Abstract
Objective
The aim was to determine whether lower visceral pain thresholds in irritable bowel syndrome (IBS) primarily reflect physiological or psychological factors.
Methods
Firstly, 121 IBS patients and 28 controls underwent balloon distensions in the descending colon using the ascending methods of limits (AML) to assess pain and urge thresholds. Secondly, sensory decision theory analysis was used to separate physiological from psychological components of perception: neurosensory sensitivity (p(A)) was measured by the ability to discriminate between 30 mm Hg vs 34 mm Hg distensions; psychological influences were measured by the report criterion—that is, the overall tendency to report pain, indexed by the median intensity rating for all distensions, independent of intensity. Psychological symptoms were assessed using the Brief Symptom Inventory (BSI).
Results
IBS patients had lower AML pain thresholds (median: 28 mm Hg vs 40 mm Hg; p<0.001), but similar neurosensory sensitivity (median p(A): 0.5 vs 0.5; p = 0.69; 42.6% vs 42.9% were able to discriminate between the stimuli better than chance) and a greater tendency to report pain (median report criterion: 4.0 (“mild” pain) vs 5.2 (“weak” pain); p = 0.003). AML pain thresholds were not correlated with neurosensory sensitivity (r = −0.13; p = 0.14), but were strongly correlated with report criterion (r = 0.67; p<0.0001). Report criterion was inversely correlated with BSI somatisation (r = −0.26; p = 0.001) and BSI global score (r = −0.18; p = 0.035). Similar results were seen for the non‐painful sensation of urgency.
Conclusion
Increased colonic sensitivity in IBS is strongly influenced by a psychological tendency to report pain and urge rather than increased neurosensory sensitivity.
Keywords: hypersensitivity, hypervigilance, perceptual response bias, irritable bowel syndrome
During balloon distension of the rectum or colon patients with irritable bowel syndrome (IBS) report pain and discomfort at abnormally low volumes or pressures.1,2,3 These lower pain thresholds have been interpreted to represent visceral hypersensitivity4,5 and have been attributed to physiological differences in IBS patients.6,7,8 Mertz et al even proposed that lower pain thresholds are “a reliable biological marker of IBS.”9 However, it is impossible to attribute lower IBS pain thresholds specifically to underlying physiological mechanisms3,10 since cognitive and psychological influences affect the reporting of pain and, by extension, affect threshold measurements.1,11,12
The physiological and psychological components that determine pain thresholds can be separately quantified by sensory decision theory analysis (SDT).13 In SDT stimuli of different intensities are presented in an unpredictable order and subjects rate the intensity of each stimulus. Statistical decision theory is then used to determine:
The discrimination index (p(A)): a measure of neurosensory sensitivity (physiological) that is based on the subject's ability to discriminate between two stimuli of similar, yet distinct, intensities. The discrimination index is reduced by local nerve blocks and analgesics, but is immune to cognitive and psychological manipulations.14,15
The report criterion (B): a measure of the subject's overall tendency to label any stimuli as weak vs intense, independent of the actual stimulus intensity. The report criterion is susceptible to cognitive and psychological manipulations such as suggestion and placebo, but is not affected by analgesics.14,15,16
The primary aim of this study was to determine whether differences in pain thresholds between patients with IBS and healthy controls are explained primarily by differences in neurosensory sensitivity (physiological differences) or differences in the overall tendency to report pain (psychological differences). The secondary aim was to determine and explain differences in urge thresholds. Ultimately, a better understanding of the factors that affect these thresholds will improve our understanding of the mechanisms responsible for hypersensitivity and might help to direct therapy. Accordingly, we used AML to compare sensory thresholds in both IBS patients and healthy controls, and SDT supplemented by psychological questionnaires to determine how physiological and psychological factors contribute to these thresholds. We hypothesised that, compared to healthy controls, IBS patients would have: (1) lower AML determined pain and urge thresholds; (2) similar levels of neurosensory sensitivity; and (3) a lower report criterion (that is, an increased overall tendency to report stimuli as intense). (4) We also hypothesised that AML pain thresholds and the report criterion would be inversely correlated with levels of psychological distress.
Methods
Subjects
Subjects were recruited by advertisements or physician referrals and screened by telephone. The study was approved by the institutional review board of the University of North Carolina (UNC) and all subjects provided informed consent.
IBS patients
The study population consisted of 132 patients (84% female; median age 35 years) who met Rome II criteria for IBS17 and had current symptom activity (abdominal pain at least once a week in the past month). Twenty‐seven IBS patients were constipation predominant (IBS‐C), 31 were diarrhoea predominant IBS (IBS‐D), and 61 were not classifiable as either. These subjects had no history of gastrointestinal resection (other than appendectomy or cholecystectomy), known IBS, coeliac disease, lactose malabsorption, heart disease, or diabetes mellitus, and they were not pregnant at the time of study. IBS patients were required to stop the following medications—antidepressants (seven days before study), antispasmodics, muscle relaxants or narcotic analgesics (three days); and non‐steroidal anti‐inflammatory agents (one day).
