate BrdU-positive cells in the gated cells. The figure shown is representative of five independent analyses.
Next, we compared the status of proliferation of Igλ+ and Igκ+ B cells in wild-type and PLCγ2-deficient mice. Following administration of BrdU for 5 days, BM was harvested from wild-type and PLCγ2-deficient mice. As expected, both PLCγ2-deficient BM Igκ+ and Igλ+ B cells incorporated markedly less BrdU than did the corresponding subpopulation of wild-type B cells (Fig. 3C). Similarly, both PLCγ2-deficient Igκ+ and Igλ+ splenic B cells incorporated clearly less BrdU than did the corresponding subpopulations of wild-type B cells (Fig. 3D). Thus, PLCγ2 deficiency impaired in vivo proliferation of both Igκ+ and Igλ+ B cells to a similar extent (Fig. 3C and D) and did not specifically decrease proliferation of Igλ+ B cells. Taken together, we conclude that reduced Igλ usage by PLCγ2-deficient B cells is not due to increased apoptosis or decreased proliferation of PLCγ2-deficient Igλ+ B cells.
PLCγ2 plays a role in receptor editing.
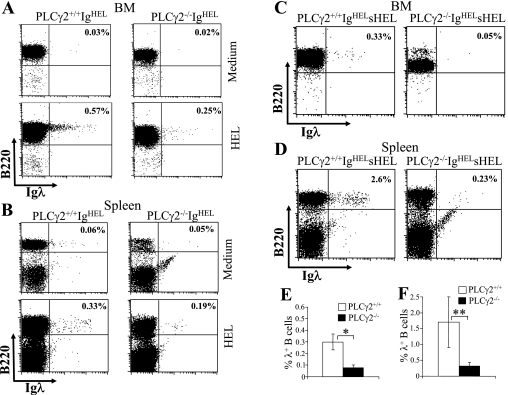
Receptor editing involves new Ig gene rearrangements, particularly at L-chain loci, and is accompanied by increased Igλ usage (5, 52, 63). The impaired Igλ usage by PLCγ2-deficient B cells prompted us to examine the role of PLCγ2 in receptor editing. PLCγ2-deficient mice were crossed with IgHEL transgenic mice, which bear rearranged H- and Igκ L-chain genes encoding a BCR that specifically recognizes HEL (13). BM cells from wild-type or PLCγ2-deficient IgHEL transgenic mice were cultured for 5 days with IL-7 to expand the virtually uniform B-cell population that expresses the IgHEL BCR (data not shown). Consistent with a previous study (65), BM-derived (Fig. 4A) and freshly isolated splenic (Fig. 4B) IgHEL B cells from wild-type transgenic mice underwent receptor editing and rearranged endogenous L-chain genes in response to HEL stimulation in vitro, resulting in a detectable fraction of cells expressing Igλ. In contrast, following HEL stimulation, BM-derived (Fig. 4A) and freshly isolated splenic (Fig. 4B) IgHEL B cells from PLCγ2-deficient mice exhibited a small but constant reduction in the population of cells expressing Igλ compared to wild-type cells. Of note, both the wild-type and PLCγ2-deficient BM-derived (Fig. 4A) and freshly isolated splenic (Fig. 4B) IgHEL B cells exhibited low background expression of endogenous Igλ when cultured with medium alone. Therefore, PLCγ2 deficiency reduces antigen-induced expression of Igλ associated with receptor editing in vitro.
FIG. 4.
Impairment of antigen-induced receptor editing in PLCγ2-deficient B cells. Antigen-induced receptor editing is impaired in PLCγ2-deficient BM-derived (A) and splenic (B) IgHEL B cells in vitro. BM cells from wild-type or PLCγ2-deficient IgHEL transgenic mice were initially cultured for 5 days with IL-7, and splenocytes were isolated from wild-type or PLCγ2-deficient IgHEL transgenic mice. The cells were then recultured without IL-7 and in the absence (medium) or presence (HEL) of HEL. After a further 2-day culture, the cells were harvested and stained with anti-B220 and anti-Igλ antibodies. Percentages indicate Igλ+ cells in the gated B220+ B lymphocytes. Data are representative of three independent experiments. Antigen-induced receptor editing is impaired in PLCγ2-deficient BM (C) and splenic (D) IgHEL B cells in vivo. BM cells from 21-week-old wild-type or PLCγ2-deficient IgHEL sHEL mice were stained with anti-B220, anti-IgM, and anti-Igλ antibodies. Fluorescence-activated cell sorting analysis with B220 and Igλ staining of IgM+ gated cells is shown. Splenocytes from 21-week-old wild-type or PLCγ2-deficient IgHEL sHEL mice were stained with anti-B220 and anti-Igλ antibodies. Percentages indicate Igλ+ cells in the gated B220+ B lymphocytes. Data are representative of three independent analyses. (E) Statistical analysis of the percentages of Igλ+ B cells in the gated live BM IgM+ B220+ lymphoid population from panel C (n = 3; *, P < 0.01). (F) Statistical analysis of the percentages of Igλ+ B cells in the gated live splenic B220+ lymphoid population from panel D (n = 3; **, P < 0.05).
