Abstract
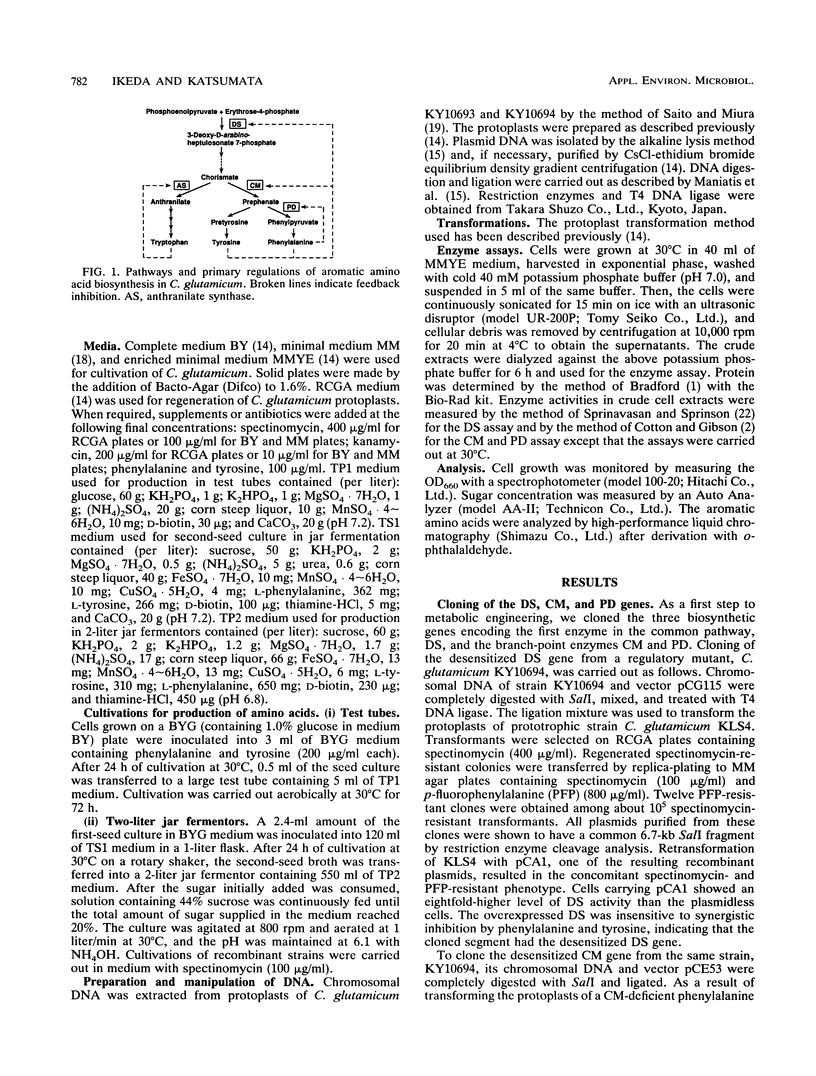
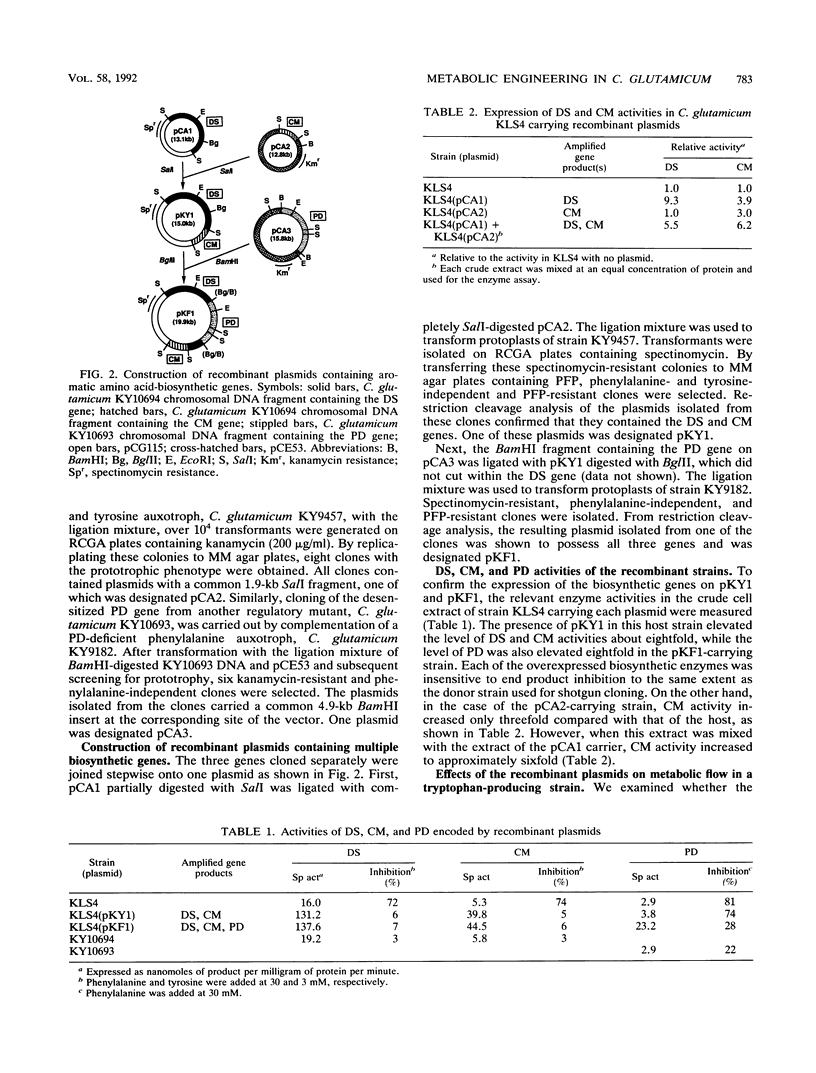
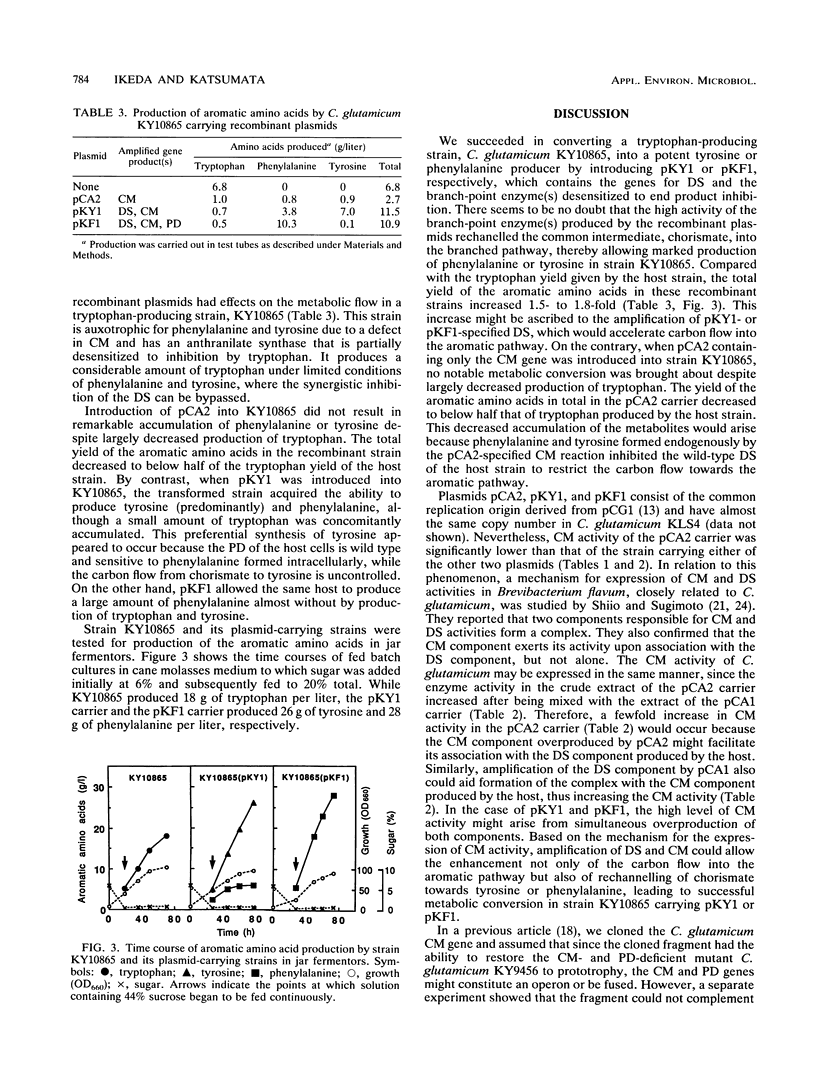
The aromatic amino acids are synthesized via a common biosynthetic pathway. A tryptophan-producing mutant of Corynebacterium glutamicum was genetically engineered to produce tyrosine or phenylalanine in abundance. To achieve this, three biosynthetic genes encoding the first enzyme in the common pathway, 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase (DS), and the branch-point enzymes chorismate mutase and prephenate dehydratase were individually cloned from regulatory mutants of C. glutamicum which have either of the corresponding enzymes desensitized to end product inhibition. These cloned genes were assembled one after another onto a multicopy vector of C. glutamicum to yield two recombinant plasmids. One plasmid, designated pKY1, contains the DS and chorismate mutase genes, and the other, designated pKF1, contains all three biosynthetic genes. The enzymes specified by both plasmids were simultaneously overexpressed approximately sevenfold relative to the chromosomally encoded enzymes in a C. glutamicum strain. When transformed with pKY1 or pKF1, tryptophan-producing C. glutamicum