Controls
The control population consisted of 31 subjects (71% female; median age 40 years) without any significant or recurring gastrointestinal symptoms; exclusion criteria were average stool frequency of less than three per week or more than three per day, abdominal pain, use of a laxative or anti‐diarrhoeal agent on more than two occasions over the previous year, history of alcohol or substance abuse, a psychiatric diagnosis, or any of the medical conditions listed above for the IBS patients. None of these healthy subjects had used any antidepressants, antispasmodics, muscle relaxants, or narcotic analgesics for at least one year. Non‐steroidal anti‐inflammatory agents were not permitted for at least one day before the study. There were no significant differences between the IBS group and healthy controls for age (p = 0.72) or sex (p = 0.12).
Psychological evaluation
On the first day of the study subjects reported to the UNC General Clinical Research Center (GCRC) at 11 am where they completed the Brief Symptom Inventory‐18 (BSI‐18). This is an 18‐item measure of psychological distress along three primary symptom dimensions: somatisation, anxiety, and depression.18 The BSI‐18 was also scored for the global severity index. The rationale for including the BSI somatisation scale is that somatic hypervigilance is hypothesised to play a part in visceral hypersensitivity.12 The BSI depression, anxiety, and global scales were included based on the convention of regarding depression and anxiety as the primary dimensions of psychological distress.
Colonic sensory testing
At approximately 4 pm subjects underwent bowel preparation with 3 oz of Fleets Phospho‐Soda followed by an overnight fast. On the morning of the second day (approximately 8 am) a barostat catheter was placed into the descending colon for sensory testing. Firstly, a guide wire was inserted to the level of the splenic flexure using a flexible sigmoidoscope. The sigmoidoscope was then withdrawn and a barostat catheter (Model No C7‐CB‐0026, Mui Scientific, Mississauga, Ontario, Canada) was inserted over the guide wire. The guide wire was then withdrawn and barostat placement was confirmed by fluoroscopy. No sedation was used throughout the duration of this procedure. A 600 ml plastic bag (Model No CT‐BP600R, Mui Scientific, Mississauga, Ontario, Canada) was attached to the catheter, and the catheter was connected to a computer controlled piston type pump (barostat) that was capable of inflating and deflating the bag at a rate of 38 ml/s (G&J Electronics, Willodale, Ontario, Canada). The pump was interfaced to a computer running a software program that recorded the pressure inside the bag 16 times per second.
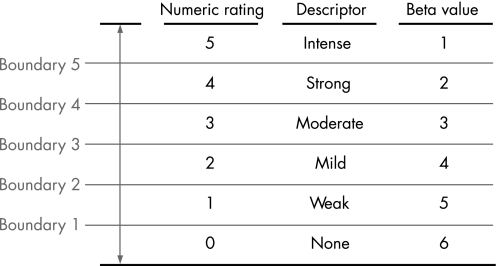
Subjects were instructed to give separate ratings of the intensity of pain and urgency to defecate experienced at the end of each distension, using a six point scale (0 = no sensation; 1 = weak; 2 = mild; 3 = moderate; 4 = strong; 5 = intense) (fig 1). The scale was visible to subjects during the procedure. Sample distensions were then performed during which the barostat bag was inflated in a stepwise fashion by increasing bag pressure by 4 mm Hg every 15 seconds until the subject reported moderate pain (rating of 3). The purpose of the sample distensions was threefold: (1) to insure that the barostat bag was unfolded; (2) to teach the subject how to use the rating scale to rate the intensity of colonic sensations; and (3) to decrease anticipatory anxiety. The barostat bag was then slowly inflated with 30 ml of air and the pressure was allowed to equilibrate for 3 minutes. The average pressure during the last 15 seconds defined the individual operating pressure (IOP): the minimum pressure required to overcome mechanical forces and inflate the bag with 30 ml of air.
Figure 1 Subjects rated the intensity of each stimulus on the six point rating scale showed above. The corresponding descriptor and beta value for each numeric rating are shown. Boundaries separate consecutive ratings.
Ascending method of limits (AML) protocol
This protocol started approximately 90 minutes following barostat placement. Phasic distensions were 30 seconds in duration and were separated by 30‐second rest intervals starting at the IOP and progressively increasing in 2 mm Hg steps until either the subject requested the research nurse to stop the protocol or 48 mm Hg was reached. The pain threshold was defined as the amount of pressure above IOP at which the subject first reported moderate pain (absolute distending pressure minus the IOP). If the subject requested that the research nurse stop the trial before moderate pain was reported (for example, because of urge to defecate) then the pain threshold was not determined. If the subject reached 48 mm Hg without reporting moderate pain, then the pain threshold was defined as 50 mm Hg minus the IOP. The urge threshold was defined analogously.
Sensory decision theory (SDT) protocol
This protocol started approximately 100 minutes following barostat placement. Subjects were instructed that the purpose was to evaluate how well they could discriminate between different balloon pressures. Twenty‐four 30‐second phasic distensions (eight at 30 mm Hg, eight at 32 mm Hg, and eight at 34 mm Hg) were presented in an unpredictable order separated by 30‐second rest intervals at the IOP. These stimulus intensities were selected to bracket the average pain threshold determined by AML in a previous study of SDT.19 The choice of 2 mm Hg increments between stimuli was based on this previous study in which this difference was found to work well (that is, subjects made some errors of classification but discrimination was better than chance).19 This protocol followed the recommendation of McNicol20 and one of the co‐investigators who is an expert on SDT (WCC). The subjects were able to stop the protocol at any time.