Importantly, the effect of PLCγ2 deficiency on receptor editing was also confirmed in vivo. When wild-type IgHEL transgenic mice were bred with transgenic mice expressing sHEL, sHEL induced receptor editing and resulted in expression of Igλ in BM and splenic B cells of 21-week-old mice (Fig. 4C to F). Strikingly, PLCγ2-deficient IgHEL sHEL mice of the same age displayed a dramatic reduction in the fraction of BM and splenic B cells expressing Igλ relative to wild-type controls (Fig. 4C to F). Thus, PLCγ2 deficiency impairs antigen-induced expression of Igλ, which is associated with receptor editing in vivo.
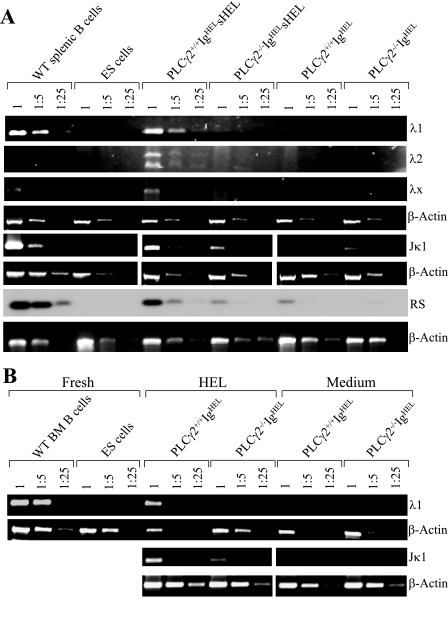
Impaired antigen-induced rearrangement of endogenous Igλ associated with PLCγ2 deficiency in vivo was further confirmed at the level of genomic DNA. Genomic DNA was isolated from wild-type IgHEL, PLCγ2-deficient IgHEL, wild-type IgHEL sHEL, and PLCγ2-deficient IgHEL sHEL mice, and levels of endogenous Igλ gene rearrangements were determined by semiquantitative PCR. Splenic B cells from wild-type mice served as a positive control, in which rearrangements of V-Jλ1, V-Jλ2, and V-Jλx were easily detectable (Fig. 5A). Embryonic stem (ES) cells served as a negative control, in which rearrangements of V-Jλ1, V-Jλ2, and V-Jλx were not detectable (Fig. 5A). As expected, both wild-type IgHEL and PLCγ2-deficient IgHEL splenic B cells had barely detectable levels of rearrangements of endogenous V-Jλ1, V-Jλ2, and V-Jλx (Fig. 5A). Interestingly, whereas splenic B cells from wild-type IgHEL sHEL mice exhibited apparent rearrangements of endogenous V-Jλ1, V-Jλ2, and V-Jλx, such rearrangements were not detectable in PLCγ2-deficient IgHEL sHEL splenic B cells (Fig. 5A).
FIG. 5.
Impairment of antigen-induced receptor editing among the endogenous λ and κ loci in PLCγ2-deficient IgHEL B cells. (A) Impairment of antigen-induced receptor editing among the endogenous λ and κ loci in PLCγ2-deficient splenic IgHEL B cells in vivo. Genomic DNA was isolated from the splenocytes derived from wild-type IgHEL, PLCγ2-deficient IgHEL, wild-type IgHEL sHEL, or PLCγ2-deficient IgHEL sHEL mice. Subsequently, the genomic DNA was subjected to a semiquantitative PCR analysis of the β-actin gene; of endogenous λ1, λ2, λx, and Jκ1 rearrangements, and of RS rearrangement. Genomic DNA of splenic B cells from wild-type mice served as a positive control, whereas genomic DNA from ES cells served as a negative control. Data are representative of three independent experiments for λ rearrangements and two independent experiments for Jκ1 and RS rearrangements. (B) Impairment of antigen-induced rearrangements of endogenous λ and κ chain in PLCγ2-deficient BM-derived IgHEL B cells in vitro. BM cells from wild-type or PLCγ2-deficient IgHEL transgenic mice were cultured for 5 days with IL-7 to expand into a B-cell population that expresses the IgHEL BCR. Following further stimulation with (HEL) or without (medium) HEL for 2 days, genomic DNA was isolated from the cells and subsequently subjected to a semiquantitative PCR analysis of the β-actin gene and of endogenous λ1 and Jκ1 rearrangements. Genomic DNA of BM B cells from wild-type mice served as a positive control, whereas genomic DNA from ES cells served as a negative control. Data are representative of three independent experiments for λ rearrangements and two independent experiments for Jκ1 rearrangement. WT, wild type.
