Abstract
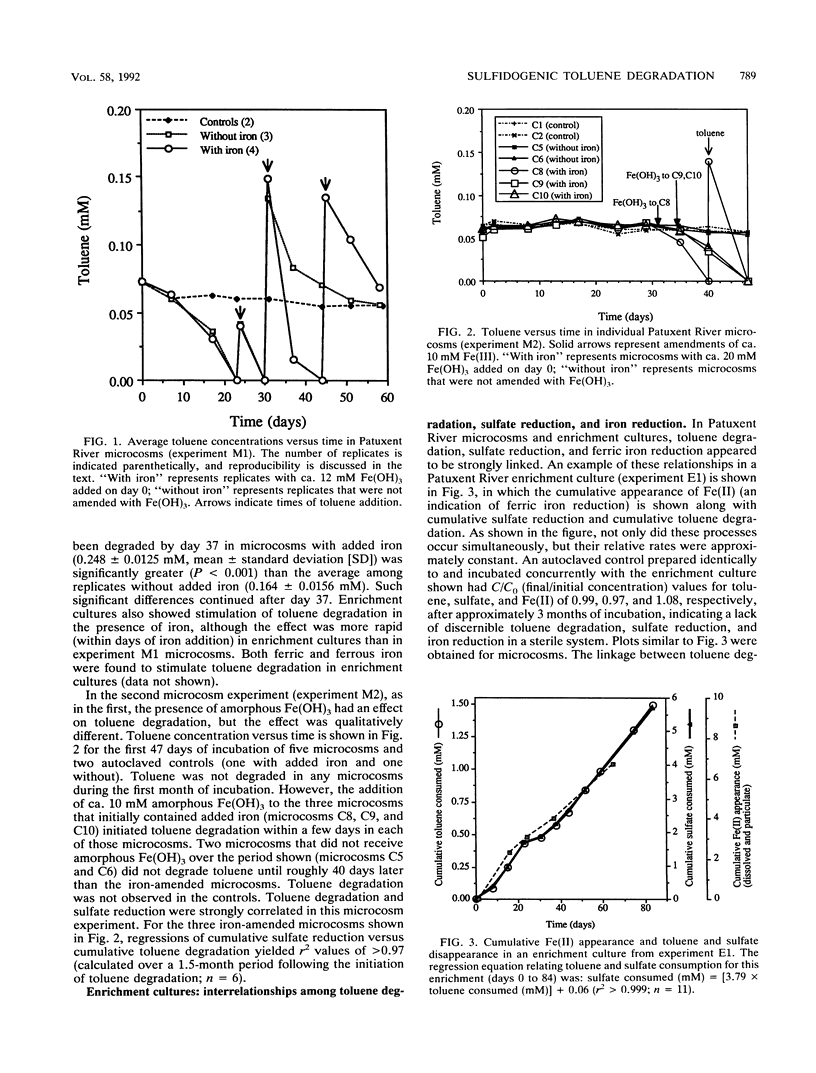
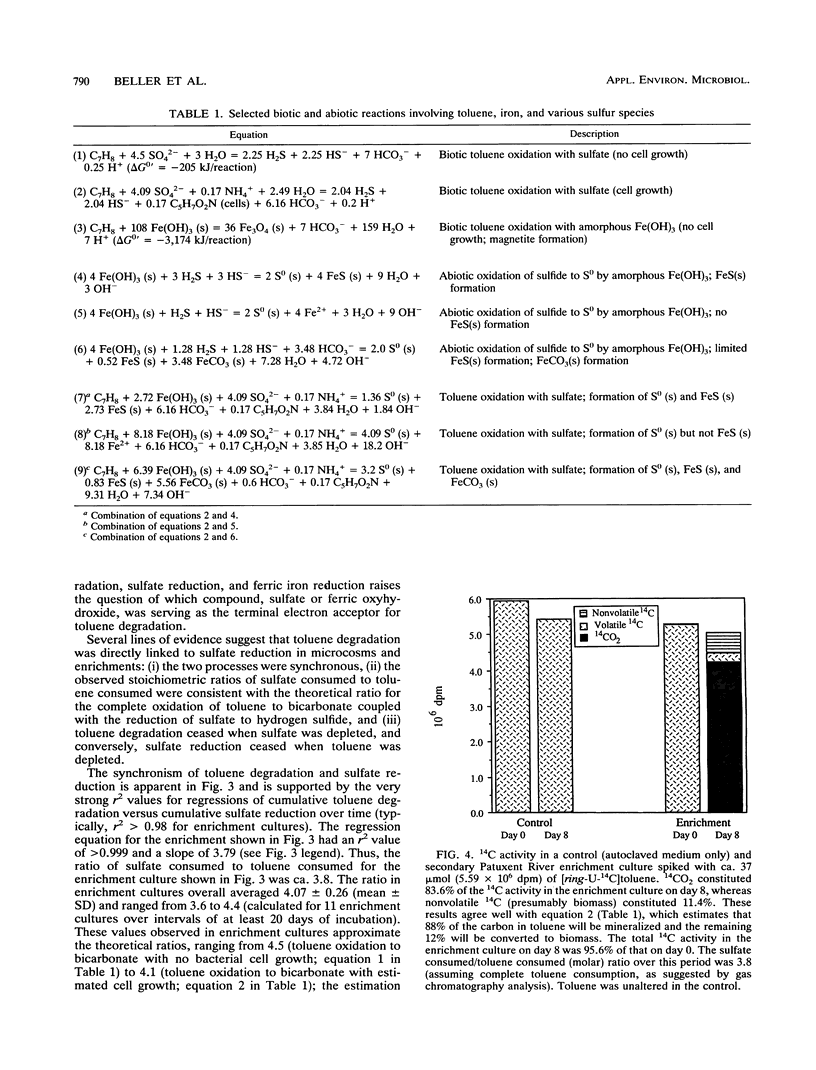
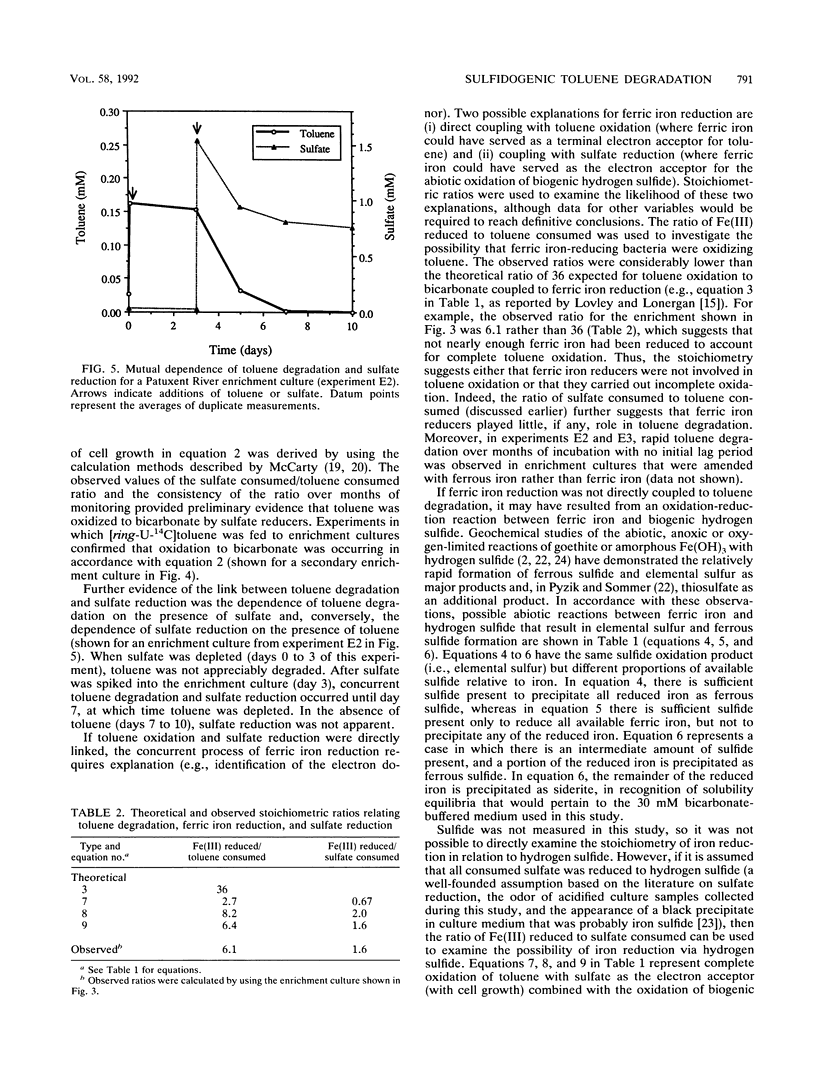
Toluene degradation occurred concomitantly with sulfate reduction in anaerobic microcosms inoculated with contaminated subsurface soil from an aviation fuel storage facility near the Patuxent River (Md.). Similar results were obtained for enrichment cultures in which toluene was the sole carbon source. Several lines of evidence suggest that toluene degradation was directly coupled to sulfate reduction in Patuxent River microcosms and enrichment cultures: (i) the two processes were synchronous and highly correlated, (ii) the observed stoichiometric ratios of moles of sulfate consumed per mole of toluene consumed were consistent with the theoretical ratio for the oxidation of toluene to CO2 coupled with the reduction of sulfate to hydrogen sulfide, and (iii) toluene degradation ceased when sulfate was depleted, and conversely, sulfate reduction ceased when toluene was depleted. Mineralization of toluene was confirmed in experiments with [ring-U-14C]toluene. The addition of millimolar concentrations of amorphous Fe(OH)3 to Patuxent River microcosms and enrichment cultures either greatly facilitated the onset of toluene degradation or accelerated the rate once degradation had begun. In iron-amended microcosms and enrichment cultures, ferric iron reduction proceeded concurrently with toluene degradation and sulfate reduction. Stoichiometric data