Discrimination index (p(A)) and report criterion (B) values for the 30 mm Hg vs 34 mm Hg stimuli were calculated for each subject using a computer program developed by MN Janal and WC Clark (personal communication). This program was based on formulas taken from McNicol for non‐parameteric SDT analysis of rating scale data.20
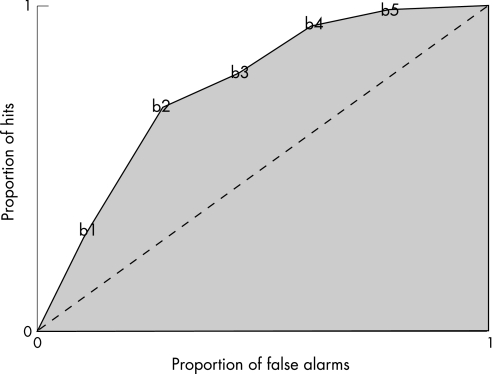
The meaning of the discrimination index (p(A)) is clear: it is a measure of the ability to distinguish between the two stimulus intensities, based on the sensory intensity ratings reported in response to them. However, the computational formula is complex: (1) ratings on the rating scale used by the subject to subjectively rate the intensity of stimuli that are presented, are separated by multiple boundaries (fig 1). (2) For each boundary one calculates the proportion of all the higher intensity stimuli (that is, 34 mm Hg distensions) that received ratings above this boundary (this is the “hit” rate for this boundary) and one separately calculates the proportion of the lower intensity stimuli (that is, 30 mm Hg distensions) that received ratings above this boundary (this is the “false alarm” rate for this boundary). Thus, in this study hit rates and false alarm rates were calculated for each of five boundaries. (3) These hit rates and false alarm rates are plotted against each other to create a receiver operator characteristic curve (ROC curve) as shown in figure 2. The curve is drawn by connecting the different intersections of hit and false alarm rates calculated for each boundary (shown by the solid line in fig 2). (4) P(A) is the total area under the ROC curve (shaded area in fig 2) expressed as a proportion of the maximum possible area. The broken diagonal line in figure 2 goes through all the points for which the hit rate and the false alarm rates are equal; this represents chance performance or no discrimination, and the index, p(A) is 0.5. All values less than 0.5 are considered chance performance and are rounded up to 0.5. Thus, p(A) is a number between 0.5 (chance) and 1.0 (perfect discrimination) that measures the ability to discriminate between the two intensities independently of what rating labels the subject uses to describe the stimuli.
Figure 2 Receiver operator characteristic curve (ROC curve): each point represents the proportion of hits and false alarms for a given boundary (b1–b5). The total area under the ROC curve represents p(A).
The report criterion (B) is the median rating assigned by the subjects to all stimuli. Firstly, the ratings assigned to the 30 mm Hg distensions were pooled with ratings for the 34 mm Hg distensions. Secondly, each response on the six‐point rating scale was assigned an individual report criterion (B) value. Based on SDT convention, a numerically low criterion means a “liberal” tendency to rate most of the stimuli as intense, whereas a numerically high criterion means a “conservative” or “stoic” tendency to label most stimuli as less intense. Therefore, higher (that is, more intense) subject ratings are assigned lower B values and vice versa (fig 2). Thirdly, the overall report criterion (B) was determined as the B value on the six point rating scale for which half of total responses to both stimulus intensities were to categories above the criterion and half were to categories below the criterion.13
There was a strong correlation between AML pain thresholds and pain report criterion (r = 0.67 p<0.0001). On the contrary, AML pain thresholds did not correlate with neurosensory sensitivity for pain (r = −0.13; p = 0.14).
Data analysis
The data were not normally distributed. Consequently, non‐parameteric statistical tests were used. Significance was set at a p value of 0.05. Firstly, Wilcoxon rank sum tests were used to compare IBS patients to controls with respect to the following measures: AML determined pain and urge thresholds; SDT determined pain and urge discrimination index (p(A)) and report criterion values (B); BSI anxiety, depression, somatisation, and global severity index scores. Secondly, Spearman correlations were used to determine associations between AML pain thresholds with SDT determined pain discrimination index (p(A)) and report criterion (B). Thirdly, Spearman correlations were used to determine associations between both AML pain thresholds and pain report criteria (B) with the following measures: p(A), BSI anxiety, depression, somatisation, and global severity index scores.
Results
Excluded subjects
In all, 119 IBS patients and 29 control subjects underwent colonic sensory testing. Of the 13 IBS patients who did not undergo colonic sensory testing, three withdrew consent after the first day, possibly because of apprehension regarding the pain test procedure, three refused flexible sigmoidoscopy, two did not tolerate sigmoidoscopy, one had an extremely elevated blood pressure, and one had colonic inflammation detected on sigmoidoscopy. Of the three excluded control subjects, one did not tolerate the flexible sigmoidoscopy and two had exclusionary medical conditions that were detected during the study (lactose intolerance in one and previous colonic surgery in the other).