Further, we examined the effect of PLCγ2 deficiency on antigen-induced rearrangement of endogenous Igκ, which is also associated with receptor editing in vivo. IgHEL transgenic mice have a rearranged V-Jκ2 transgene (60). Thus, we examined the rearrangement of endogenous V-Jκ1 in genomic DNA derived from wild-type IgHEL, PLCγ2-deficient IgHEL, wild-type IgHEL sHEL, and PLCγ2-deficient IgHEL sHEL mice by semiquantitative PCR. Both wild-type IgHEL and PLCγ2-deficient IgHEL splenic B cells had low levels of rearrangements of endogenous V-Jκ1 (Fig. 5A). Notably, antigen-induced rearrangement of endogenous V-Jκ1 was reduced in splenic B cells derived from PLCγ2-deficient IgHEL sHEL mice relative to wild-type controls (Fig. 5A). Moreover, RS rearrangement between an intron RS within the Jκ-Cκ intron and an RSS located downstream of Cκ, a critical mechanism for the receptor editing of Igκ (52), was reduced in splenic B cells derived from PLCγ2-deficient IgHEL sHEL mice relative to wild-type controls (Fig. 5A). Therefore, PLCγ2 contributes not only to Igλ but also to Igκ rearrangement.
Impaired antigen-induced rearrangements of Igλ and Igκ genes in PLCγ2-deficient B cells were also confirmed at genomic DNA levels in an in vitro receptor editing experiment. BM cells from wild-type or PLCγ2-deficient IgHEL transgenic mice were cultured for 5 days with IL-7 to expand the uniform B-cell population that expresses the IgHEL BCR. Following further stimulation with HEL or medium, genomic DNA was isolated from the cells and levels of endogenous gene Igλ and Igκ rearrangements were determined by semiquantitative PCR. BM B cells from wild-type mice served as a positive control, in which V-Jλ1 rearrangements were easily detectable (Fig. 5B). ES cells served as a negative control, in which V-Jλ1 rearrangements were not detectable (Fig. 5B). Both wild-type and PLCγ2-deficient BM-derived IgHEL B cells had low background levels of rearrangements of endogenous Igλ and Igκ genes when cultured with medium alone (Fig. 5B). Upon HEL stimulation, wild-type BM-derived IgHEL B cells underwent receptor editing such that rearrangements of endogenous V-Jλ1 and V-Jκ1 gene were easily detectable (Fig. 5B), consistent with a previous study (65). In contrast, PLCγ2-deficient BM-derived IgHEL B cells displayed a barely detectable level of rearrangement of V-Jλ1 and a reduced level of rearrangements of V-Jκ1 following HEL stimulation (Fig. 5B). These genomic data further confirm that PLCγ2 deficiency impairs activation of Igλ and Igκ locus. Taken together, our results demonstrate that PLCγ2 plays a role in rearrangements of Igλ and Igκ, which are associated with receptor editing.
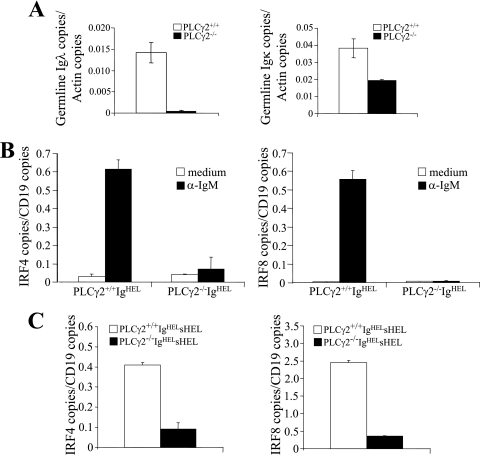
Ig L recombination requires accessibility of both the V region and J region to the recombination machinery, and germ line transcription of Vλ and Vκ positively correlates with their accessibility (3). Thus, we compared germ line transcription of Vλ and Vκ genes in wild-type and PLCγ2-deficient pro/pre-B cells. B220low IgM− pro/pre-B cells were sorted out from BM of wild-type or PLCγ2-deficient mice. The levels of Vλ and Vκ germ line transcription in these cells were quantitated by real-time PCR. The level of Vλ germ line transcription was markedly decreased in PLCγ2-deficient pro/pre-B cells compared to wild-type controls (Fig. 6A). Of note, the level of Vκ germ line transcription was also decreased in PLCγ2-deficient pro/pre-B cells compared to wild-type controls (Fig. 6A). Thus, consistent with an important role for PLCγ2 in rearrangements of Igλ and Igκ, PLCγ2 deficiency reduces the germ line transcription of both Vλ and Vκ genes, especially the former.
FIG. 6.
Impairment of germ line transcription of light-chain genes and BCR-mediated expression of IRF-4/IRF-8 in PLCγ2-deficient B cells. (A) Reduction of germ line transcription of λ and κ chain in PLCγ2-deficient pro/pre-B cells. The pro/pre-B (B220low IgM−) cells were sorted from BM of 4-month-old PLCγ2+/+ and PLCγ2−/− mice. Then, total RNA was isolated from the cells and subjected to real-time RT-PCR analysis of germ line transcription of λ and κ chain. Data are a combination of results from two independent experiments. (B) BCR-induced expression of IRF-4 and IRF-8 is impaired in PLCγ2-deficient BM-derived IgHEL B cells in vitro. BM cells were isolated from wild-type (PLCγ2+/+ IgHEL) and PLCγ2-deficient (PLCγ2−/− IgHEL) IgHEL mice and were initially cultured with IL-7 for 5 days. The cells were then stimulated with (α-IgM) or without (medium) anti-IgM for 2 h. Total RNA was isolated from the cells and subjected to real-time RT-PCR analysis of IRF-4 and IRF-8 gene expression. The figures shown are representative of three independent experiments. (C) Impairment of BCR-induced expression of IRF-4 and IRF-8 in PLCγ2-deficient splenic IgHEL B cells in vivo. Splenic B cells were isolated from wild-type and PLCγ2-deficient IgHEL sHEL mice. Total RNA was isolated from the cells and subjected to real-time RT-PCR analysis of IRF-4 and IRF-8 gene expression. The figure shown is representative of four independent experiments.
