Abstract
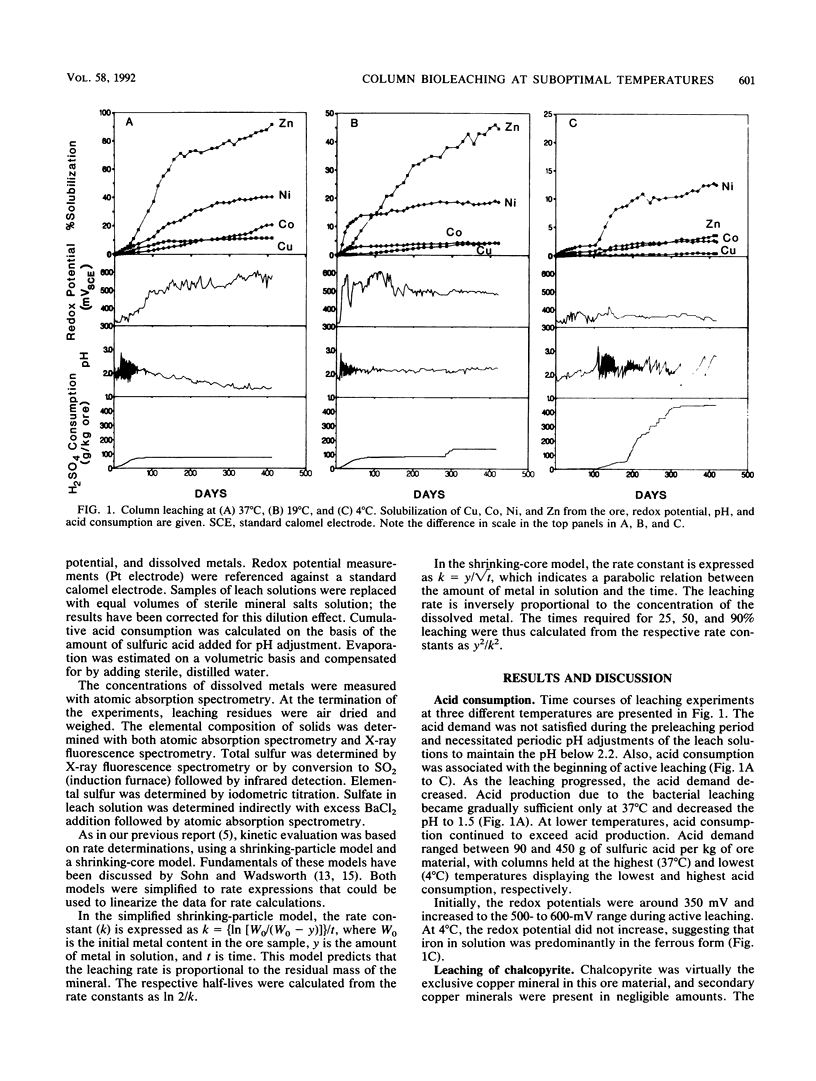
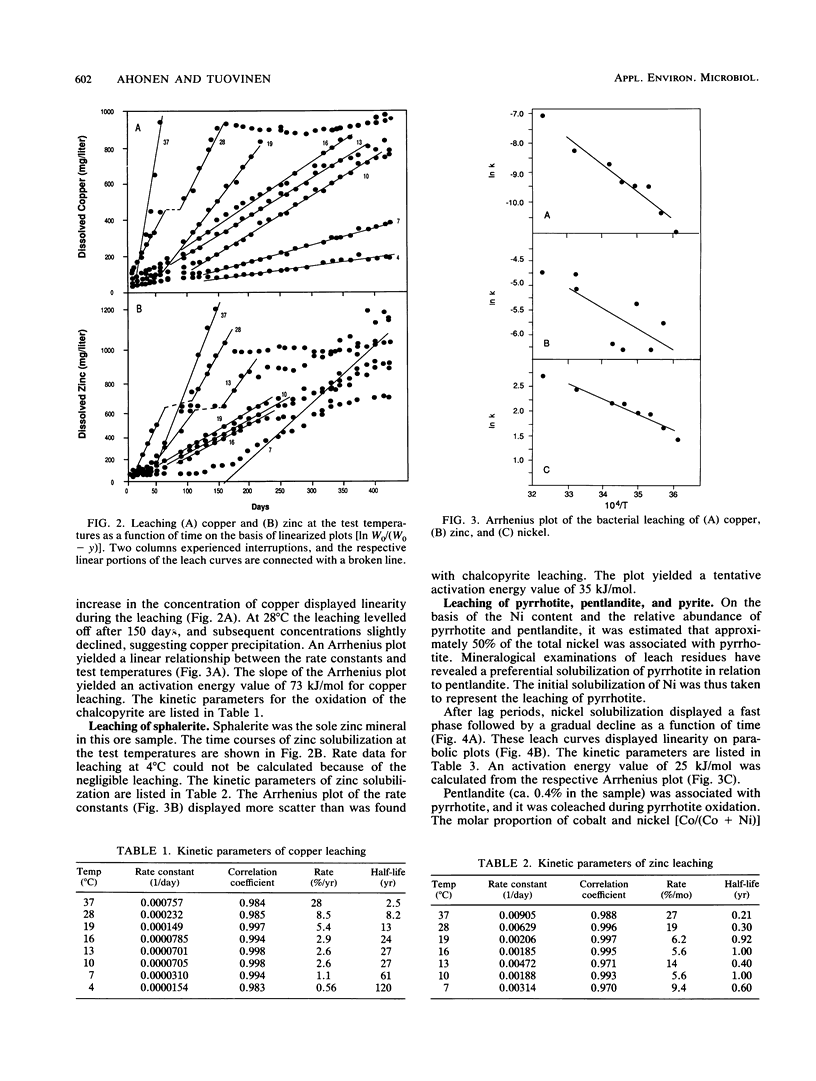
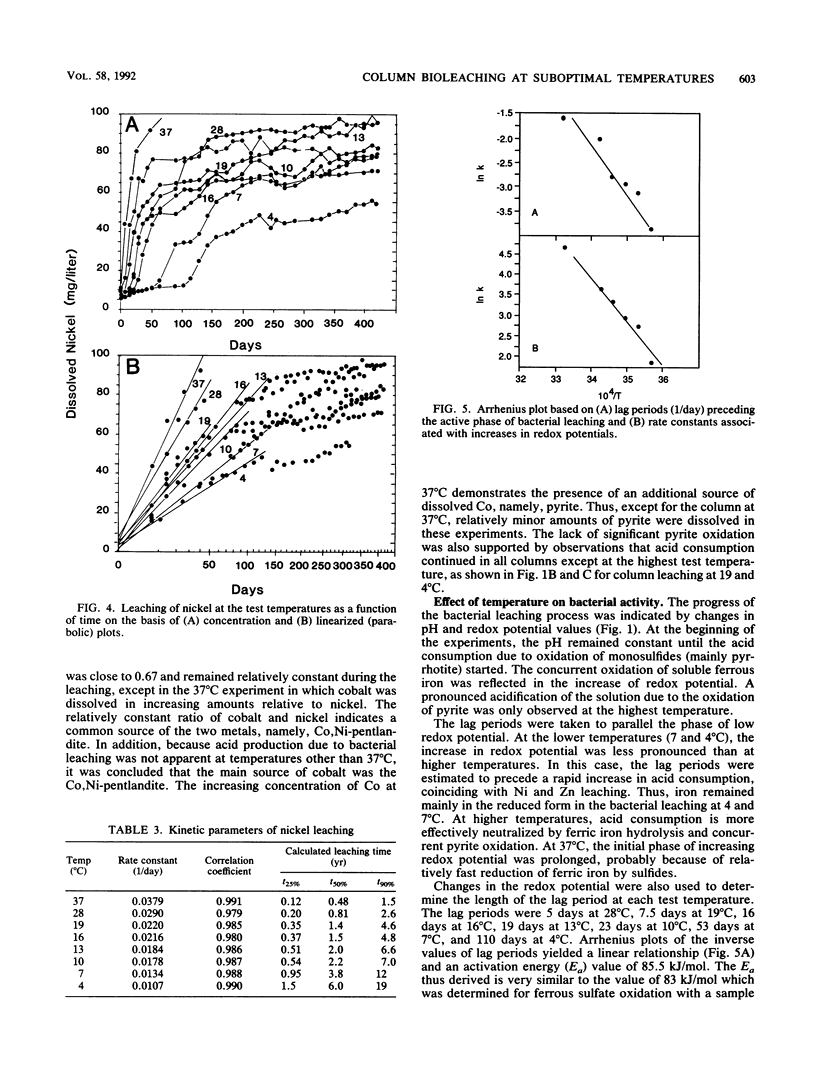
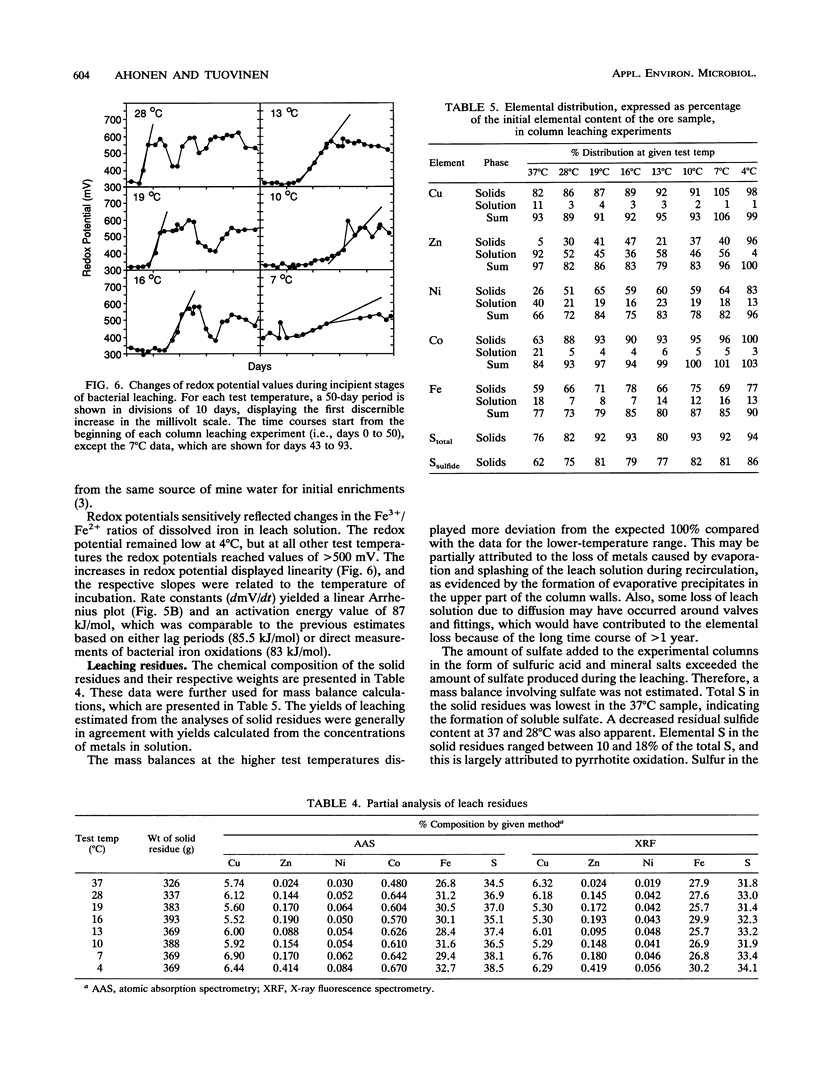
The purpose of the work was to quantitatively characterize temperature effects on the bacterial leaching of sulfide ore material containing several sulfide minerals. The leaching was tested at eight different temperatures in the range of 4 to 37°C. The experimental technique was based on column leaching of a coarsely ground (particle diameter, 0.59 to 5 mm) ore sample. The experimental data were used for kinetic analysis of chalcopyrite, sphalerite, and pyrrhotite oxidation. Chalcopyrite yielded the highest (73 kJ/mol) and pyrrhotite yielded the lowest (25 kJ/mol) activation energies. Especially with pyrrhotite, diffusion contributed to rate limitation. Arrhenius plots were also linear for the reciprocals of lag periods and for increases of redox potentials (dmV/dt). Mass balance analysis based on total S in leach residue was in agreement with the highest rate of leaching at 37 and 28°C. The presence of elemental S in leach residues was attributed to pyrrhotite oxidation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonen L., Tuovinen O. H. Kinetics of sulfur oxidation at suboptimal temperatures. Appl Environ Microbiol. 1990 Feb;56(2):560–562. doi: 10.1128/aem.56.2.560-562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen L., Tuovinen O. H. Microbiological oxidation of ferrous iron at low temperatures. Appl Environ Microbiol. 1989 Feb;55(2):312–316. doi: 10.1128/aem.55.2.312-316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen L., Tuovinen O. H. Temperature effects on bacterial leaching of sulfide minerals in shake flask experiments. Appl Environ Microbiol. 1991 Jan;57(1):138–145. doi: 10.1128/aem.57.1.138-145.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