Pain thresholds, neurosensory sensitivity, and report criterion
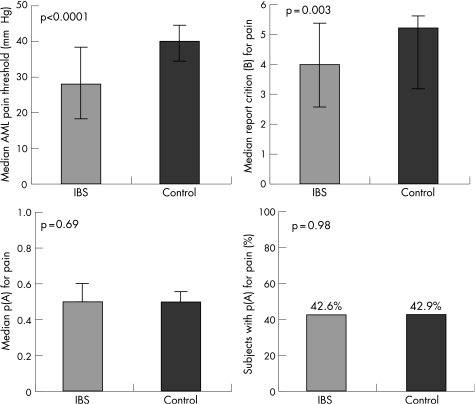
On the AML protocol IBS patients had lower pain thresholds (median 28 mm Hg vs 40 mm Hg; p = 0.0002). On sensory decision theory analysis there were no differences in pain neurosensory sensitivity (median p(A): 0.5 vs 0.5; p = 0.69; 42.6% of IBS patients vs 42.9% of healthy controls had p(A) >0.5 (chance); p = 0.98). Conversely, IBS patients had a lower pain report criterion, which represents their increased tendency to report stimuli as being relatively painful irrespective of the actual intensity of the stimulus (median B: 4.0 (median response = mild pain) vs 5.2 (median response = weak pain); p = 0.003) (fig 3).
Figure 3 (Top left) Median AML pain thresholds: thresholds were significantly higher in healthy controls than in IBS subjects. (Top right) The pain report criterion (B) across both 30 mm Hg and 34 mm Hg stimuli: IBS patients had a lower criterion, which reflects their increased tendency to report pain irrespective of stimulus intensity. (Bottom left) The median pain neurosensory sensitivity (p(A)). There were no differences between the two groups. (Bottom right) The percentage of subjects whose ability to discriminate painful sensations between 30 mm Hg and 34 mm Hg stimuli was better than chance (p(A)>0.5): there was no difference between the two groups. The bars on each graph represent the interquartile range.
Psychometric scores and pain report criterion
IBS patients scored higher than controls on all psychometric scales (table 1). There were modest inverse correlations between pain report criterion (B) and BSI global score (r = −0.18; p = 0.035) and BSI somatisation (r = −0.26; p = 0.001) (table 2). Higher psychological distress correlated with an increased tendency to report pain.
Table 1 Psychological profiles of IBS and control populations.
| IBS median (range) | Controls median (range) | p Value | |
|---|---|---|---|
| BSI global severity index | 49 (33–78) | 42 (33–63) | <0.0001 |
| BSI anxiety | 50 (38–74) | 39 (38–61) | <0.0001 |
| BSI depression | 48 (40–81) | 42 (40–61) | = 0.006 |
| BSI somatisation | 55 (41–74) | 41 (41–66) | <0.0001 |
Table 2 Spearman's correlations: AML pain threshold and pain report criterion (B).
| Correlation (rho) with AML pain threshold | Correlation (rho) with SDT pain report criterion (B) | |
|---|---|---|
| Pain p(A) | −0.13 p = 0.1 | −0.16 p = 0.04 |
| Pain B | 0.67 p<0.0001 | — |
| BSI global severity index | −0.22 p = 0.01 | −0.18 p = 0.04 |
| BSI anxiety | −0.11 p = 0.2 | −0.04 p = 0.7 |
| BSI depression | −0.11 p = 0.2 | −0.07 p = 0.4 |
| BSI somatisation | −0.28 p = 0.001 | −0.26 p = 0.001 |
Urge thresholds, neurosensory sensitivity, and report criterion
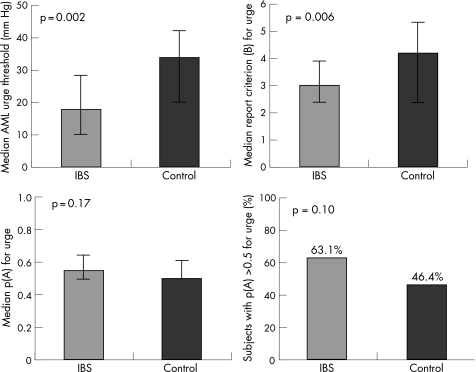
Sensory thresholds for urge were lower than those for pain. On the AML protocol IBS patients had lower urge thresholds than controls (median: 18 mm Hg vs 34 mm Hg; p = 0.002), but on sensory decision theory analysis there were no differences in urge neurosensory sensitivity (median p(A): 0.55 vs 0.50; p = 0.17; 63.1% of IBS patients vs 46.4% of healthy controls had urge p(A) >0.5 (chance); p = 0.10). Conversely, IBS patients had a lower urge report criterion, which represents their increased tendency to report relatively intense urge irrespective of the actual intensity of the stimulus (median B: 3.0 (median response = “moderate” urge) vs 4.2 (median response = “mild”); p = 0.006) (fig 4).
Figure 4 (Top left) Median AML urge thresholds: thresholds were significantly higher in healthy controls than IBS subjects. (Top right) The median urge report criterion (B) to 30 mm Hg and 34 mm Hg stimuli: IBS patients had a lower criterion which reflects their increased tendency to report urge irrespective of stimulus intensity. (Bottom left) The median urge neurosensory sensitivity (p(A)). There were no differences between the two groups. (Bottom right) The percentage of subjects whose ability to discriminate urge sensations between 30 mm Hg and 34 mm Hg stimuli was better than chance (p(A)>0.5): there was no difference between the two groups. The bars on each graph represent the interquartile range.