PLCγ2 deficiency impairs BCR-induced expression of IRF-4 and IRF-8.
IRF-4 and IRF-8 are transcription factors whose expression can be induced by BCR engagement (9, 35, 62). Both transcription factors play essential roles in rearrangement of Ig L-chain genes during B-cell development, and their deficiencies block rearrangement of κ and λ chain genes (31). Thus, we examined the effect of PLCγ2 deficiency on BCR-mediated induction of IRF-4 and IRF-8 expression. BM cells from wild-type or PLCγ2-deficient IgHEL transgenic mice were cultured for 5 days with IL-7 to expand the IgHEL BCR-expressing uniform B-cell progenitors. Cross-linking of the BCR dramatically induced expression of IRF-4 and IRF-8 in wild-type BM-derived IgHEL B cells (Fig. 6B). In contrast, BCR-induced expression of IRF-4 and IRF-8 was markedly impaired in PLCγ2-deficient BM-derived IgHEL B cells (Fig. 6B). In addition, we examined the effect of PLCγ2 deficiency on BCR engagement-induced expression of IRF-4 and IRF-8 in vivo. Splenic B cells were isolated from wild-type or PLCγ2-deficient IgHEL transgenic mice that also express sHEL, and expression of IRF-4 and IRF-8 in these cells was determined by real-time RT-PCR. The expression of IRF-4 and IRF-8 was markedly reduced in PLCγ2-deficient IgHEL sHEL B cells compared to wild-type IgHEL sHEL splenic B cells (Fig. 6C). Thus, PLCγ2 deficiency severely impairs antigen-induced expression of IRF-4 and IRF-8 both in vitro and in vivo.
DISCUSSION
Although previous studies have shown that PLCγ2 plays an important role in early B-cell development at the pro-B to pre-B transition and is essential for maturation of T2 to follicular B cells (68-70), our current studies discovered that PLCγ2 deficiency impairs rearrangements of Igλ and Igκ L-chain genes, resulting in an impairment of antigen-induced receptor editing. Although the impairment of Igκ rearrangement is not obvious in nontransgenic PLCγ2-deficient mice, it is clearly detected in the PLCγ2-deficient IgHEL sHEL transgenic mice. Previous studies have demonstrated that rearrangements of Igκ and Igλ occur independently with different kinetics, such that the κ locus is activated and the λ locus is not subsequently activated until rearrangements of κ genes fail to yield a functional Igκ L chain (2, 10, 39, 72). A different regulation of the activation of these two loci for recombination might explain that differential effect of PLCγ2 deficiency on the κ versus the λ locus. PLCγ2 provides an important signal involved in the activation of both the λ and κ loci, although the signal seems to be more essential for the activation of λ. Interestingly, the two signaling molecules that are upstream of PLCγ2 in BCR signaling, the adapter molecule BLNK and the tyrosine kinase Btk, have also been shown to play roles in the activation of the L-chain loci (6, 19). Similar to deficiency of PLCγ2, lack of BLNK impairs the activation of both Igλ and Igκ loci, leading to an impairment of antigen-induced receptor editing (19). In contrast, Btk deficiency has no effect on antigen-induced receptor editing, although it severely reduces the expression of Igλ chain (6). One possible explanation is that PLCγ2 and BLNK initiate additional Btk-independent signals that are required for receptor editing.
The ratio of Igκ+ versus Igλ+ mature B cells is ultimately determined by the kinetics and efficiency of activation of κ and λ loci, the number of functional Vκ and Vλ gene segments, and the survival and proliferation rates of B cells that have undergone L-chain gene rearrangement (28, 72). Although PLCγ2 is known to be involved in the survival and proliferation of transitional and mature B cells (68, 70), our analysis of apoptosis and proliferation rates in Igλ+ and Igκ+ B-cell populations derived from wild-type and PLCγ2−/− mice demonstrates that PLCγ2 deficiency does not specifically increase apoptosis or decrease proliferation of Igλ+ B cells relative to Igκ+ B cells. Therefore, the reduced usage of Igλ relative to Igκ in nontransgenic PLCγ2-deficient B cells is not due to a shortened life span or decreased proliferative capacity of Igλ+ B cells relative to Igκ+ B cells. Instead, the reduced usage of Igλ by PLCγ2-deficient B cells appears to be due solely to the absence of signals from PLCγ2 that differentially regulate activation of the Igλ and Igκ loci. Consistently, overexpression of the antiapoptotic molecule Bcl-2 protects Btk-deficient B cells from apoptosis but does not alter the frequency of Igλ expression in the mutant B cells (6).
