Abstract
Objective
To determine the effects of primary antiphospholipid syndrome (PAPS)‐derived anti‐β2GPI antibodies on gene expression in human umbilical vein endothelial cells (HUVEC) by gene profiling using microarrays.
Methods
Anti‐β2GPI antibodies purified from sera of patients with PAPS or control IgG isolated from normal subjects were incubated with HUVEC for 4 h before isolation of RNA and processing for hybridisation to Affymetrix Human Genome U133A‐2.0 arrays. Data were analysed using a combination of the MAS 5.0 (Affymetrix) and GeneSpring (Agilent) software programmes. For selected genes microarray data were confirmed by real‐time PCR analysis or at the protein level by ELISA.
Results
A total of 101 genes were found to be upregulated and 14 genes were downregulated twofold or more in response to anti‐β2GPI antibodies. A number of novel genes not previously associated with APS were induced, including chemokines CCL20, CXCL3, CX3CL1, CXCL5, CXCL2 and CXCL1, the receptors Tenascin C, OLR1, IL‐18 receptor 1, and growth factors CSF2, CSF3 IL‐6, IL1β and FGF18. The majority of downregulated genes were transcription factors/signalling molecules including ID2. Quantitative real‐time RT‐PCR analysis confirmed the microarray results for selected genes (CSF3, CX3CL1, FGF18, ID2, SOD2, Tenascin C).
Conclusions
This study reveals a complex gene expression response in HUVEC to anti‐β2GPI antibodies with multiple chemokines, pro‐inflammatory cytokines, pro‐thrombotic and pro‐adhesive genes regulated by these antibodies in vitro. Some of these newly identified anti‐β2GPI antibody‐regulated genes could contribute to the vasculopathy associated with this disease.
Antiphospholipid syndrome (APS) is characterised by thrombosis, thrombocytopenia and recurrent foetal loss.1 Two forms of the syndrome have been described; the “primary” syndrome (PAPS), where there is no evidence of any other underlying disease and “secondary” syndrome that is mainly associated with systemic lupus erythematosus (SLE). Elevated serum titres of antiphospholipid antibodies (aPL) correlate with thrombotic events in APS2 and there is strong evidence that aPL display a pathogenic role in APS.3,4 β2‐glycoprotein I (β2GPI) binds to negatively charged phospholipids through a positively charged lysine‐rich sequence of amino acids in its fifth domain5 and is now recognised as the primary aPL target in APS.5,6,7,8 Anti‐β2GPI antibodies bind to the β2GPI protein adherent to the endothelial cell (EC) surface and induce EC activation.9
Anti‐β2GPI antibodies might exert a direct pathogenic effect in APS by perturbing homeostatic reactions that take place on the surface of EC.10 A number of in vitro studies have reported that anti‐β2GPI antibodies can activate EC as shown by early increases in monocyte adhesion and the expression of E‐selectin, vascular cell adhesion molecule‐1 (VCAM‐1), and intracellular adhesion molecule‐1 (ICAM‐1).9,11,12 In vivo, aPL infused into naïve mice caused increased adhesion of monocytes and formation of sustained and larger thrombi when compared to normal control IgG.13
In addition, recent studies have reported that nuclear factor kappa B (NF‐κB) translocation, the myeloid differentiation primary response gene 88 (MyD88) pathway and p38 mitogen‐activated protein kinase (MAPK) phosphorylation are involved in EC and monocyte activation by anti‐β2GPI antibodies.14,15,16 However, the extent and diversity of anti‐β2GPI‐mediated gene regulation in EC cells is not yet well characterised. The present study was undertaken to examine the profile and diversity of early gene regulation in EC in response to polyclonal patient‐derived anti‐β2GPI antibodies using Affymetrix microarray gene profiling.
Methods
Patient group
Ethical approval for the collection of sera from PAPS patients was obtained prior to the initiation of the study from the St. Thomas' Hospital Research Ethics Committee. Following written patient consent, sera were collected from a total of five patients with PAPS. All 5 patients had high levels of IgG aPL and strong lupus anti‐coagulant activity. Anticardiolipin activity in the patients was β2GPI dependent (data not shown). The clinical profiles of patients from whom polyclonal anti‐β2GPI antibody preparations were isolated and used in this study are shown in table 1. All 5 patients fulfilled the Sapporo classification criteria for definitive PAPS.17
Table 1 Clinical profiles of patients from whom polyclonal anti‐β2GPI antibody preparations were made.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Sex/age | F/33 | F/53 | F/54 | F/38 | F/59 |
| Diagnosis | PAPS | PAPS | PAPS | PAPS | PAPS |
| Clinical features of APS | 1 DVT, 1 PE, PET, TIAs and stroke | 1 DVT, 3 PE | 1 DVT, 2 stillbirths, 1 PE, CVD, catastrophic APS | PVD, TIAs, brachial artery thrombosis | 3 Foetal losses, microinfarct CNS, MI, abnormal MRI, aortic stenosis |
| IgG aCL (GPL U/ml) | 350 | 223 | 142 | 257 | 308 |
| Lupus anticoagulant | + | + | + | + | + |
| Experimental procedures | Microarray, real time RT‐PCR, ELISA | Microarray, real time RT‐PCR, ELISA | Microarray, ELISA | Microarray, real time RT‐PCR, ELISA | Real time RT‐PCR |
aCL, anticardiolipin; CVD, cerebral vascular disease; DVT, deep vein thrombosis; MI, myocardial infarction; PE, pulmonary embolism; PET, pre‐eclampsia; PVD, peripheral vascular disease; TIA, transient ischemic attack.
Purification of normal IgG and anti‐β2GPI antibodies from sera
IgG from patients or normal age and sex‐matched subjects were purified using a HiTrap Protein G HP affinity column (GE Healthcare, Buckinghamshire, UK) as per the manufacturer's instructions. Purified human β2GPI protein was purchased from SCIPAC Ltd. (Sittingborne, Kent, UK.) The protein was coupled to a HiTrap NHS‐activated HP column as recommended by the manufacturer (GE Healthcare). A 1/8 dilution of serum in starting buffer was applied to the column and affinity‐purified antibody was eluted in 0.1 M glycine‐HCL pH 2.7 and neutralised with 1 M Tris‐HCL pH 9.0. The purification was carried out on an AKTA prime 3 system (GE Healthcare). Protein concentration of IgG and affinity purified antibodies was determined by Bicinchoninic protein assay (Sigma).