There was a strong inverse correlation between AML urge thresholds and urge report criterion (r = −0.51; p<0.0001) and a weaker but significant inverse correlation with neurosensory sensitivity to urge (r = −0.22; p = 0.007).
Psychometric scores and urge report criterion
There were modest inverse correlations between urge report criterion (B) and BSI global score (r = −0.19; p = 0.03), BSI somatisation (r = −0.18; p = 0.04), and BSI anxiety (r = −0.17; p = 0.05) (table 3). Higher psychological distress correlated with an increased tendency to report urge.
Table 3 Spearman's correlations: AML urge threshold and urge report criterion (B).
| Correlation (rho) with AML urge threshold | Correlation (rho) with SDT urge report criterion (B) | |
|---|---|---|
| Urge p(A) | −0.22 p = .007 | −0.09 p = 0.3 |
| Urge B | −0.51 p<0.0001 | – |
| BSI global severity index | −0.19 p = 0.03 | −0.18 p = 0.04 |
| BSI anxiety | −0.17 p = 0.05 | −0.15 p = 0.07 |
| BSI depression | −0.07 p = 0.4 | −0.12 p = 0.15 |
| BSI somatisation | −0.18 p = 0.04 | −0.16 p = 0.06 |
Additional analyses of SDT data
There was a moderately strong positive correlation between pain and urge discrimination (p(A)) (r = 0.50; p<0.0001). Similarly, there was a moderately strong positive correlation between pain and urge report criteria (B) r = 0.44; p<0.0001).
The SDT test involved 24 distensions at pressures, which were painful for most subjects, and consequently some subjects did not complete all trials. The accuracy of discrimination index (p(A)) and report criterion (B) values in subjects who underwent fewer SDT distension trials might have been lower because of increased variance. We therefore excluded subjects who completed fewer than one‐half (<12) of all trials (33 IBS, 4 controls, p = 0.158) and repeated the comparison between IBS patients and controls for pain p(A) and report criterion (B). The pattern of results and the significance of the differences did not change for pain p(A) (median p(A) 0.5 vs 0.5; p = 0.31; % with pain p(A) > chance: IBS = 47.1%; control = 41.7%; p = 0.63;) or pain report criterion (median B: IBS = 4.4; control = 5.4; p = 0.0001).
Repeated distension of the colon has been previously shown to induce hyperalgesia (“sensitisation”) in IBS patients.8 Thus, it is possible that as a result of this potential sensitisation, the intensity ratings made by IBS patients to late SDT trials may have been affected. In order to test for this we first determined the change in pain intensity ratings between the first and the last 30 mm Hg and 34 mm Hg trials (change in ratings = pain intensity rating to the last 30 mm Hg stimuli plus pain intensity rating to the last 34 mm Hg stimuli minus pain intensity ratings to the first 30 mm Hg stimuli minus pain intensity rating to the first 34 mm Hg stimuli). We then used the Wilcoxon rank sum test of differences to compare change in intensity ratings between IBS patients and controls who completed at least one‐half (⩾12) of all trials. There was no difference between the two groups (p = 0.22).
Finally, the intensities of the three SDT stimuli (30 mm Hg, 32 mm Hg, 34 mm Hg) were below AML pain thresholds for some subjects (mostly controls) and above threshold for other subjects (mostly IBS patients). Therefore, it was possible that certain subjects failed to demonstrate discrimination (p(A)) because they assigned the same ratings to all stimuli (either calling all of them “intense” or calling all of them non‐painful). We identified nine (7.4%) IBS patients and nine (35%) healthy controls who rated each SDT stimulus as zero pain intensity. One IBS patient rated all stimuli as “intense.” All other subjects varied their pain intensity ratings. When we excluded the 10 IBS patients and nine healthy controls who did not vary their pain intensity ratings and repeated the analysis, the pattern of results and the significance of the differences did not change for pain p(A) (median p(A) 0.5 vs 0.52; p = 0.8); percentage with pain p(A) > chance: IBS = 45.6%; control = 52.2%; p = 0.57) or pain report criterion (median B: IBS = 3.9; control = 4.52; p = 0.04).
Discussion
In this study we first used AML to measure pain and urge thresholds and we then used SDT to determine the two components of these thresholds: physiologically determined neurosensory sensitivity and psychologically determined report criterion. Using these techniques, we demonstrated that lower AML determined pain and urge thresholds in patients with IBS are explained primarily by an increased tendency to report pain and urge, not increased neurosensory sensitivity. Since this lower report criterion reflects psychological phenomena, increased colonic sensitivity in IBS appears to be determined more by psychological factors than by physiological factors.
Pain is a complex perceptual experience that can only be measured indirectly.21 Gastrointestinal pain sensitivity is typically measured by pain thresholds, which are defined as the lowest stimulus intensity to which subjects report pain. However, pain thresholds are not equivalent with painful sensations since pain reports are influenced by non‐neurosensory factors such as placebo, emotion, attention, and distraction.13
SDT is an alternative pain measurement technique that separately quantifies the individual components of the pain response: neurosensory sensitivity (p(A)), a measure of neurosensory function based on the ability to discriminate between stimuli; and report criterion (B), a measure of stoicism based on the overall tendency to report pain.13 Importantly, previous research has shown that only the criterion is susceptible to changes in cognitive or psychological variables.13,14,15 The discrimination index, p(A), changes in response to analgesic drugs but is not influenced by psychological manipulations.15,16 In this study, IBS patients had similar pain neurosensory sensitivity and lower pain report criterion compared to healthy controls. In other words, their tendency to report pain at lower thresholds related not to increased neural sensitivity, but rather to their predilection towards reporting pain.