and other observations indicate that ferric iron reduction was not directly coupled to toluene oxidation but was a secondary, presumably abiotic, reaction between ferric iron and biogenic hydrogen sulfide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dolfing J., Zeyer J., Binder-Eicher P., Schwarzenbach R. P. Isolation and characterization of a bacterium that mineralizes toluene in the absence of molecular oxygen. Arch Microbiol. 1990;154(4):336–341. doi: 10.1007/BF00276528. [DOI] [PubMed] [Google Scholar]
- Edwards E. A., Wills L. E., Reinhard M., Grbić-Galić D. Anaerobic degradation of toluene and xylene by aquifer microorganisms under sulfate-reducing conditions. Appl Environ Microbiol. 1992 Mar;58(3):794–800. doi: 10.1128/aem.58.3.794-800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. J., Mang D. T., Kim K. S., Young L. Y. Anaerobic degradation of toluene by a denitrifying bacterium. Appl Environ Microbiol. 1991 Apr;57(4):1139–1145. doi: 10.1128/aem.57.4.1139-1145.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbić-Galić D., Vogel T. M. Transformation of toluene and benzene by mixed methanogenic cultures. Appl Environ Microbiol. 1987 Feb;53(2):254–260. doi: 10.1128/aem.53.2.254-260.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E. P., Zeyer J., Eicher P., Schwarzenbach R. P. Anaerobic degradation of alkylated benzenes in denitrifying laboratory aquifer columns. Appl Environ Microbiol. 