Abstract
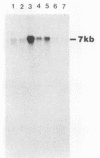
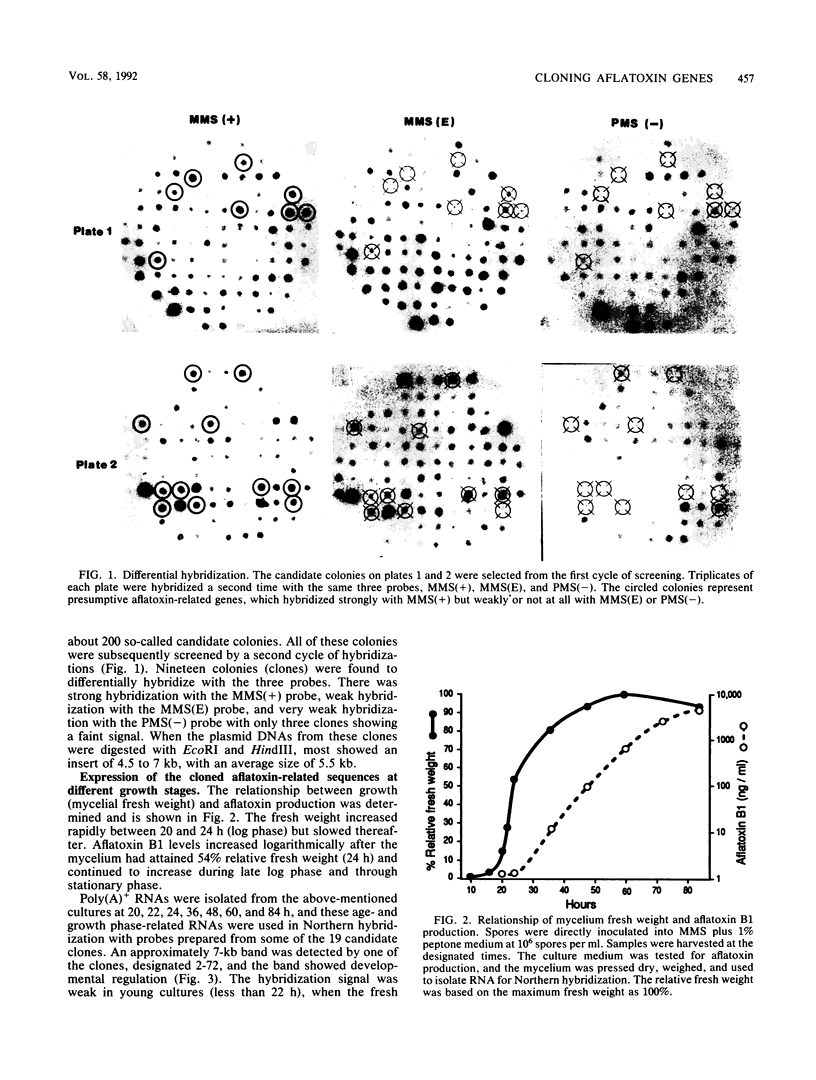
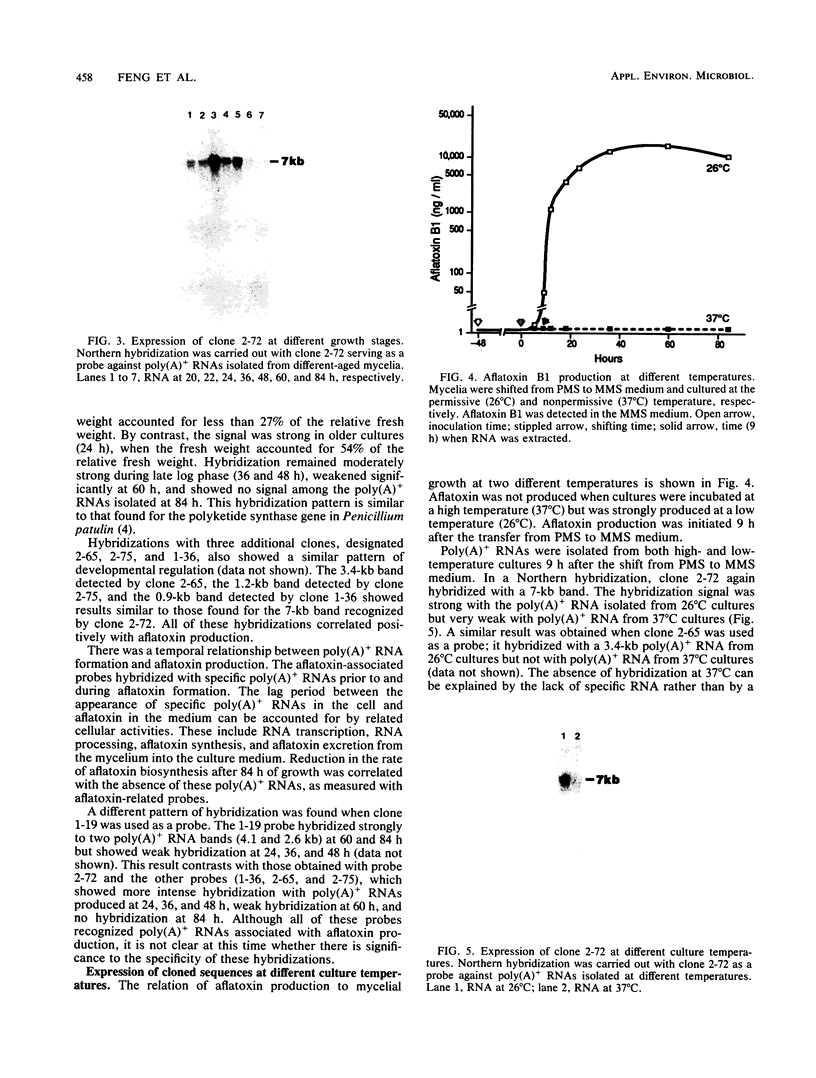
A differential hybridization strategy was used to clone genes associated with aflatoxin biosynthesis. A genomic library, formed between nuclear DNA and the pUC19 plasmid, was screened with three different cDNA probes by the colony hybridization procedure. Nineteen clones were selected; all were positively correlated with and presumably enriched with genes associated with aflatoxin production. Some of these clones were further characterized by using them as probes in Northern (RNA blot) hybridizations. Five clones hybridized strongly with some polyadenylated RNAs formed during the transition to or during idiophase when aflatoxin was produced. However, little or no corresponding hybridization occurred with polyadenylated RNAs formed in early and mid-log growth phase. Two of the clones were further used as probes to hybridize with polyadenylated RNAs formed under aflatoxin-permissive and nonpermissive temperatures. Hybridization occurred with RNA species formed under the permissive temperature only.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J., Ripka S., Siegner A., Schiltz E., Schweizer E. The multifunctional 6-methylsalicylic acid synthase gene of Penicillium patulum. Its gene structure relative to that of other polyketide synthases. Eur J Biochem. 1990 Sep 11;192(2):487–498. doi: 10.1111/j.1432-1033.1990.tb19252.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Christensen S. B. New perspectives on aflatoxin biosynthesis. Adv Appl Microbiol. 1983;29:53–92. doi: 10.1016/s0065-2164(08)70354-x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar D., Cleveland T. E., Lillehoj E. B. Enzymes in aflatoxin B1 biosynthesis: strategies for identifying pertinent genes. Mycopathologia. 1989 Sep;107(2-3):75–83. doi: 10.1007/BF00707542. [DOI] [PubMed] [Google Scholar]