Binding of self antigen to self-reactive B cells can initiate new Ig gene rearrangements, especially at L-chain loci, to replace a self-reactive receptor with a new receptor (5, 7, 12, 48, 63). The BCR signaling pathway that specifically regulates receptor editing is not clear, although BLNK has been shown to play an important role in receptor editing (19). Although development of anergy is a dominant phenotype in the IgHEL sHEL transgenic mode, this model can be used to study receptor editing (66). Previous studies have demonstrated that sHEL is able to induce autoreactive IgHEL B cells to undergo receptor editing in vitro (65, 66). Consistent with these previous studies, we found that both BM culture-derived and primary splenic B cells from wild-type IgHEL mice undergo sHEL-induced Igλ expression in vitro. However, sHEL-induced expression of Igλ is severely impaired in PLCγ2−/− IgHEL B cells. Expression of sHEL as a “self antigen” in mice that express IgHEL might be expected to induce editing of the “autoreactive” IgHEL receptor and consequent expression of endogenous Igλ in vivo. Whereas a previous study failed to detect elevated levels of Igλ expression in B cells from young (<12 weeks of age) IgHEL sHEL double-transgenic mice in vivo (65), we constantly observed Igλ expression in B cells from older (>13 weeks of age), but not younger (data not shown), IgHEL sHEL double-transgenic mice in vivo. Importantly, Igλ expression was much lower in B cells from older PLCγ2-deficient mice than in B cells from wild-type IgHEL sHEL double-transgenic mice. Taken together, our results show that PLCγ2 signaling downstream of BCR engagement plays a role in receptor editing both in vitro and in vivo.
IRF-4 and IRF-8 are two members of the IRF family of transcription factors that are essential for down-regulation of both surrogate L-chain expression and rearrangement of Igκ and Igλ chain genes during B-cell development (32). In the absence of IRF-4 and IRF-8, rearrangement of both Igκ and Igλ chain genes is blocked (32). We show in the present studies that the PLCγ2 pathway plays a critical role in BCR-induced expression of IRF-4 and IRF-8. Nevertheless, although PLCγ2 deficiency severely impairs up-regulation of both transcription factors, it more severely impairs rearrangement of Igλ than Igκ chain genes in nontransgenic mice. One possible explanation for this observation is that high levels of IRF-4 and IRF-8 may be required for Igλ chain gene rearrangement whereas low levels of IRF-4 and IRF-8 may be sufficient for Igκ chain gene rearrangement. Thus, the complete absence of IRF-4 and IRF-8 caused by targeted gene disruption would be expected to block rearrangement of both Igκ and Igλ chain genes, whereas reduced expression of IRF-4 and IRF-8, for example as a consequence of PLCγ2 deficiency, would be expected to affect rearrangement of Igλ more than that of Igκ. In fact, a recent study has demonstrated that IRF-4 is expressed in a graded manner in differentiating B cells and that different concentrations of IRF-4 regulate expression of distinct genes that coordinate isotype switching during plasma cell differentiation (58). Moreover, both IRF-4 and IRF-8 are essential for germ line transcription of Vκ and Vλ (31). Levels of germ line transcription of Vκ and Vλ positively correlate with their accessibility to the recombination machinery (3). The impaired expression of IRF-4 and IRF-8 as a consequence of PLCγ2 deficiency should contribute to the reduction of the germ line transcription of Vκ and particularly Vλ genes. Nonetheless, further studies are required to fully understand the mechanism by which the PLCγ2 pathway regulates L-chain gene rearrangement and receptor editing.
Acknowledgments
This work is supported in part by NIH grants R01 AI52327 (R.W.) and R01 HL073284 (D.W.) and by American Cancer Society grant RSG CCG-106204 (D.W.).
We gratefully acknowledge the help from Guoping Fu, Yang Xu, Matthew A. Inlay, and Tongxiang Lin. We are grateful to Harinder Singh for providing primers for real-time PCR analysis of IRF-4 and IRF-8. We thank Debra K. Newman for critical review of the manuscript and for helpful discussion.
Footnotes
Published ahead of print on 25 June 2007.