Characterisation of patient‐derived anti‐β2GPI antibodies
Following isolation, patient‐derived anti‐β2GPI antibodies were tested for binding to β2GPI by enzyme‐linked immunosorbant assay (ELISA) using a previously described method.18 All antibodies were also tested in an anti‐cardiolipin ELISA.19
Human umbilical vein endothelial cell isolation and culture
Human umbilical cords were obtained from the Labour Ward at St. Thomas' Hospital London following ethical approval and written patient consent. Human umbilical vein endothelial cells (HUVEC) were isolated from normal full term umbilical cord vein using collagenase enzyme (Sigma) and cultured as previously described at 37°C in a humidified incubator.20 For cell stimulation experiments HUVEC were incubated for 4 h with different antibody preparations. In all experiments, polymixin B (5 μg/ml) was included to exclude the possibility of endotoxin effects as previously described.15
Isolation of RNA from HUVEC treated with anti‐β2GPI antibodies or normal control IgG for microarray analysis
Confluent HUVEC at passage 3 were incubated with the four PAP‐derived anti‐β2GPI antibody preparations (P1, P2, P3, P4, 50 μg/ml) preparations or four normal control IgG (N1, N2, N3, N4, 50 μg/ml) for 4 h at 37°C in a humidified incubator. Total HUVEC RNA was then extracted using the RNeasy Kit (Qiagen, Crawley, West Sussex, UK). The quality of the RNA was checked using a 1% agarose gel. Three independent experiments using HUVEC from three different donors were carried out on different occasions.
Preparation of target biotinylated cRNA and hybridisation
cRNA samples for microarray hybridisation were prepared following the manufacturer's instructions (Affymetrix, Santa Clara, California). Fragmented cRNA was hybridised overnight to gene chip arrays at 45°C for 18 hours. Control cRNAs were then added to the hybridisation mix. Human Genome U133A‐2.0 gene chips containing probe sets for 18 400 human transcripts were used. In one of the three independent experiments, one anti‐β2GPI antibody (P2) treated sample and one control IgG treated (N2) sample were not processed beyond initial RNA quantitation due to low RNA yield. Therefore, a total of 22 chips were hybridised and scanned. Gene chips were washed and stained on the Gene Chip Fluidics Station 400 (Affymetrix). Fluorescent signals were detected using the HP G2500A Gene Array Scanner.
Statistical analysis of microarray data
After scanning the gene chips, images were analysed using the Affymetrix microarray suite (MAS) 5.0 (Affymetrix, Santa Clara, California, USA) to generate raw data in the form of “.cel” files. Further analysis was carried out using a combination of the MAS 5.0 and GeneSpring (Agilent Technologies, Santa Clara, California, USA) software programmes. The detection of a particular gene as “present, absent or marginal” was carried out using the MAS 5.0 software. The .cel files were imported into GeneSpring and normalised by GC‐Robust Multichip Average (GCRMA), an algorithm that normalises the data by quantile normalisation, in order to minimise the biological variation between samples. Further analysis was carried out on genes identified as present or marginal. Genes with statistically different expression between the control IgG and the anti‐β2GPI antibody treated cells (p<0.05) were identified by the Kruskal–Wallis test (non‐parametric one way analysis of variance (ANOVA)) with the Benjamin and Hochberg multiple testing correction.21 Filtering the gene list on the criteria of a twofold or more increase or decrease in expression identified a panel of genes that were significantly changed in HUVEC by anti‐β2GPI antibody treatment compared to normal control IgG treatment. Average‐linkage hierarchical clustering (using the Pearson Correlation) was carried out separately on the genes and the samples generating a genetree and condition tree, respectively, to highlight any distinct patterns in gene expression and the relationships between the samples.
Quantitative real‐time RT‐PCR analysis of gene expression
Quantitative real time PCR was used to confirm the microarray results for the expression levels of selected genes. The primer pairs used for the following genes were: CSF3, forward 5′‐CGCTCCAGGAGAAGCTGT‐3′ and reverse 5′‐CCAGAGAGTGTCCGAGCAG‐3′, CX3CL1, forward 5′‐ATCTCTGTCGTGGCTGCTC‐3′ and reverse 5′‐TCACACCGTGGTGCTGTC‐3′, E‐selectin, forward 5′‐TGAAGCTCCCACTGAGTCCAA‐3′ and reverse 5′‐ GGTGCTAATGTCAGGAGGGAGA‐3′, FGF18, forward 5′‐CTCTACAGCCGGACCAGTG‐3′ and reverse 5′‐CCGAAGGTGTCTGTCTCCAC‐3′, ID2, forward 5′‐CAGCATCCTGTCCTTGCAG‐3′ and reverse 5′‐AAAGAAATCATGAACACCGCTTA‐3′, SOD2, forward 5′‐CAAATTGCTGCTTGTCCAAA‐3′ and reverse 5′‐CGTGCTCCCACACATCAAT‐3′, Tenascin C, forward 5′‐GCTCAAAGCAGCCACTCATT‐3′ and reverse, 5′‐CCCATATCTGGAACCTCCTCT‐3′, and β‐actin, forward 5′‐CCAACCGCGAGAAGATGA‐3′ and reverse 5′‐CCAGAGGCGTACAGGGATAG‐3′. β‐Actin was used as an internal control as no changes were found in levels of expression of this housekeeping gene when cells were treated with anti‐β2GPI antibodies in microarray experiments. Primers for the genes were designed using the Roche universal probe library. One µg of total RNA from HUVEC incubated for 4 h in medium alone (blank), or with 2 normal control IgG preparations (N3, N4, 50 μg/ml) or 4 anti‐β2GPI antibody preparations (P1, P2, P4, P5, 50 μg/ml), or with TNFα was reverse transcribed into cDNA with the Quantitect reverse transcription kit (Qiagen) using oligo‐dT primers. Antibody preparations N3, N4, P1, P2 and P4 were previously used in the microarray experiments. Quantitative real‐time PCR was carried out with the QuantiTect SYBR Green PCR Kit (Qiagen) in the ABI 7000 sequence detector (Applied Biosystems). Gene expression levels were calculated with the absolute quantitation method,22 and normalised to the β‐actin level. All PCR reactions were carried out in duplicate, and repeated at least twice for each gene. The specificity of the PCR reactions was verified with dissociation curve analysis.
Enzyme‐linked immunosorbant assay to detect E‐selectin and IL‐8 expression levels in HUVEC
E‐selectin cell surface expression was evaluated by a cell ELISA.9 Unstimulated cells were used as a negative control and TNFα (10 ng/ml, R&D Systems) was used a positive control stimulus.15 IL‐8 levels in cell supernatants were determined using a human IL‐8 ELISA kit according to the manufacturer's instructions (BD Biosciences, Cowley, Oxon, UK).