1988 Feb;54(2):490–496. doi: 10.1128/aem.54.2.490-496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Lonergan D. J. Anaerobic Oxidation of Toluene, Phenol, and p-Cresol by the Dissimilatory Iron-Reducing Organism, GS-15. Appl Environ Microbiol. 1990 Jun;56(6):1858–1864. doi: 10.1128/aem.56.6.1858-1864.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal potomac river. Appl Environ Microbiol. 1986 Oct;52(4):751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986 Apr;51(4):683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell S., Bak F., Pfennig N. Anaerobic degradation of aniline and dihydroxybenzenes by newly isolated sulfate-reducing bacteria and description of Desulfobacterium anilini. Arch Microbiol. 1989;152(6):556–563. doi: 10.1007/BF00425486. [DOI] [PubMed] [Google Scholar]
- Smolenski W. J., Suflita J. M. Biodegradation of cresol isomers in anoxic aquifers. Appl Environ Microbiol. 1987 Apr;53(4):710–716. doi: 10.1128/aem.53.4.710-716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T. M., Grbìc-Galìc D. Incorporation of Oxygen from Water into Toluene and Benzene during Anaerobic Fermentative Transformation. Appl Environ Microbiol. 1986 Jul;52(1):200–202. doi: 10.1128/aem.52.1.200-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyer J., Kuhn E. P., Schwarzenbach R. P. Rapid microbial mineralization of toluene and 1,3-dimethylbenzene in the absence of molecular oxygen. Appl Environ Microbiol. 1986 Oct;52(4):944–947. doi: 10.1128/aem.52.4.944-947.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