REFERENCES
- 1.Alt, F. W., T. K. Blackwell, and G. D. Yancopoulos. 1987. Development of the primary antibody repertoire. Science 238:1079-1087. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa, H., T. Shimizu, and S. Takeda. 1996. Re-evaluation of the probabilities for productive arrangements on the kappa and lambda loci. Int. Immunol. 8:91-99. [DOI] [PubMed] [Google Scholar]
- 3.Bassing, C. H., W. Swat, and F. W. Alt. 2002. The mechanism and regulation of chromosomal V(D)J recombination. Cell 109:S45-S55. [DOI] [PubMed] [Google Scholar]
- 4.Brass, A. L., A. Q. Zhu, and H. Singh. 1999. Assembly requirements of PU.1-Pip (IRF-4) activator complexes: inhibiting function in vivo using fused dimers. EMBO J. 18:977-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casellas, R., T. A. Shih, M. Kleinewietfeld, J. Rakonjac, D. Nemazee, K. Rajewsky, and M. C. Nussenzweig. 2001. Contribution of receptor editing to the antibody repertoire. Science 291:1541-1544. [DOI] [PubMed] [Google Scholar]
- 6.Dingjan, G. M., S. Middendorp, K. Dahlenborg, A. Maas, F. Grosveld, and R. W. Hendriks. 2001. Bruton's tyrosine kinase regulates the activation of gene rearrangements at the lambda light chain locus in precursor B cells in the mouse. J. Exp. Med. 193:1169-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edry, E., and D. Melamed. 2004. Receptor editing in positive and negative selection of B lymphopoiesis. J. Immunol. 173:4265-4271. [DOI] [PubMed] [Google Scholar]
- 8.Ehlich, A., and R. Kuppers. 1995. Analysis of immunoglobulin gene rearrangements in single B cells. Curr. Opin. Immunol. 7:281-284. [DOI] [PubMed] [Google Scholar]
- 9.Eisenbeis, C. F., H. Singh, and U. Storb. 1995. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 9:1377-1387. [DOI] [PubMed] [Google Scholar]
- 10.Engel, H., A. Rolink, and S. Weiss. 1999. B cells are programmed to activate kappa and lambda for rearrangement at consecutive developmental stages. Eur. J. Immunol. 29:2167-2176. [DOI] [PubMed] [Google Scholar]
- 11.Fang, W., B. C. Weintraub, B. Dunlap, P. Garside, K. A. Pape, M. K. Jenkins, C. C. Goodnow, D. L. Mueller, and T. W. Behrens. 1998. Self-reactive B lymphocytes overexpressing Bcl-xL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity 9:35-45. [DOI] [PubMed] [Google Scholar]
- 12.Gay, D., T. Saunders, S. Camper, and M. Weigert. 1993. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 177:999-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodnow, C. C., J. Crosbie, S. Adelstein, T. B. Lavoie, S. J. Smith-Gill, R. A. Brink, H. Pritchard-Briscoe, J. S. Wotherspoon, R. H. Loblay, and K. Raphael. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334:676-682. [DOI] [PubMed] [Google Scholar]
- 14.Gorman, J. R., N. van der Stoep, R. Monroe, M. Cogne, L. Davidson, and F. W. Alt. 1996. The Ig(kappa) enhancer influences the ratio of Ig(kappa) versus Ig(lambda) B lymphocytes. Immunity 5:241-252. [DOI] [PubMed] [Google Scholar]
- 15.Grawunder, U., T. M. Leu, D. G. Schatz, A. Werner, A. G. Rolink, F. Melchers, and T. H. Winkler. 1995. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity 3:601-608. [DOI] [PubMed] [Google Scholar]
- 16.Hardy, R. R., and K. Hayakawa. 2001. B cell development pathways. Annu. Rev. Immunol. 19:595-621. [DOI] [PubMed] [Google Scholar]
- 17.Hartley, S. B., J. Crosbie, R. Brink, A. B. Kantor, A. Basten, and C. C. Goodnow. 1991. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 353:765-769. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto, A., K. Takeda, M. Inaba, M. Sekimata, T. Kaisho, S. Ikehara, Y. Homma, S. Akira, and T. Kurosaki. 2000. Cutting edge: essential role of phospholipase C-gamma 2 in B cell development and function. J. Immunol. 165:1738-1742. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi, K., T. Nojima, R. Goitsuka, and D. Kitamura. 2004. Impaired receptor editing in the primary B cell repertoire of BASH-deficient mice. J. Immunol. 173:5980-5988. [DOI] [PubMed] [Google Scholar]
- 20.Healy, J. I., and C. C. Goodnow. 1998. Positive versus negative signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 16:645-670. [DOI] [PubMed] [Google Scholar]
- 21.Hertz, M., and D. Nemazee. 1997. BCR ligation induces receptor editing in IgM+IgD− bone marrow B cells in vitro. Immunity 6:429-436. [DOI] [PubMed] [Google Scholar]
- 22.Hombach, J., T. Tsubata, L. Leclercq, H. Stappert, and M. Reth. 1990. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature 343:760-762. [DOI] [PubMed] [Google Scholar]
- 23.Inlay, M. A., T. Lin, H. H. Gao, and Y. Xu. 2006. Critical roles of the immunoglobulin intronic enhancers in maintaining the sequential rearrangement of IgH and Igk loci. J. Exp. Med. 203:1721-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karasuyama, H., A. Kudo, and F. Melchers. 1990. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J. Exp. Med. 172:969-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 26.Kurosaki, T. 1999. Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol. 17:555-592. [DOI] [PubMed] [Google Scholar]