Statistical analysis
The non‐parametric Mann–Whitney U Test was used to compare E‐selectin and IL‐8 levels between cells incubated with anti‐β2GPI antibodies and normal control IgG preparations in ELISA experiments.
Results
Anti‐β2GPI‐induced gene expression in endothelial cells
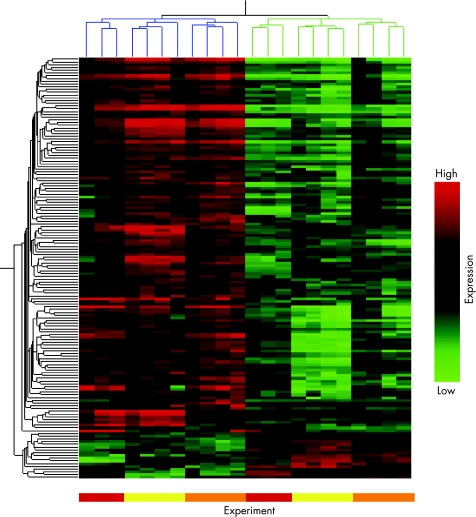
Sera were collected from four PAPs patients and anti‐β2GPI antibodies purified by protein G and β2GPI affinity column isolation. All patient derived anti‐β2GPI antibodies bound to β2GPI by ELISA and were also positive in a modified anti‐cardiolipin ELISA with but not without co‐factor, carried out as previously described (data not shown).8 Following gene chip hybridisation and scanning, HUVEC were found to express 13 727 out of 18 400 transcripts. Genes that were significantly changed (p<0.05, up or down) twofold or more were filtered and categorised as anti‐β2GPI antibody‐regulated genes. A total of 101 genes were upregulated by at least twofold or more by anti‐β2GPI antibodies (fig 1 and table 2). Figure 1 shows a hierarchical cluster analysis of upregulated and downregulated genes. Genes were clustered according to their patterns of expression (vertical axis) and also per condition (similarities between total gene expression profiles in different samples). It is noteworthy that in the dendrogram, similarities in level of gene expression are grouped (see branching) per independent experiment (that is per separate HUVEC population) rather than per antibody preparation. This implies that the greatest source of variation, in terms of genes regulated by anti‐β2GPI antibody, is determined by individual HUVEC populations rather than between individual anti‐β2GPI antibody preparations. It is likely that inter‐experiment variation masked any subtle differences in the upregulation/downregulation of genes by anti‐β2GPI antibody from different patients.
Figure 1 Hierarchical clustering of HUVEC genes changed twofold or more in expression (p<0.05) by treatment with anti‐β2GPI antibodies. In three independent experiments on different HUVEC preparations, cells were incubated for 4 h with either anti‐β2GPI antibodies (P1, P2, P3, P4, 50 μg/ml) or normal control IgG (N1, N2, N3, N4, 50 μg/ml). mRNA was isolated and processed for microarray hybridisation and analysis. A genetree and condition tree were created in GeneSpring software by average linkage hierarchical clustering using the Pearson Correlation. Each column represents results from an individual microarray chip (n = 22), each horizontal row represents a gene. Genes have been clustered according to similarities in patterns of expression (vertical axis) as well as per condition (horizontal axis). Branches are colour‐coded for anti‐β2GPI antibody treated (blue) and control IgG (green) treated samples. Coloured bars below figure also indicate location of results from the three independent experiments. Differences in expression level between anti‐β2GPI antibody treated HUVEC and those incubated with normal control IgG are clearly distinguishable on the heat map. Genes with high expression levels are in red, intermediate‐level expression in black and low‐level expression in green.
Table 2 Anti‐β2GPI antibody‐induced gene expression in HUVEC.
| Gene | Induction (‐fold) | Accession no. | Gene description | Gene | Induction (‐fold) | Accession no. | Gene description |
|---|---|---|---|---|---|---|---|
| Apoptosis/anti‐apoptosis | Metabolism | ||||||
| BCL2A1 | 14.4 | NM_004049 | BCL2‐related protein A1 | SLC7A5 | 4.7 | AB018009 | Solute carrier family 7 (cationic amino acid transporter) |
| TNAIP8 | 3.8 | NM_014350 | Tumour necrosis factor α‐induced protein 8 | PPAP2B | 4.2 | AB000889 | Phosphatidic acid phosphatase type 2B |
| TNFAIP3 | 3.6 | NM_006290 | Tumour necrosis factor α‐induced protein 3 | ASNS | 3.5 | NM_001673 | Asparagine synthetase |
| TRAF1 | 3.5 | NM_005658 | TNF receptor‐associated factor | INDO | 3.3 | M34455 | Indoleamine‐pyrrole 2,3 dioxygenase |
| BIRC3 | 3.4 | U37546 | Baculoviral IAP repeat‐containing 3 | GCH1 | 2.9 | NM_000161 | GTP cyclohydrolase 1 (dopa‐responsive dystonia) |
| CARD15 | 3.0 | NM_022162 | Caspase recruitment domain family, member 15 | S100A3 | 2.8 | NM_002960 | S100 calcium binding protein A3 |
| RIPK2 | 2.5 | AF027706 | Receptor‐interacting serine‐threonine kinase 2 | SDC4 | 2.6 | NM_002999 | Syndecan 4 (amphiglycan, ryudocan) |
| TRIB3 | 2.3 | NM_021158 | Tribbles homolog 3 (Drosophila) | MSCP | 2.5 | BE677761 | Solute carrier family 25, member 37 |
| GFPT2 | 2.5 | NM_005110 | Glutamine‐fructose‐6‐phosphate transaminase 2 | ||||
| Adhesion molecules/receptors | MT1X | 2.3 | NM_002450 | Metallothionein 1X | |||
| TNFAIP6 | 21.3 | NM_007115 | Tumour necrosis factor α‐induced protein 6 | MTIE | 2.3 | BF217861 | Metallothionein 1E (functional) |
| TNFRSF1 | 5.0 | NM_002546 | Tumour necrosis factor receptor superfamily, member 11b | PDLM4 | 2.3 | AF153882 | PDZ and LIM domain 4 |
| LLT1 | 4.7 | NM_013269 | C‐type lectin domain family 2, member D | LIPG | 2.3 | NM_006033 | Lipase, endothelial |
| BDKRB2 | 4.7 | NM_000623 | Bradykinin receptor B2 | KCNMB1 | 2.2 | U61536 | Potassium large conductance calcium‐activated channel |
| TNC | 4.5 | NM_002160 | Tenascin C (hexabrachion) | OASL | 2.2 | NM_00373 | 2′‐5′‐Oligoadenylate synthetase‐like |