- Bhatnagar D., Ullah A. H., Cleveland T. E. Purification and characterization of a methyltransferase from Aspergillus parasiticus SRRC 163 involved in aflatoxin biosynthetic pathway. Prep Biochem. 1988;18(3):321–349. doi: 10.1080/00327488808062532. [DOI] [PubMed] [Google Scholar]
- Chu F. S., Fan T. S., Zhang G. S., Xu Y. C., Faust S., McMahon P. L. Improved enzyme-linked immunosorbent assay for aflatoxin B1 in agricultural commodities. J Assoc Off Anal Chem. 1987 Sep-Oct;70(5):854–857. [PubMed] [Google Scholar]
- Chuturgoon A. A., Dutton M. F., Berry R. K. The preparation of an enzyme associated with aflatoxin biosynthesis by affinity chromatography. Biochem Biophys Res Commun. 1990 Jan 15;166(1):38–42. doi: 10.1016/0006-291x(90)91908-b. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E., Bhatnagar D. Evidence for de novo synthesis of an aflatoxin pathway methyltransferase near the cessation of active growth and the onset of aflatoxin biosynthesis in Aspergillus parasiticus mycelia. Can J Microbiol. 1990 Jan;36(1):1–5. doi: 10.1139/m90-001. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E., Lax A. R., Lee L. S., Bhatnagar D. Appearance of enzyme activities catalyzing conversion of sterigmatocystin to aflatoxin B1 in late-growth-phase Aspergillus parasiticus cultures. Appl Environ Microbiol. 1987 Jul;53(7):1711–1713. doi: 10.1128/aem.53.7.1711-1713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dons J. J., Springer J., de Vries S. C., Wessels J. G. Molecular cloning of a gene abundantly expressed during fruiting body initiation in Schizophyllum commune. J Bacteriol. 1984 Mar;157(3):802–808. doi: 10.1128/jb.157.3.802-808.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton M. F. Enzymes and aflatoxin biosynthesis. Microbiol Rev. 1988 Jun;52(2):274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loros J. J., Denome S. A., Dunlap J. C. Molecular cloning of genes under control of the circadian clock in Neurospora. Science. 1989 Jan 20;243(4889):385–388. doi: 10.1126/science.2563175. [DOI] [PubMed] [Google Scholar]