- 27.Lam, K. P., R. Kuhn, and K. Rajewsky. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90:1073-1083. [DOI] [PubMed] [Google Scholar]
- 28.Lang, J., B. Arnold, G. Hammerling, A. W. Harris, S. Korsmeyer, D. Russell, A. Strasser, and D. Nemazee. 1997. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells. J. Exp. Med. 186:1513-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loder, F., B. Mutschler, R. J. Ray, C. J. Paige, P. Sideras, R. Torres, M. C. Lamers, and R. Carsetti. 1999. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J. Exp. Med. 190:75-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loffert, D., A. Ehlich, W. Muller, and K. Rajewsky. 1996. Surrogate light chain expression is required to establish immunoglobulin heavy chain allelic exclusion during early B cell development. Immunity 4:133-144. [DOI] [PubMed] [Google Scholar]
- 31.Lu, R., K. L. Medina, D. W. Lancki, and H. Singh. 2003. IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev. 17:1703-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu, Y. F., M. Singh, and J. Cerny. 2001. Canonical germinal center B cells may not dominate the memory response to antigenic challenge. Int. Immunol. 13:643-655. [DOI] [PubMed] [Google Scholar]
- 33.Ma, A., P. Fisher, R. Dildrop, E. Oltz, G. Rathbun, P. Achacoso, A. Stall, and F. W. Alt. 1992. Surface IgM mediated regulation of RAG gene expression in E mu-N-myc B cell lines. EMBO J. 11:2727-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin, F., and J. F. Kearney. 2001. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 13:195-201. [DOI] [PubMed] [Google Scholar]
- 35.Matsuyama, T., A. Grossman, H. W. Mittrucker, D. P. Siderovski, F. Kiefer, T. Kawakami, C. D. Richardson, T. Taniguchi, S. K. Yoshinaga, and T. W. Mak. 1995. Molecular cloning of LSIRF, a lymphoid-specific member of the interferon regulatory factor family that binds the interferon-stimulated response element (ISRE). Nucleic Acids Res. 23:2127-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melamed, D., R. J. Benschop, J. C. Cambier, and D. Nemazee. 1998. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell 92:173-182. [DOI] [PubMed] [Google Scholar]
- 37.Melamed, D., and D. Nemazee. 1997. Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc. Natl. Acad. Sci. USA 94:9267-9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melchers, F., E. ten Boekel, T. Seidl, X. C. Kong, T. Yamagami, K. Onishi, T. Shimizu, A. G. Rolink, and J. Andersson. 2000. Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells. Immunol. Rev. 175:33-46. [PubMed] [Google Scholar]
- 39.Melchers, F., E. ten Boekel, T. Yamagami, J. Andersson, and A. Rolink. 1999. The roles of preB and B cell receptors in the stepwise allelic exclusion of mouse IgH and L chain gene loci. Semin. Immunol. 11:307-317. [DOI] [PubMed] [Google Scholar]
- 40.Muljo, S. A., and M. S. Schlissel. 2003. A small molecule Abl kinase inhibitor induces differentiation of Abelson virus-transformed pre-B cell lines. Nat. Immunol. 4:31-37. [DOI] [PubMed] [Google Scholar]
- 41.Nadel, B., A. M. Drapier, P. A. Cazenave, and P. Sanchez. 1993. Available lambda B cell repertoire in the mouse: evidence of positive selection by environmental factors. Eur. J. Immunol. 23:537-543. [DOI] [PubMed] [Google Scholar]
- 42.Nemazee, D. 2000. Receptor selection in B and T lymphocytes. Annu. Rev. Immunol. 18:19-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nemazee, D. A., and K. Burki. 1989. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature 337:562-566. [DOI] [PubMed] [Google Scholar]
- 44.Niiro, H., and E. A. Clark. 2002. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2:945-956. [DOI] [PubMed] [Google Scholar]
- 45.Nossal, G. J. 1994. Negative selection of lymphocytes. Cell 76:229-239. [DOI] [PubMed] [Google Scholar]
- 46.Pongubala, J. M., S. Nagulapalli, M. J. Klemsz, S. R. McKercher, R. A. Maki, and M. L. Atchison. 1992. PU.1 recruits a second nuclear factor to a site important for immunoglobulin κ 3′ enhancer activity. Mol. Cell. Biol. 12:368-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prak, E. L., M. Trounstine, D. Huszar, and M. Weigert. 1994. Light chain editing in kappa-deficient animals: a potential mechanism of B cell tolerance. J. Exp. Med. 180:1805-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radic, M. Z., J. Erikson, S. Litwin, and M. Weigert. 1993. B lymphocytes may escape tolerance by revising their antigen receptors. J. Exp. Med. 177:1165-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radic, M. Z., and M. Zouali. 1996. Receptor editing, immune diversification, and self-tolerance. Immunity 5:505-511. [DOI] [PubMed] [Google Scholar]