| TNFRSF9 | 4.4 | NM_001561 | Tumour necrosis factor receptor superfamily, member 9 | Miscellaneous | |||
| OLR1 | 4.4 | AF035776 | Oxidised low density lipoprotein (lectin‐like) receptor 1 | TNFAIP2 | 9.7 | NM_006291 | Tumour necrosis factor α‐induced protein 2 |
| IL18R1 | 4.3 | NM_003855 | Interleukin 18 receptor 1 | DD1T4 | 2.8 | NM_019058 | DNA‐damage‐inducible transcript 4 |
| E‐Selectin | 3.8 | NM_000450 | Selectin E (endothelial adhesion molecule 1) | IFIT3 | 2.5 | NM_001549 | Interferon‐induced protein with tetratricopeptide repeats 3 |
| ICAM | 3.4 | NM_000201 | Intercellular adhesion molecule 1 (CD54) | IFIT2 | 2.3 | BE888744 | Interferon‐induced protein with tetratricopeptide repeats 2 |
| ICOSL | 3.1 | AL355690 | Inducible T‐cell co‐stimulator ligand | MOX2 | 2.2 | H23979 | CD200 antigen |
| CCRL2 | 3.0 | AF015524 | Chemokine (C‐C motif) receptor‐like 2 | ZC3HV1 | 2.2 | NM_020119 | Zinc finger CCCH‐type, antiviral 1 |
| PDZK3 | 2.8 | AF338650 | PDZ domain containing 3 | CDC42 | 2.1 | AI754416 | CDC42 effector protein (Rho GTPase binding) 3 |
| CD69 | 2.6 | L07555 | CD69 antigen (p60, early T‐cell activation antigen) | MSCP | 2.1 | NM_018579 | Solute carrier family 25, member 37 |
| IFRG28 | 2.5 | NM_022147 | 28kD Interferon responsive protein | AIMI | 2.1 | U83115 | Absent in melanoma 1 |
| JAG1 | 2.3 | U61276 | Jagged 1 (Alagille syndrome) | CHST5 | 2.1 | N32257 | Carbohydrate metabolism N‐acetylglucosamine metabolism |
| PTHLH | 2.2 | BC005961 | Parathyroid hormone‐like hormone | NAV3 | 2.0 | NM_014903 | Neuron navigator 3 |
| EB13 | 2.2 | NM_005755 | Epstein‐Barr virus induced gene 3 | ST5 | 2.0 | NM_005418 | Suppression of tumourigenicity 5 |
| VCAM | 2.2 | NM_001078 | Vascular cell adhesion molecule 1 | FLJ23231 | 2.0 | NM_025079 | Zinc finger CCCH‐type containing 12A |
| CD83 | 2.1 | NM_004233 | CD83 antigen (immunoglobulin superfamily) | ||||
| PDGFRA | 2.1 | NM_006206 | Platelet‐derived growth factor receptor α polypeptide | Transcription factors/signalling | |||
| HRH1 | 2.1 | D28481 | Histamine receptor H1 | NKX31 | 6.3 | AF247704 | Transcription factor related, locus 1 (Drosophila) |
| IL1R1 | 2.1 | NM_000877 | Interleukin 1 receptor, type I | SOD2 | 5.7 | AL050388 | Superoxide dismutase 2, mitochondrial |
| CEBPD | 5.6 | NM_005195 | CCAAT/enhancer binding protein (C/EBP), delta | ||||
| Coagulation | HIVER2 | 4.4 | AL023584 | Human immunodeficiency virus type I enhancer binding protein 2 | |||
| F3 | 3.3 | NM_001993 | Coagulation factor III (tissue factor) | DSCR1 | 3.7 | NM_004414 | Down syndrome critical region gene 1 |
| Transcription factors signalling continued RAPGEF5 | 3.4 | NM_012294 | Rap guanine nucleotide exchange factor (GEF) 5 | ||||
| Cytokines/chemokines | STC2 | 3.2 | BC000658 | Stanniocalcin 2 | |||
| CCL20 | 27.0 | NM_004591 | Macrophage inflammatory protein‐ MIP‐3 | NCF4 | 2.9 | NM_013416 | Neutrophil cytosolic factor 4, 40 kDa |
| CXCL3 | 14.7 | NM_002090 | Chemokine (C‐X‐C motif) ligand 3 | APOL3 | 2.7 | NM_014349 | Apolipoprotein L3 |
| CSF2 | 11.6 | M11734 | Colony stimulating factor 2 (granulocyte‐macrophage) | SNFT | 2.6 | NM_018664 | Jun dimerisation protein p21SNFT |
| CX3CL1 | 10.5 | NM_002996 | Fractalkine | RND1 | 2.6 | U69563 | Rho family GTPase 1 |
| CSF3 | 6.9 | NM_000759 | Colony stimulating factor 3 (granulocyte) | MAP3K8 | 2.6 | NM_005204 | Mitogen‐activated protein kinase kinase kinase 8 |
| IL6 | 6.0 | NM_000600 | Interleukin 6 (interferon β2) | FOXF1 | 2.5 | NM_001451 | Forkhead box F1 |
| CXCL5 | 5.6 | AK026546 | Chemokine (C‐X‐C motif) ligand 5 | IRF1 | 2.5 | NM_002198 | Interferon regulatory factor 1 |
| IL1β | 5.1 | NM_000576 | Interleukin 1β | MSC | 2.4 | AF060154 | Musculin (activated B‐cell factor‐1) |
| CXCL2 | 4.1 | M57731 | Chemokine (C‐X‐C motif) ligand 2 | ||||
| FGF18 | 4.1 | NM_003862 | Fibroblast growth factor 18 | RGS2 | 2.3 | NM_002923 | Regulator of G‐protein signalling 2, 24 kDa |
| CXCL1 | 4.0 | NM_001511 | Chemokine (C‐X‐C motif) ligand 1 | STAT5A | 2.2 | NM_003152 | Signal transducer and activator of transcription 5A |
| LIF | 3.6 | NM_002309 | Leukaemia inhibitory factor (cholinergic differentiation factor) | MEOX1 | 2.2 | NM_004527 | Mesenchyme homeo box1 |
| CXCL10 | 3.3 | NM_001565 | Chemokine (C‐X‐C motif) ligand 10 | NFKB1 | 2.2 | M55643 | Nuclear factor of kappa light polypeptide gene enhancer |
| CXCL11 | 2.3 | AF030514 | Chemokine (C‐X‐C motif) ligand 11 | ISG20 | 2.2 | NM_002201 | Interferon stimulated exonuclease gene 20 kDa |
| LTB | 2.3 | NM_002341 | Lymphotoxin β (TNF superfamily, member 3) | ABTB2 | 2.2 | AL050374 | Ankyrin repeat and BTB (POZ) domain containing 2 |
| IL8 | 2.2 | AF043337 | Interleukin 8 | ||||
| CCL5 | 2.1 | NM_002985 | Chemokine (C‐C motif) ligand 5 | ||||
| CCL8 | 2.1 | AI984980 | Chemokine (C‐C motif) ligand 8 | ||||
| PBEF1 | 2.0 | BF575514 | Pre‐B‐cell colony enhancing factor 1 | ||||
| CSF1 | 2.0 | M37435 | Colony stimulating factor 1 | ||||
Confirmation of microarray data by real‐time RT‐PCR and ELISA
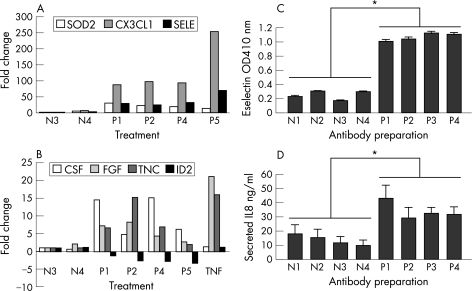
