Abstract
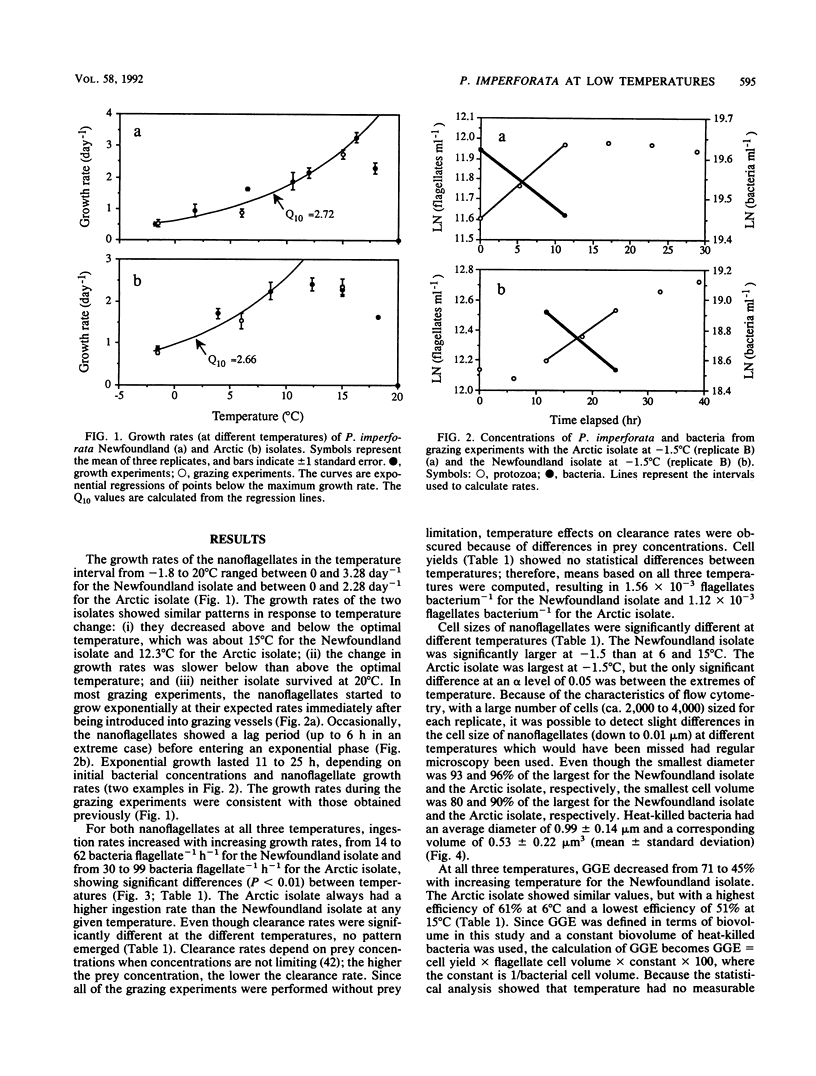
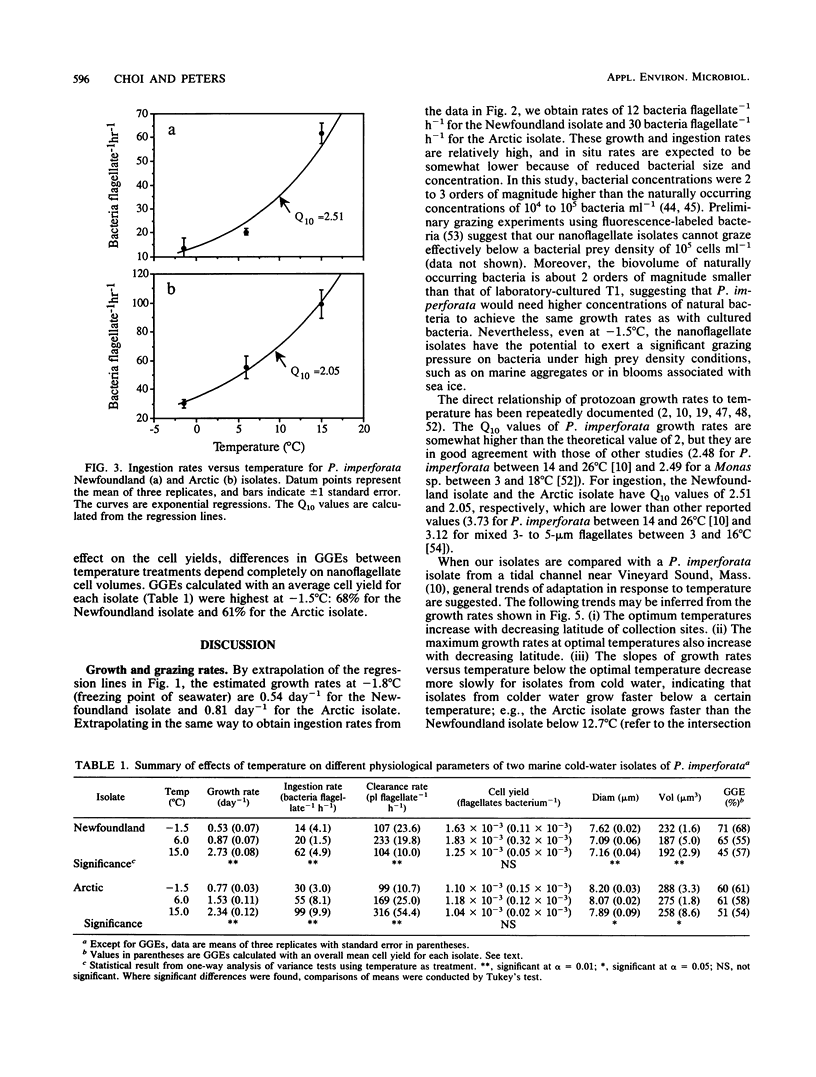
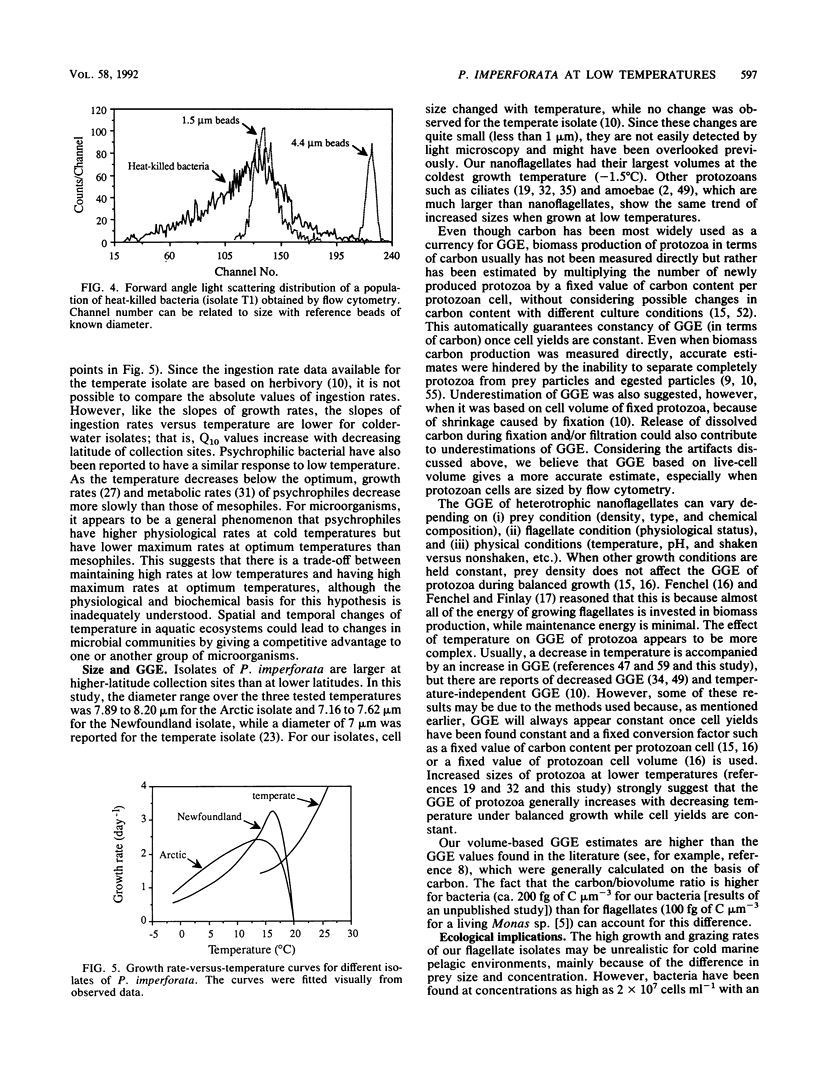
Two different psychrophilic types of the heterotrophic nanoflagellate Paraphysomonas imperforata were isolated from Newfoundland coastal waters and the Arctic Ocean. When fed bacteria without food limitation, both isolates were able to grow at temperatures from -1.8 to 20°C, with maximum growth rates of 3.28 day-1 at 15°C and 2.28 day-1 at 12.3°C for the Newfoundland and the Arctic isolates, respectively. Ingestion rates increased with temperature from 14 to 62 bacteria flagellate-1 h-1 for the Newfoundland isolate and from 30 to 99 bacteria flagellate-1 h-1 for the Arctic isolate. While temperature did not affect cell yields (number of protozoa produced divided by number of bacteria consumed), it affected flagellate sizes. This differential effect of temperature on cell yield and cell size resulted in a changing gross growth efficiency (GGE) in terms of biovolume; colder temperatures favored higher GGEs. The comparison of Q10 values for growth rates and ingestion rates between the isolates shows that the Arctic isolate is better adapted to extremely cold temperature than the Newfoundland isolate. At seawater-freezing temperature (-1.8°C), the estimated maximum growth rates and maximum ingestion rates are 0.81 day-1 and 30 bacteria flagellate-1 h-1 for the Arctic isolate and 0.54 day-1 and 12 bacteria flagellate-1 h-1 for the Newfoundland isolate. Our findings about psychrophilic nanoflagellates fit