Whereas SDT has been widely used in somatic pain research13 it has been used only rarely in previously published studies on visceral pain sensitivity in functional gastrointestinal disorders. Bradley et al observed lower AML pain thresholds, similar neurosensory sensitivity, and decreased report criterion for balloon distensions of the oesophagus in patients with non‐cardiac chest pain,22 which is similar to the findings of this study. Whitehead et al observed lower AML pain thresholds and similar neurosensory sensitivity for rectal distensions in women with IBS,19 which is also similar to the findings of this study. However, they did not measure the report criterion.
Similar to pain, our findings also suggest that lower AML determined urge thresholds in patients with IBS are largely explained by an increased tendency to report urge. However, the finding that urge thresholds and urge neurosensory sensitivity were inversely correlated (r = −0.22, p<0.005) suggests that lower urge thresholds in IBS may also be attributable—albeit to a lesser extent—to increased urge neurosensory sensitivity. These findings contrast with those reported by Corsetti et al who, using non‐painful, barely perceivable balloon distensions, found that patients with IBS had increased neurosensory sensitivity and similar report criterion. However, unlike our study, their study involved a small population (22 patients and 13 controls) in which there were no psychological differences between the IBS and control groups.23
The increased tendency to report pain and urge in patients with IBS may be the downstream result of multiple cognitive and psychological processes. Firstly, patients with IBS appear to be hypervigilant to gastrointestinal sensations.12,24 For example, on functional brain imaging they show similar, abnormal cortical responses to both actual and anticipated (sham) distensions.25,26 Secondly, hypervigilance may reduce the intensity at which they notice gut distensions28 and sensations. Thirdly, once perceived, subjects with IBS interpret these sensations through a generally negative schema (framework for explaining reality),28 which leads them to attribute their sensations to disease.29 Finally, disease attribution in turn further increases attention to gastrointestinal symptoms30 through which a cycle of gastrointestinal sensory amplification is ultimately established.31 Along these lines, in our study somatisation was more common in IBS and was correlated inversely with pain thresholds and directly with the response criterion. This is similar to findings that in Gulf War veterans with IBS, lower pain thresholds could be largely explained by increased somatic focus.32 Other investigators have also found that global psychological distress is correlated with the amount of brain activation in response to painful rectal distension33 and is inversely correlated with tolerance for painful balloon distension of the rectum.34
In order to assess visceral sensitivity independently from these cognitive processes, some have proposed measuring cortical activity during subliminal distensions (that is, not consciously perceived).35,36 Lawal et al used this approach and found increased cortical activation in subjects with IBS. They interpreted this as evidence for neural hypersensitivity that is independent of cognitive input.37 However, it is unclear whether these distensions were truly subliminal since most individuals can perceive distensions as small as 5 mm Hg38; the distensions in their study ranged from 10 mm Hg to 20 mm Hg. Secondly, their observation that cerebral activation in IBS patients did not increase in a positive dose‐response fashion suggests that IBS patients were globally hypersensitive at baseline. This global hypersensitivity was attributed by Naliboff and Mayer to cognitive and psychological processes such as uncertain expectation and hypervigilance, that could not be completely controlled for in the study.39
Although our data demonstrate that psychological phenomena strongly influence pain thresholds, our experimental methods may not have been sensitive enough to detect subtle differences in neurosensory sensitivity. Thus, we cannot rule out the effects of peripheral physiological mechanisms, such as sensitisation of colonic afferent pathways.6,42,43 This afferent hypersensitivity has been credited to inflammation based on evidence that experimentally induced colonic inflammation lowers rectal pain thresholds in animal models.42 Nonetheless, inflammation has not been shown to explain lower thresholds in IBD patients.43,44
Study limitations
Two potential limitations to this study were posed by the repeated balloon distensions required by the SDT protocol. Firstly, certain subjects failed to complete all 24 SDT trials because of intolerable levels of pain or urge. We estimated the effects of this by repeating our analyses without including those subjects who completed fewer than half of the trials. The results were the same. Secondly, the process of repeated very intense colonic distensions (60 mm Hg) has been previously shown to induce rectal hypersensitivity in subjects with IBS.8 We estimated the effects of this by comparing the change in pain intensity ratings between early and late stimuli in IBS patients and healthy controls. There was no difference between the two groups.
SDT, which quantifies the ability of subjects to discriminate between very similar stimuli, required that we use stimulus intensities that were very close to each other (30 mm Hg vs 34 mm Hg). This might have been too close to allow for adequate discrimination—that is, the measurement of neural sensitivity may have been insensitive. However, most subjects can perceive a 5 mm Hg increase in stimulus intensity.19 In this study 43% of both IBS patients and healthy controls were able to discriminate between the 30 mm Hg and 34 mm Hg distensions at better than chance levels (p(A) values above 0.5).