Real‐time RT‐PCR analysis was carried out for selected novel anti‐β2GPI antibody regulated genes, covering a range of different levels of regulation. Genes included in this analysis were CSF3, CX3CL1, FGF18, SOD2 and Tenascin C plus E‐selectin as a positive control gene. We also included the downregulated gene ID2 in these experiments. The results of these experiments are shown in fig 2. All six upregulated genes (CSF3, CX3CL1, E‐Selectin, FGF18, Tenascin C and SOD2) were also found to be upregulated by real time PCR analysis. Levels of upregulation were variable, but CX3CL1 was the highest‐fold upregulated gene of the six selected genes by microarray analysis (fig 2A). ID2 was downregulated 2.3‐fold by microarray analysis and this was very similar to the level of downregulation by real‐time PCR analysis. (fig 2B). In the present study increased mRNA levels of E‐selectin and IL‐8 following anti‐β2GPI antibody treatment and microarray analysis were consistent with increased protein levels following antibody treatment (figs 1, 2C, D).
Figure 2 Quantitative real‐time RT‐PCR and ELISA analysis of anti‐β2GPI antibody‐mediated gene regulation in HUVEC. Cells were incubated with different anti‐β2GPI antibody preparations (P1, P2, P4, P5, 50 μg/ml) or with control normal IgG (N3, N4, 50 μg/ml) or, TNFα for 4 h and total RNA isolated and processed for real‐time PCR analysis. Antibody preparations N3, N4, P1, P2 and P4 were previously used in the microarray experiments. Gene expression levels were normalised to the β‐actin mRNA level. The results show change in expression level relative to control normal IgG (N3) level and represent the mean of duplicate samples from two independent experiments. (A) shows data for SOD2, CX3CL1 and E‐selectin. TNFα induced high‐level expression of these genes but induction levels were off the scale and omitted from the figure, (B) shows data for CSF, FGF, Tenascin C (TNC) and ID2. TNFα‐regulated changes in levels of expression are included for comparison. The effect of four control normal IgG and four APS‐derived anti‐β2GPI antibody preparations (used in microarray experiments) on E‐selectin expression (C) and IL‐8 secretion (D) was determined by ELISA. Antibodies were incubated with the cells for 4 h at 50 μg/ml. Results show mean ±SEM of triplicate samples from a representative experiment (one of three). * = p<0.03 as determined by two‐tailed Mann–Whitney U Test. TNFα also induced high levels of E‐selectin and IL‐8 in HUVEC as measured by ELISA (data not shown).
We assigned the 101 upregulated genes to the following APS relevant functional groups; cell receptors/adhesion molecules, cytokines/chemokines, coagulation genes, apoptosis/anti‐apoptosis, transcription factors/signalling, metabolism and miscellaneous genes (table 2). Of particular note are the high level of induction of the chemokines CCL20, CXCL3, CX3CL1, CXCL5, CXCL2 and CXCL1 as well as genes classically associated with pro‐inflammatory cytokine TNFα signalling such as TNFAIP6, TNFAIP2, TNFAIP8, TNFAIP3 and the anti‐apoptotic gene BCL2A1. Cell receptors induced included Tenascin C, OLR1 (oxLDL receptor) and IL‐18 receptor 1. Other induced genes of interest included growth factors CSF2, CSF3, IL‐6, IL‐1β and FGF18. The list of upregulated genes includes some previously identified anti‐β2GPI‐induced genes such as E‐selectin, Tissue Factor (TF), ICAM‐1 and VCAM‐110 but the majority of the genes we have identified represent anti‐β2GPI‐induced genes not previously reported.
Anti‐β2GPI‐mediated downregulation of gene expression in endothelial cells
A smaller panel of anti‐β2GPI antibody‐regulated genes in EC were downregulated (fig 2 and table 3). None of these genes has previously been reported to be anti‐β2GPI antibody‐regulated genes in EC. The majority of the 14 downregulated genes encode signalling and transcription factors/signalling molecules. Two receptor/adhesion molecules were also downregulated. GJA4 (connexin 37) is a gap junctional protein and OCLN (Occludin) is a structural protein of tight junctions.
Table 3 Anti‐β2GPI antibody downregulation of gene expression in HUVEC.
| Gene | Reduction (‐fold) | Accession no. | Gene description |
|---|---|---|---|
| Adhesion molecules/receptors | |||
| GJA4 | 2.9 | NM_002060 | Gap junction protein α4, 37 kDa (connexin 37) |
| OCLN | 2.1 | U53823 | Occludin |
| Cytokine/chemokine | |||
| BDNF | 2.1 | NM_001709 | Brain‐derived neurotrophic factor |
| Metabolism | |||
| GFOD1 | 2.0 | NM_018988 | Glucose‐fructose oxidoreductase domain containing 1 |
| Miscellaneous | |||
| 13CDNA73 | 2.2 | NM_023037 | Hypothetical protein CG003 |
| Transcription factors/signalling | |||
| MEOX2 | 3.1 | NM_005924 | Mesenchyme homeo box 2 |
| MAF | 3.0 | NM_005360 | v‐maf Musculoaponeurotic fibrosarcoma oncogene homolog (avian) |
| BMP4 | 2.8 | D30751 | Bone morphogenetic protein 4 |
| TXB1 | 2.8 | AF012130 | T‐box 1 |
| ID2 | 2.3 | NM_002166 | Inhibitor of DNA binding 2 |
| DACH1 | 2.2 | NM_004392 | Dachshund homolog 1 (Drosophila) |
| RUNX1T1 | 2.2 | NM_004349 | Runt‐related transcription factor 1 |
| MAFB | 2.0 | NM_005461 | v‐maf Musculoaponeurotic fibrosarcoma oncogene homolog B |
| ZNF365 | 2.0 | NM_014951 | Zinc finger protein 365 |
Discussion
The most striking feature of this study is the extent and diversity of anti‐β2GPI antibody regulated genes in EC. The results reveal induction of a complex pro‐inflammatory, as well as, a pro‐adhesive and pro‐coagulant milieu by these antibodies, which could potentially be involved in the pathogenesis of PAPs.