- 50.Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature 381:751-758. [DOI] [PubMed] [Google Scholar]
- 51.Reth, M., J. Wienands, and W. W. Schamel. 2000. An unsolved problem of the clonal selection theory and the model of an oligomeric B-cell antigen receptor. Immunol. Rev. 176:10-18. [DOI] [PubMed] [Google Scholar]
- 52.Retter, M. W., and D. Nemazee. 1998. Receptor editing occurs frequently during normal B cell development. J. Exp. Med. 188:1231-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhee, S. G., and Y. S. Bae. 1997. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 272:15045-15048. [DOI] [PubMed] [Google Scholar]
- 54.Rhee, S. G., and K. D. Choi. 1992. Regulation of inositol phospholipid-specific phospholipase C isozymes. J. Biol. Chem. 267:12393-12396. [PubMed] [Google Scholar]
- 55.Sanchez, P., B. Nadel, and P. A. Cazenave. 1991. V lambda-J lambda rearrangements are restricted within a V-J-C recombination unit in the mouse. Eur. J. Immunol. 21:907-911. [DOI] [PubMed] [Google Scholar]
- 56.Sandel, P. C., and J. G. Monroe. 1999. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity 10:289-299. [DOI] [PubMed] [Google Scholar]
- 57.Schweitzer, B. L., and R. P. DeKoter. 2004. Analysis of gene expression and Ig transcription in PU.1/Spi-B-deficient progenitor B cell lines. J. Immunol. 172:144-154. [DOI] [PubMed] [Google Scholar]
- 58.Sciammas, R., A. L. Shaffer, J. H. Schatz, H. Zhao, L. M. Staudt, and H. Singh. 2006. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity 25:225-236. [DOI] [PubMed] [Google Scholar]
- 59.Shaffer, A. L., A. Peng, and M. S. Schlissel. 1997. In vivo occupancy of the kappa light chain enhancers in primary pro- and pre-B cells: a model for kappa locus activation. Immunity 6:131-143. [DOI] [PubMed] [Google Scholar]
- 60.Smith-Gill, S. J., C. Mainhart, T. B. Lavoie, R. J. Feldmann, W. Drohan, and B. R. Brooks. 1987. A three-dimensional model of an anti-lysozyme antibody. J. Mol. Biol. 194:713-724. [DOI] [PubMed] [Google Scholar]
- 61.Takeda, S., E. Sonoda, and H. Arakawa. 1996. The kappa:lambda ratio of immature B cells. Immunol. Today 17:200-201. [DOI] [PubMed] [Google Scholar]
- 62.Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623-655. [DOI] [PubMed] [Google Scholar]
- 63.Tiegs, S. L., D. M. Russell, and D. Nemazee. 1993. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 177:1009-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsubata, T., and M. Reth. 1990. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J. Exp. Med. 172:973-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tze, L. E., E. A. Baness, K. L. Hippen, and T. W. Behrens. 2000. Ig light chain receptor editing in anergic B cells. J. Immunol. 165:6796-6802. [DOI] [PubMed] [Google Scholar]
- 66.Tze, L. E., K. L. Hippen, and T. W. Behrens. 2003. Late immature B cells (IgMhighIgDneg) undergo a light chain receptor editing response to soluble self-antigen. J. Immunol. 171:678-682. [DOI] [PubMed] [Google Scholar]
- 67.Verkoczy, L. K., B. J. Stiernhdm, and N. L. Berinstein. 1995. Up-regulation of recombination activating gene expression by signal transduction through the surface Ig receptor. J. Immunol. 154:5136-5143. [PubMed] [Google Scholar]
- 68.Wang, D., J. Feng, R. Wen, J. C. Marine, M. Y. Sangster, E. Parganas, A. Hoffmeyer, C. W. Jackson, J. L. Cleveland, P. J. Murray, and J. N. Ihle. 2000. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity 13:25-35. [DOI] [PubMed] [Google Scholar]
- 69.Wen, R., Y. Chen, J. Schuman, G. Fu, S. Yang, W. Zhang, D. K. Newman, and D. Wang. 2004. An important role of phospholipase Cgamma1 in pre-B-cell development and allelic exclusion. EMBO J. 23:4007-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen, R., Y. Chen, L. Xue, J. Schuman, S. Yang, S. W. Morris, and D. Wang. 2003. Phospholipase Cgamma2 provides survival signals via Bcl2 and A1 in different subpopulations of B cells. J. Biol. Chem. 278:43654-43662. [DOI] [PubMed] [Google Scholar]
- 71.Xue, L., S. W. Morris, C. Orihuela, E. Tuomanen, X. Cui, R. Wen, and D. Wang. 2003. Defective development and function of Bcl10-deficient follicular, marginal zone and B1 B cells. Nat. Immunol. 4:857-865. [DOI] [PubMed] [Google Scholar]
- 72.Yamagami, T., E. ten Boekel, J. Andersson, A. Rolink, and F. Melchers. 1999. Frequencies of multiple IgL chain gene rearrangements in single normal or kappaL chain-deficient B lineage cells. Immunity 11:317-327. [DOI] [PubMed] [Google Scholar]
- 73.Zou, Y. R., S. Takeda, and K. Rajewsky. 1993. Gene targeting in the Ig kappa locus: efficient generation of lambda chain-expressing B cells, independent of gene rearrangements in Ig kappa. EMBO J. 12:811-820. [DOI] [PMC free article] [PubMed] [Google Scholar]