Calculation of the report criterion required us to use the same stimuli for all subjects, irrespective of their AML thresholds. As a result, the ability of some subjects to discriminate between SDT stimuli might have been affected either because the test stimuli were well above their pain threshold or they were so far below their pain threshold that none of them were perceived as painful. We tested for this by excluding subjects who rated all stimuli as equally painful and repeating the analysis. The results did not change. Furthermore, in our previous smaller study where we individualised SDT stimulus intensities for each patient based on their AML determined pain threshold (though we did not compute a report criterion), we still found that subjects with IBS and healthy controls had similar neurosensory sensitivity to pain.19
A theoretical limitation is that we used pressure rather than volume based balloon distensions. Some investigators prefer volume based distensions or indices that integrate pressure and volume into estimates of wall tension.45 We followed the recommendations of an international consensus committee46 by scaling our distensions in pressure rather than volume because it is recognised that volume thresholds are influenced by muscle tone, which varies from hour to hour in response to meal ingestion and anxiety. Individual differences in pain thresholds are believed to be more stable and reproducible when measured on a pressure scale rather than a volume scale.
Conclusion
These data show that lower pain and urge thresholds in subjects with IBS are strongly influenced by cognitive and psychological factors. Peripheral physiological events such as inflammation42 and temporal summation8 have also been shown to influence pain sensitivity. However, these data suggest that, when explaining the differences between IBS patients and healthy controls, the contribution of peripheral physiological events may be relatively small compared to the cognitive and psychological influences that are reflected in the report criterion index, which reflects the generalised tendency to report pain. The implications of this finding are far reaching. Firstly, it underscores the importance of accounting for psychological factors when interpreting tests of sensory function. Secondly, it highlights the important part played by centrally mediated processes in the pathophysiology of visceral sensitivity in IBS and suggests that novel therapies for pain in IBS should target centrally mediated mechanisms.
Acknowledgements
Supported by grants R24 DK67674, R01 DK31369, RR00046, and T32DK7634 from the National Institutes of Health in the United States.
Abbreviations
AML - ascending methods of limits
BSI - Brief Symptom Inventory
IBS - irritable bowel syndrome
IBS‐C - constipation predominant irritable bowel syndrome
IBS‐D - diarrhoea predominant irritable bowel syndrome
IOP - individual operating pressure
ROC - receiver operator characteristic
SDT - sensory decision theory analysis
References
- 1.Whitehead W E, Palsson O S. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology 19981151263–1271. [DOI] [PubMed] [Google Scholar]
- 2.Gebhart G F. Visceral pain‐peripheral sensitisation. Gut 200047(Suppl 4)iv54–iv55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut 200251(Suppl 1)i67–i71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azpiroz F. Hypersensitivity in functional gastrointestinal disorders. Gut 200251(Suppl 1)i25–i28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M, Coulie B, Tack J F. Visceral hypersensitivity: facts, speculations, and challenges. Gut 200148125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lembo T, Munakata J, Mertz H.et al Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology 19941071686–1696. [DOI] [PubMed] [Google Scholar]
- 7.Mayer E A, Gebhart G F. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 1994107271–293. [DOI] [PubMed] [Google Scholar]
- 8.Munakata J, Naliboff B, Harraf F.et al Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology 199711255–63. [DOI] [PubMed] [Google Scholar]
- 9.Mertz H, Naliboff B, Munakata J.et al Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 199510940–52. [DOI] [PubMed] [Google Scholar]
- 10.Naliboff B, Mayer E A. Sensational developments in the irritable bowel. Gut 199639770–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aziz Q. Visceral hypersensitivity: fact or fiction. Gastroenterology 2006131661–664. [DOI] [PubMed] [Google Scholar]
- 12.Naliboff B D, Munakata J, Fullerton S.et al Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut 199741505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark W C. Pain sensitivity and the report of pain: an introduction to sensory decision theory. Anesthesiology 197440272–287. [DOI] [PubMed] [Google Scholar]
- 14.Clark W C. Sensory‐decision theory analysis of the placebo effect on the criterion for pain and thermal sensitivity. J Abnorm Psychol 196974363–371. [DOI] [PubMed] [Google Scholar]
- 15.Clark W C, Yang J C. Acupunctural analgesia? Evaluation by signal detection theory. Science 19741841096–1098. [DOI] [PubMed] [Google Scholar]
- 16.Yang J C, Clark W C, Ngai S H.et al Analgesic action and pharmacokinetics of morphine and diazepam in man: an evaluation by sensory decision theory. Anesthesiology 197951495–502. [DOI] [PubMed] [Google Scholar]