It is intriguing that many of the most highly upregulated genes in the present study are chemokines such as CCL20, CXCL3, CX3CL1 (fractalkine), CXCL5, CXCL2 and CXCL1, which are involved in recruitment, chemotaxis and proliferation of mononuclear cells and/or granulocytes. These findings are consistent with a number of in vitro and in vivo studies reporting that anti‐β2GPI antibodies increased monocyte adhesion to EC.9,11,12 Moreover, placental biopsies from APS patients had a higher concentration of inflammatory cells particularly macrophages23 and an association has been found between neutrophil recruitment and foetal loss in APS.24
CX3CL1 (fractalkine) and its receptor CX3CR1 are expressed in atherosclerotic lesions of humans and mice25 and in CX3CL1‐deficient mice there is a major reduction of atherosclerosis.26 Roughly one third of PAPS patients have atherosclerosis and a direct association of aPL with the pathogenesis of accelerated atherosclerosis in APS patients has been reported.27,28 aPLs are thought to accelerate this process by activating EC. β2GPI has also been demonstrated in high concentration in atherosclerotic plaque.29 Other cytokine and adhesion molecules found to be upregulated by anti‐β2GPI antibody could also have a role in the development of atherosclerosis. Monocytes have been shown to strongly express IL‐18 in atheromatous lesions in situ30 and EC expression of IL‐18R was increased 4.3‐fold in our study. Gerdes et al.31 suggested an IL‐18 mediated paracrine proinflammatory pathway involving monocytes ECs and smooth muscle cells in association with atherogenesis.
Expression of oxLDL receptor OLR1 was upregulated over fourfold in our study (table 2). OLR1 expressed on vascular EC is involved in binding, internalisation and degradation of oxLDL and might therefore play a significant role in atherogenesis.32,33 Anti‐β2GPI antibodies bind to β2GPI‐oxLDL complexes and have been shown in vitro to enhance uptake into monocytes/macrophages potentially accelerating the lesion formation.34 GJA4 (connexin 37), a gap junction protein, was downregulated by anti‐β2GPI antibodies and polymorphisms in this protein have been associated with the development of arteriosclerotic plaques in human subjects.35
We have confirmed by gene microarray profiling anti‐β2GPI mediated upregulation of molecules, previously reported to be upregulated at the protein level, including TF, E‐selectin, ICAM, and VCAM‐1.9,11,12,36,37,38 It is tempting to speculate that a combination of increased adhesion molecules, pro‐inflammatory cytokines and chemokines in addition to increased TF expression could strongly support development of thrombosis and contribute to the advancement of atherosclerotic lesions in response to anti‐β2GPI antibodies.
A number of pro‐angiogenic cytokines/chemokines such as IL‐8 and fibroblast growth factor, which were upregulated by anti‐β2GPI antibodies in the present study, might contribute to the hyperplasia associated with PAPS. APS is associated with EC proliferation and fibrosis characterised by intimal hyperplasia within the lumen of micro‐capillaries typically within the kidney or skin.39 It is also associated with cardiac lesions involving thickening of heart valves with deposition of aPL in the subendothelial layers.40 Histologic examination of renal biopsies from 16 patients with PAPs showed small vessel vaso‐occlusive lesions associated with myofibroblastic intimal cellular proliferation and thrombosis, five patients showed endothelialised channels indicating recanalising thrombosis and EC proliferation.41
β2GPI has been reported to bind to cells in a number of different ways. For example, it can bind to anionic membrane molecules such as heparan sulphate and it has also been reported to bind as a ligand to the annexin II receptor.10,42 Intriguingly, an indirect mechanism for EC stimulation by anti‐β2GPI antibody could exist. Annexin II is also a high affinity receptor for Tenascin C, a component of the extracellular matrix that functions as an adhesion molecule, shown in this study to be upregulated by anti‐β2GPI antibody.43
One possibility that has to be considered, however, is that our regulated genes might include some or many genes not regulated by anti‐β2GPI antibody directly but rather indirectly by autocrine cytokine/chemokine production produced by EC cells shortly after anti‐β2GPI antibody exposure. We chose to study gene expression at 4 h after exposure to anti‐β2GPI antibody in order to study the early gene expression profile. However, many cytokines/chemokines are induced rapidly and they could themselves then induce gene expression in EC by binding to high affinity receptors on the EC. This possibility should be addressed in future studies aimed at investigating the signal transduction mechanisms responsible for anti‐β2GPI antibody mediated gene regulation in EC. The anti‐β2GPI antibody induced gene panel described here is largely distinct to those described in gene profiling studies on HUVEC with different cytokines or LPS.44,45,46 Nonetheless, a small subset of anti‐β2GPI antibody‐induced genes (for example; E‐selectin, IL‐8, VCAM‐1, TNFAIP6, TNFAIP2, TNFAIP8, TNFAIP3), have been shown to be induced by cytokine and/or LPS‐induced gene profiling of HUVEC.44,45,46 A study profiling monoclonal anti‐β2GPI antibody mediated gene regulation in human monocytes found upregulation of a number of genes using cDNA arrays, such as, IL‐1β and TF, which were also identified in our study.16 However many more anti‐β2GPI antibody regulated genes in HUVEC were found in the present study probably due to the much larger number of genes represented on the Affymetrix chips in comparison to the cDNA arrays used in the earlier study.16
An important question in relation to our findings is how they relate to the in vivo situation. A recent study, measuring a limited number of parameters of EC function, concluded that aPL were unable to support a full‐blown endothelial perturbation in vivo.47 There is evidence however from other studies for increased circulating levels of TF, IL‐6, TNFα48 and VCAM‐149 in APS patients. Our studies suggest increased levels of some cytokines might, at least in part, be EC derived and therefore evidence of endothelial perturbation in vivo.