the general characteristics of cold-water-dwelling organisms: reduced physiological rates and higher GGEs at lower temperatures. Because of the large and persistent differences between the isolates, we conclude that they are ecotypes adapted to specific environmental conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown C. M., Rose A. H. Effects of temperature on composition and cell volume of Candida utilis. J Bacteriol. 1969 Jan;97(1):261–270. doi: 10.1128/jb.97.1.261-272.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron D. A., Goldman J. C., Dennett M. R. Effect of temperature on growth, respiration, and nutrient regeneration by an omnivorous microflagellate. Appl Environ Microbiol. 1986 Dec;52(6):1340–1347. doi: 10.1128/aem.52.6.1340-1347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. W., Stoecker D. K. Effects of fixation on cell volume of marine planktonic protozoa. Appl Environ Microbiol. 1989 Jul;55(7):1761–1765. doi: 10.1128/aem.55.7.1761-1765.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curds C. R., Cockburn A. Studies on the growth and feeding of Tetrahymena pyriformis in axenic and monoxenic culture. J Gen Microbiol. 1968 Dec;54(3):343–358. doi: 10.1099/00221287-54-3-343. [DOI] [PubMed] [Google Scholar]
- Harder W., Veldkamp H. Competition of marine psychrophilic bacteria at low temperatures. Antonie Van Leeuwenhoek. 1971;37(1):51–63. doi: 10.1007/BF02218466. [DOI] [PubMed] [Google Scholar]