- 17.Thompson W G, Longstreth G F, Drossman D A.et al Functional bowel disorders and functional abdominal pain. Gut 199945(Suppl 2)ii43–ii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derogatis L R.Brief Symptom Inventory (BSI) 18: Administration, scoring and procedures manual. Minneapolis: NCS Pearson Inc, George Allen and Unwin, 2000
- 19.Whitehead W E, Crowell M D, Davidoff A L.et al Pain from rectal distension in women with irritable bowel syndrome: relationship to sexual abuse. Dig Dis Sci 199742796–804. [DOI] [PubMed] [Google Scholar]
- 20.McNicol D.A primer of signal detection theory. London: George Allen and Unwin, 1972
- 21.Chapman C R, Casey K L, Dubner R.et al Pain measurement: an overview. Pain 1985221–31. [DOI] [PubMed] [Google Scholar]
- 22.Bradley L A, Richter J E, Scarinci I C.et al Mechanisms of altered pain perception in non‐cardiac chest pain patients. Gastroenterology 1993104A482 [Google Scholar]
- 23.Corsetti M, Ogliari C, Marino B.et al Perceptual sensitivity and response bias during rectal distension in patients with irritable bowel syndrome. Neurogastroenterol Motil 200517541–547. [DOI] [PubMed] [Google Scholar]
- 24.Naliboff B D, Berman S, Suyenobu B.et al Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology 2006131352–365. [DOI] [PubMed] [Google Scholar]
- 25.Naliboff B D, Derbyshire S W G, Munakata J.et al Cerebral activation in irritable bowel syndrome patients and control subjects during rectosigmoid stimulation. Psychosom Med 200163365–375. [DOI] [PubMed] [Google Scholar]
- 26.Silverman D H S, Munakata J A, Ennes H.et al Regional cerebral activity in normal and pathologic perception of visceral pain. Gastroenterology 199711264–72. [DOI] [PubMed] [Google Scholar]
- 27.Accarino A M, Azpiroz F, Malagelada J R. Attention and distraction: effects on gut perception. Gastroenterology 1997113415–422. [DOI] [PubMed] [Google Scholar]
- 28.Gomborone J E, Dewsnap P A, Libby G W.et al Selective affective biasing in recognition memory in the irritable bowel syndrome. Gut 1993341230–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitehead W E, Winget C, Fedoravicius A S.et al Learned illness behavior in patients with irritable bowel syndrome and peptic ulcer. Dig Dis Sci 198227202–208. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs‐Gallagher N, Palsson O S, Levy R L.et al Selective recall of gastrointestinal‐sensation words: evidence for a cognitive‐behavioral contribution to irritable bowel syndrome. Am J Gastroenterol 2001961133–1138. [DOI] [PubMed] [Google Scholar]
- 31.Barsky A J. Amplification, somatization, and the somatoform disorders. Psychosomatics 19923328–34. [DOI] [PubMed] [Google Scholar]
- 32.Dunphy R C, Bridgewater L, Price D D.et al Visceral and cutaneous hypersensitivity in Persian Gulf war veterans with chronic gastrointestinal symptoms. Pain 200310279–85. [DOI] [PubMed] [Google Scholar]
- 33.Drossman D A, Ringel Y, Vogt B A.et al Alterations of brain activity associated with resolution of emotional distress and pain in a case of severe irritable bowel syndrome. Gastroenterology 2003124754–761. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie E, Barlow J, Fernandes L.et al Changes in tolerance to rectal distension correlate with changes in psychological state in patients with severe irritable bowel syndrome. Psychosom Med 200466578–582. [DOI] [PubMed] [Google Scholar]
- 35.Kern M K, Shaker R. Cerebral cortical registration of subliminal visceral stimulation. Gastroenterology 2002122290–298. [DOI] [PubMed] [Google Scholar]
- 36.Sidhu H, Kern M, Shaker R. Absence of increasing cortical fMRI activity volume in response to increasing visceral stimulation in IBS patients. Am J Physiol Gastrointest Liver Physiol 2004287G425–G435. [DOI] [PubMed] [Google Scholar]
- 37.Lawal A, Kern M, Sidhu H.et al Novel evidence for hypersensitivity of visceral sensory neural circuitry in irritable bowel syndrome patients. Gastroenterology 200613026–33. [DOI] [PubMed] [Google Scholar]
- 38.Whitehead W E, Engel B T, Schuster M M. Irritable bowel syndrome: physiological and psychological differences between diarrhea‐predominant and constipation‐predominant patients. Dig Dis Sci 198025404–413. [DOI] [PubMed] [Google Scholar]
- 39.Naliboff B D, Mayer E A. Brain imaging in IBS: drawing the line between cognitive and non‐cognitive processes. Gastroenterology 2006130267–270. [DOI] [PubMed] [Google Scholar]
- 40.Accarino A M, Azpiroz F, Malagelada J R. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology 1995108636–643. [DOI] [PubMed] [Google Scholar]
- 41.Coffin B, Bouhassira D, Sabate J M.et al Alteration of the spinal modulation of nociceptive processing in patients with irritable bowel syndrome. Gut 2004531465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bueno L, Fioramonti J. Visceral perception: inflammatory and non‐inflammatory mediators. Gut 200251(Suppl 1)i19–i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernstein C N, Niazi N, Robert M.et al Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain 199666151–161. [DOI] [PubMed] [Google Scholar]
- 44.Chang L, Munakata J, Mayer E A.et al Perceptual responses in patients with inflammatory and functional bowel disease. Gut 200047497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Distrutti E, Azpiroz F, Soldevilla A.et al Gastric wall tension determines perception of gastric distention. Gastroenterology 19991161035–1042. [DOI] [PubMed] [Google Scholar]
- 46.Whitehead W E, Delvaux M. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. The Working Team of Glaxo‐Wellcome Research, UK. Dig Dis Sci 199742223–241. [DOI] [PubMed] [Google Scholar]