In conclusion, global gene expression profiling using microarray technology has been used for the first time to examine the extent and diversity of PAPs patient‐derived anti‐β2GPI antibody mediated gene regulation in HUVEC. These studies have identified important anti‐β2GPI antibody regulated EC genes that might contribute to the vasculopathy in PAPs. Further studies on signal transduction mechanisms responsible for anti‐β2GPI antibody mediated gene regulation and the role of individual anti‐β2GPI antibody gene targets in APS pathogenesis should provide opportunities for new therapeutic strategies by either inhibiting the expression of particular (pathogenic) genes or the activation of corresponding signalling pathways. Moreover, these findings could have wider implications for other autoimmune diseases, where anti‐β2GPI antibodies have been described.
Acknowledgements
We would like to thank Beverley Hunt for providing sera samples from patients and Ewan Hunter for help and advice with analysis of microarray data. We also thank Pier‐Luigi Meroni for help and advice on isolating antibodies from patient sera, Phil Marsh for help and advice on real‐time PCR analysis and Helen Collins and Steve Thompson for helpful discussions on the manuscript.
Footnotes
This work was supported by Lupus UK and the Arthritis Research Campaign.
Competing interests: None.
References
- 1.Cameron J S, Frampton G. The antiphospholipid syndrome and the lupus anticoagulant. Pediatr Nephro 19904663–678. [DOI] [PubMed] [Google Scholar]
- 2.Roubey R A S. Immunology of the antiphospholipid antibody syndrome. Arthritis Rheum 1996391444–1454. [DOI] [PubMed] [Google Scholar]
- 3.Blank M, Cohen J, Toder V, Shoenfeld Y. Induction of anti‐phospholipid syndrome in naïve mice with lupus monoclonal and human polyclonal anticardiolipin antibodies. Proc Natl Acad Sci USA 1991883069–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritchie D S, Sainani A, D'Souza A, Grigg A P. Passive donor‐to‐recipient transfer of antiphospholipid syndrome following allogeneic stem‐cell transplantation. Am J Hematol 200579299–302. [DOI] [PubMed] [Google Scholar]
- 5.Del Papa N, Sheng Y H, Raschi E, Kandiah D A, Tincani A, Khamashta M A.et al Human β2−glycoprotein I binds to endothelial cells through a cluster of lysine residues that are critical for anionic phospholipid binding and offers epitopes for anti‐β2−glycoprotein I antibodies. J Immunol 19981605572–5578. [PubMed] [Google Scholar]
- 6.Ichikawa K, Khamashta M A, Koiki T, Matsura E, Hughes G R V. β2‐Glycoprotein I reactivity of monoclonal anticardiolipin antibodies from patients with the antiphospholipid syndrome. Arthritis Rheum 1994371453–1461. [DOI] [PubMed] [Google Scholar]
- 7.Del Papa N, Guidali L, Spatola L, Bonara P, Borghi M O, Tincani A.et al Relationship between anti‐phospholipid and anti‐endothelial antibodies III: β2‐glycoprotein I mediates the antibody binding to endothelial membranes and induces the expression of adhesion. Clin Exp Rheumatol 199513179–185. [PubMed] [Google Scholar]
- 8.George J, Blank M, Levy Y, Meroni P L, Damianovich M, Tincani A.et al Differential effects of anti‐β2‐glycoprotein I antibodies on endothelial cells and on the manifestations of experimental antiphospholipid syndrome. Circulation 199897900–906. [DOI] [PubMed] [Google Scholar]
- 9.Del Papa N, Guidali L, Sala A, Buccellati C, Khamashta M A, Ichikawa K.et al Endothelial cells as target for antiphospholipid antibodies. Human polyclonal and monoclonal anti‐β2‐glycoprotein I antibodies react in vitro with endothelial cells through adherent β2‐glycoprotein I and induce endothelial activation. Arthritis Rheum 199740551–561. [DOI] [PubMed] [Google Scholar]
- 10.Meroni P, Ronda N, Raschi E, Borghi M O. Humoral autoimmunity against endothelium: theory or reality? Trends Immunol 200526275–281. [DOI] [PubMed] [Google Scholar]
- 11.Pierangeli S S, Colden‐Stanfield M, Liu X, Barker J H, Anderson G L, Harris E N. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation 1999991997–2002. [DOI] [PubMed] [Google Scholar]
- 12.Simantov R, LaSala J M, Lo S K, Gharavi A E, Sammaritano L R, Salmon J E.et al Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest 1995962211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierangeli S S, Gharavi A E, Harris E N. Experimental thrombosis and antiphospholipid antibodies: new insights. J Autoimmun 200015241–247. [DOI] [PubMed] [Google Scholar]
- 14.Dunoyer‐Geindre S, de Moerloose P, Galve‐de Rochemonteix B, Reber G, Kruithof E K. NFkappaB is an essential intermediate in the activation of endothelial cells by anti‐β(2)‐glycoprotein I antibodies. Thromb Haemost 200288851–857. [PubMed] [Google Scholar]
- 15.Raschi E, Testoni C, Bosisio D, Borghi M O, Koike T, Mantovani A.et al Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood 20031013495–00. [DOI] [PubMed] [Google Scholar]
- 16.Bohgaki M, Atsumi T, Yamashita Y, Yasuda S, Sakai Y, Furusaki A.et al The p38 mitogen‐activated protein kinase (MAPK) pathway mediates induction of the tissue factor gene in monocytes stimulated with human monoclonal anti‐β2glycoprotein I antibodies. Int Immunol 2004161633–1641. [DOI] [PubMed] [Google Scholar]
- 17.Wilson W A, Gharavi A E, Koike T, Lockshin M D, Branch D W, Piette J C.et al International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum 1999421309–1311. [DOI] [PubMed] [Google Scholar]
- 18.Wang M X, Kandiah D A, Ichikawa K, Khamashta M A, Hughes G, Koike T.et al Epitope specificity of monoclonal anti‐β2‐glycoprotein I antibodies derived from patients with the antiphospholipid syndrome. J Immunol 19951551629–1636. [PubMed] [Google Scholar]
- 19.Harris E N, Gharavi A E, Patel S P, Hughes G R. Evaluation of the anti‐cardiolipin antibody test: report of an international workshop held 4 April 1986. Clin Exp Immunol 198768215–222. [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 1973522745–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B (Methodological) 199557289–00. [Google Scholar]
- 22.Bustin S A. Absolute quantification of mRNA using real‐time reverse transcription polymerase chain reactions assays. J Mol Endocrin 200025169–193. [DOI] [PubMed] [Google Scholar]