- Herbert R. A., Bell C. R. Growth characteristics of an obligately psychrophilic Vibrio sp. Arch Microbiol. 1977 Jun 20;113(3):215–220. doi: 10.1007/BF00492028. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAHAM J. L., BAILEY G. F. Comparative study of effect of temperature on metabolism of psychrophilic and mesophilic bacteria. J Bacteriol. 1959 May;77(5):609–613. doi: 10.1128/jb.77.5.609-613.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES T. W., READ C. P. The effect of incubation temperature on the cell size of Tetrahymena pyriformis. Exp Cell Res. 1957 Dec;13(3):510–516. doi: 10.1016/0014-4827(57)90080-0. [DOI] [PubMed] [Google Scholar]
- MORITA R. Y., ALBRIGHT L. J. CELL YIELDS OF VIBRIO MARINUS, AN OBLIGATE PSYCHROPHILE, AT LOW TEMPERATURE. Can J Microbiol. 1965 Apr;11:221–227. doi: 10.1139/m65-028. [DOI] [PubMed] [Google Scholar]
- Morita R. Y. Psychrophilic bacteria. Bacteriol Rev. 1975 Jun;39(2):144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Berman T. Grazing, growth, and ammonium excretion rates of a heterotrophic microflagellate fed with four species of bacteria. Appl Environ Microbiol. 1983 Apr;45(4):1196–1201. doi: 10.1128/aem.45.4.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Fallon R. D. Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol. 1987 May;53(5):958–965. doi: 10.1128/aem.53.5.958-965.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Rassoulzadegan F. Rates of digestion of bacteria by marine phagotrophic protozoa: temperature dependence. Appl Environ Microbiol. 1988 May;54(5):1091–1095. doi: 10.1128/aem.54.5.1091-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