KY10865, with the ability to produce 18 g of tryptophan per liter, was altered to produce a large amount of tyrosine (26 g/liter) or phenylalanine (28 g/liter), respectively, because the accelerated carbon flow through the common pathway was redirected to tyrosine or phenylalanine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. Plasmid vehicles for direct cloning of Escherichia coli promoters. J Bacteriol. 1979 Nov;140(2):400–407. doi: 10.1128/jb.140.2.400-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Dayan J., Sprinson D. B. Enzyme alterations in tyrosine and phenylalanine auxotrophs of Salmonella typhimurium. J Bacteriol. 1971 Dec;108(3):1174–1180. doi: 10.1128/jb.108.3.1174-1180.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follettie M. T., Sinskey A. J. Molecular cloning and nucleotide sequence of the Corynebacterium glutamicum pheA gene. J Bacteriol. 1986 Aug;167(2):695–702. doi: 10.1128/jb.167.2.695-702.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S. W., Pittard J. Phenylalanine biosynthesis in Escherichia coli K-12: mutants derepressed for chorismate mutase P-prephenate dehydratase. J Bacteriol. 1971 Jun;106(3):784–790. doi: 10.1128/jb.106.3.784-790.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumata R., Ozaki A., Oka T., Furuya A. Protoplast transformation of glutamate-producing bacteria with plasmid DNA. J Bacteriol. 1984 Jul;159(1):306–311. doi: 10.1128/jb.159.1.306-311.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Ozaki A., Katsumata R., Oka T., Furuya A. Functional expression of the genes of Escherichia coli in gram-positive Corynebacterium glutamicum. Mol Gen Genet. 1984;196(1):175–178. doi: 10.1007/BF00334113. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- Shiio I., Sugimoto S. Two components of chorismate mutase in Brevibacterium flavum. J Biochem. 1979 Jul;86(1):17–25. [PubMed] [Google Scholar]
- Sugimoto S., Shiio I. Purification and properties of bifunctional 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase-chorismate mutase component A from Brevibacterium flavum. J Biochem. 1980 Mar;87(3):881–890. doi: 10.1093/oxfordjournals.jbchem.a132818. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Yoshihama M., Higashiro K., Rao E. A., Akedo M., Shanabruch W. G., Follettie M. T., Walker G. C., Sinskey A. J. Cloning vector system for Corynebacterium glutamicum. J Bacteriol. 1985 May;162(2):591–597. doi: 10.1128/jb.162.2.591-597.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