- 23.Stone S, Pijnenborg R, Vercruysse L, Poston R, Khamashta M A, Hunt B J.et al The placental bed in pregnancies complicated by primary antiphospholipid syndrome. Placenta 200627457–467. [DOI] [PubMed] [Google Scholar]
- 24.Girardi G, Berman J, Redecha P, Spruce L, Thurman J M, Kraus D.et al Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 20031121644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas A D, Bursill C, Guzik T J, Sadowski J, Channon K M, Greaves D R. Smooth muscle cells in human atherosclerotic plaques express the fractalkine receptor CX3CR1 and undergo chemotaxis to the CX3C chemokine fractalkine (CX3CL1). Circulation 20031082498–2504. [DOI] [PubMed] [Google Scholar]
- 26.Teupser D, Pavlides S, Tan M, Gutierrez‐Ramos J‐C, Kolbeck R, Breslow J L. Major reduction of atherosclerosis in fractalkine (CX3CL1)‐deficient mice is at the brachiocephalic artery, not the aortic root. PNAS 200410117795–17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuura E, Kobayashi K, Yasuda T, Koike T. Antiphospholipid antibodies and atherosclerosis. Lupus 19987S135–S139. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura E, Koike T. Accelerated atheroma and anti‐β2‐glycoprotein I antibodies. Lupus 20009210–216. [DOI] [PubMed] [Google Scholar]
- 29.George J, Harats D, Gilburd B, Afek A, Levy Y, Schneiderman J.et al Immunolocalization of β2‐glycoprotein i (apolipoprotein H) to human atherosclerotic plaques: potential implications for lesion progression. Circulation 1999992227–2230. [DOI] [PubMed] [Google Scholar]
- 30.Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y.et al Expression of interleukin‐18 in human atherosclerotic plaques and relation to plaque instability. Circulation 20011041598–1603. [DOI] [PubMed] [Google Scholar]
- 31.Gerdes N, Sukhova G K, Libby P, Reynolds R S, Young J L, Schonbeck U. Expression of interleukin (IL)‐18 and functional IL‐18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med 2002195245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kume N, Murase T, Moriwaki H, Aoyama T, Sawamura T, Masaki T.et al Inducible expression of lectin‐like oxidized LDL receptor‐1 in vascular endothelial cells. Circ Res 1998833322–327. [DOI] [PubMed] [Google Scholar]
- 33.Mango R, Clementi F, Borgiani P, Forleo G B, Federici M, Contino G.et al Association of single nucleotide polymorphisms in the oxidized LDL receptor 1 (OLR1) gene in patients with acute myocardial infarction. J Med Genet 200340933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasunuma Y, Matsuura E, Makita Z, Katahira T, Nishi S, Koike T. Involvement of β2‐glycoprotein I and anticardiolipin antibodies in oxidatively modified low‐density lipoprotein uptake by macrophages. Clin Exp Immunol 1997107(3)569–573. [DOI] [PubMed] [Google Scholar]
- 35.Boerma M, Forsberg L, Van Zeijl L, Morgenstern R, De Faire U, Lemne C.et al A genetic polymorphism in connexin 37 as a prognostic marker for atherosclerotic plaque development. J Intern Med 1999246211–218. [DOI] [PubMed] [Google Scholar]
- 36.Cuadrado M J, Lopez‐Pedrera C, Khamashta M A, Camps M T, Tinahones F, Torres A.et al Thrombosis in primary antiphospholipid syndrome: a pivotal role for monocyte tissue factor expression. Arthritis Rheum 199740834–841. [DOI] [PubMed] [Google Scholar]
- 37.Kornberg A, Blank M, Kaufman S, Shoenfeld Y. Induction of tissue factor‐like activity in monocytes by anti‐cardiolipin antibodies. J Immunol 19941531328–1332. [PubMed] [Google Scholar]
- 38.Lopez‐Pedrera C H, Buendia P, Aguirre M A, Velasco F, Cuadrado M J. Antiphospholipid syndrome and tissue factor: a thrombotic couple. Lupus 200615161–166. [DOI] [PubMed] [Google Scholar]
- 39.Frampton G, Hicks J, Cameron J S. Significance of anti‐phospholipid antibodies in patients with lupus nephritis. Kidney Int 1991391225–1231. [DOI] [PubMed] [Google Scholar]
- 40.Nesher G, Ilany J, Rosenmann D, Abraham A S. Valvular dysfunction in antiphospholipid syndrome: prevalence, clinical features, and treatment. Semin Arthritis Rheum 19972727–35. [DOI] [PubMed] [Google Scholar]
- 41.Nochy D, Daugas E, Droz D, Beaufils H, Grunfeld J P, Piette J C.et al The intrarenal vascular lesions associated with primary antiphospholipid syndrome. J Am Soc Nephrol 199910507–518. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, McCrae K R. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti‐β2 glycoprotein I antibodies. Blood 20051051964–1969. [DOI] [PubMed] [Google Scholar]
- 43.Chung C Y, Murphy‐Ullrich J E, Erickson H P. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin‐C interacting with its cell surface receptor, annexin II. Mol Biol Cell 19967883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao B, Bowden R A, Stavchansky S A, Bowman P D. Human endothelial cell response to gram‐negative lipopolysaccharide assessed with cDNA microarrays. Am J Physiol Cell Physiol 20012811587–1595. [DOI] [PubMed] [Google Scholar]
- 45.Zhao B, Stavchansky S A, Bowden R A, Bowman P D. Effect of interleukin‐1β and tumour necrosis factor‐α on gene expression in human endothelial cells. Am J Physiol Cell Physiol 20032841577–1583. [DOI] [PubMed] [Google Scholar]
- 46.Mayer H, Bilban M, Kurtev V, Gruber F, Wagner O, Binder B R.et al Deciphering regulatory patterns of inflammatory gene expression from interleukin‐1‐stimulated human endothelial cells. Arterioscler Thromb Vasc Biol 2004241192–1198. [DOI] [PubMed] [Google Scholar]
- 47.Meroni P L, Borghi M O, Raschi E, Ventura D, Sarzi Puttini P C, Atzeni F.et al Inflammatory response and the endothelium. Throm Res 2004114329–334. [DOI] [PubMed] [Google Scholar]
- 48.Forastiero R R, Martinuzzo M E, de Larrañaga G F. Circulating levels of tissue factor and proinflammatory cytokines in patients with primary antiphospholipid syndrome or leprosy related antiphospholipid antibodies. Lupus 200514129–136. [DOI] [PubMed] [Google Scholar]
- 49.Kaplanski G, Cacoub P, Farnarier C, Marin V, Gregoire R, Gatel A.et al Increased soluble vascular cell adhesion molecule 1 concentrations in patients with primary or systemic lupus erythematosus‐related antiphospholipid syndrome: correlations with the severity of thrombosis. Arthritis Rheum 20004355–64. [DOI] [PubMed] [Google Scholar]