- Payne G. A., Woloshuk C. P. Transformation of Aspergillus flavus to study aflatoxin biosynthesis. Mycopathologia. 1989 Sep;107(2-3):139–144. doi: 10.1007/BF00707551. [DOI] [PubMed] [Google Scholar]
- Schindler A. F., Palmer J. G., Eisenberg W. V. Aflatoxin Production by Aspergillus flavus as Related to Various Temperatures. Appl Microbiol. 1967 Sep;15(5):1006–1009. doi: 10.1128/am.15.5.1006-1009.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Horng J. S., Pestka J. J., Linz J. E. Transformation of Aspergillus parasiticus with a homologous gene (pyrG) involved in pyrimidine biosynthesis. Appl Environ Microbiol. 1990 Nov;56(11):3315–3320. doi: 10.1128/aem.56.11.3315-3320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Burnham M. K., Bull J. H., Hodgson J. E., Ward J. M., Browne P., Brown J., Barton B., Earl A. J., Turner G. Beta-lactam antibiotic biosynthetic genes have been conserved in clusters in prokaryotes and eukaryotes. EMBO J. 1990 Mar;9(3):741–747. doi: 10.1002/j.1460-2075.1990.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. Isolation of galactose-inducible DNA sequences from Saccharomyces cerevisiae by differential plaque filter hybridization. Cell. 1979 Feb;16(2):443–452. doi: 10.1016/0092-8674(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980 Aug;78(2):497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- Woloshuk C. P., Seip E. R., Payne G. A., Adkins C. R. Genetic transformation system for the aflatoxin-producing fungus Aspergillus flavus. Appl Environ Microbiol. 1989 Jan;55(1):86–90. doi: 10.1128/aem.55.1.86-90.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann C. R., Orr W. C., Leclerc R. F., Barnard E. C., Timberlake W. E. Molecular cloning and selection of genes regulated in Aspergillus development. Cell. 1980 Oct;21(3):709–715. doi: 10.1016/0092-8674(80)90434-1. [DOI] [PubMed] [Google Scholar]