Abstract
Objective
Prospective studies show a 10% incidence of sternal wound infection (SWI) after 90 days of follow‐up, compared with infection rates of 5% reported by the National Nosocomial Infections Surveillance System after only 30 days of follow‐up. This incidence increases 2–3 times in high‐risk patients.
Design
Prospective randomised double‐blind controlled clinical trial.
Setting
Cardiothoracic centre, UK.
Patients
Patients were eligible if they were undergoing median sternotomy for primary isolated coronary artery bypass grafting, with at least one internal thoracic artery used for coronary grafting and having one or more of the following three risk factors: (1) obesity, defined as body mass index 30 kg/m2; (2) diabetes mellitus; or (3) bilateral internal thoracic artery grafts (ie, the use of the other internal thoracic artery).
Interventions
The study group received a single dose of gentamicin 2 mg/kg, rifampicin 600 mg and vancomycin 15 mg/kg, with three further doses of 7.5 mg/kg at 12‐hour intervals. The control group received cefuroxime 1.5 g at induction and three further doses of 750 mg at 8‐hour intervals.
Main outcome measures
The primary end point was the incidence of SWI at 90 days. The secondary end point was the antibiotic and hospital costs.
Results
During the study period, 486 patients underwent isolated coronary artery bypass grafting with a 30‐day SWI of 7.6%. 186 high‐risk patients were recruited and analysed: 87 in the study group and 99 in the control group. 90‐day SWI was significantly reduced in 8 patients in the study group (9.2%; 95% CI 3.5% to 15.3%) compared with 25 patients in the control group (25.2%; 95% CI 19.5% to 39.4%; p = 0.004). The study group had a significantly lower cost of antibiotics (21.2% reduction—US$96/patient; p<0.001), and a significantly lower hospital cost (20.4% reduction in cost—US$3800/patient; p = 0.04).
Conclusions
Longer and broader‐spectrum antibiotic prophylaxis significantly reduces the incidence of SWI in high‐risk patients, with a significant economic benefit in costs of antibiotics as well as hospital costs.
Median sternotomy as access for cardiothoracic procedures was first reported by Milton in 18971 and became the standard incision for cardiac surgery in the following century. Subsequently, sternal wound infection (SWI) was recognised as a complication, and by the late 1970s, antibiotic prophylaxis was shown to reduce the incidence of SWI from 50% to about 20%, with widespread use by the 1990s.2 SWI has significant clinical and economic implications, particularly where centres across the world are still reporting 90‐day infection rates up to 10%.3,4,5,6
Over the past decade, there has been a degree of discrepancy over the incidence and risks of SWI between the National Nosocomial Infections Surveillance System (NNIS)7,8 and other cardiac surgical reports.4,5,9 The NNIS defines surgical site infection as infection occurring within 30 days of surgery and stratifies the risk into four categories based on three parameters—the American Society of Anaesthesiologists score, the type of surgery (clean or contaminated) and whether duration of surgery is prolonged (>5 h for cardiac surgery). For most elective cardiac surgery procedures, the American Society of Anaesthesiologists score is three and the surgery is clean. Hence, the predicted NNIS incidence of SWI is dependent only on the duration of surgery—that is, whether it is >5 h.7,10 Recently published NNIS data8 suggest that the risk of infection by 30 days would be 3.5% for operations lasting <5 h and 5.5% for operations lasting >5 h. In the UK, a similar assessment, the Surgical Site Infection Surveillance Service (SSISS),11 has been derived from the NNIS.
Large studies focusing on cardiac surgery have established that the incidence and risk of SWI are associated with a number of patient‐related and procedure‐related factors.4,5,9 Furthermore, these risk factors are not merely additive but multiplied,3,9 and SWI may present up to 90 days following cardiac surgery rather than the 30‐day limit suggested by the Centers for Disease Control and Prevention (CDCP), Atlanta, Georgia, USA.12
Following a perceived increase in crude infection rates, surgical activity at our institution was closed in March 2002 for 1 month while all surgical, anaesthetic, intensive care and ward procedures and plant were reviewed. The whole surgical process was reviewed by two separate external investigators. A series of changes in practice and to the building ensued, with the culmination of a new theatre suite constructed and put in use by May 2003. Changes included standardisation of diagnosis and subsequent management of SWI by infection control personnel and plastic surgeons. Our study commenced 1 month after all these changes had been completed.
Patients and methods
Study design
This is a 12‐month prospective randomised double‐blind controlled clinical trial conducted from June 2003 to the end of May 2004 in a cardiothoracic centre following institutional ethical approval. Eligible patients were scheduled to undergo median sternotomy for primary isolated coronary artery bypass grafting (CABG), with at least one internal thoracic artery and having one or more of the following three risk factors3,4,5: (1) obesity, defined as a body mass index >30 kg/m2; (2) diabetes mellitus; or (3) the use of the other internal thoracic artery, so the patient undergoes bilateral internal thoracic artery grafts. Patients were excluded if they were allergic to penicillin or the study drugs, if they were taking antibiotics during the week before surgery for any reason and if they had a high serum creatinine (>180 μmol/l) preoperatively.
Following the surgical decision of operative strategy and operative consent, eligible patients were approached for inclusion in the study. After giving their written informed consent, patients were randomised (but only after the surgical strategy had been determined) using a computerised random number generator and allocated to one of the groups (by KD and MC). No placebo preparation was used; however, all staff involved in the diagnosis and management of wound infection, including the infection control team, plastic surgeons, pharmacist, hospital accountant and primary care physician, were blinded to the antibiotic regimen used, which was administered by the anaesthetist.
Antibiotic regimens
All patients were shaved with clippers, where necessary, and had chlorhexidine showers on the day of surgery. The control group received perioperative antibiotic prophylaxis of cefuroxime: 1.5 g given on induction, 750 mg coinciding with the reversal of anticoagulation with protamine, and two doses of 750 mg at 8 and 16 h postoperatively. The study group received rifampicin 600 mg orally with their premedication, 1 h preoperatively, and then received gentamicin 2 mg/kg and vancomycin 15 mg/kg after induction of anaesthesia—a regimen that was suggested by previous studies.14 A further three doses of vancomycin 7.5 mg/kg at 12‐hour intervals were given postoperatively.
Definitions and surveillance
All patients were ascribed an infection risk score using the NNIS criteria. A second score, the sternal wound infection risk score (SWIRS), was calculated using an empirical Bayesian approach, with likelihood ratios derived from the existing literature5 (appendix A).
The primary end point was SWI, as defined by the CDCP,4 as evidenced by the presence of wound infection and/or positive blood cultures and/or positive swab cultures. However, the duration of surveillance was continued for a period of 90 days following surgery and not the 30 days suggested by the CDCP.4 The secondary end point was the 90‐day antibiotic and hospital costs.
All wounds were actively surveyed while the patient was on the ward and were referred to the infection control team—who were blinded to the study drugs—when infection was suspected. Wounds were classified according to CDCP criteria as organ‐space, deep or superficial.7 All wounds treated by the primary care physician with no surgical intervention or hospital referral were assumed to be superficial. Routine follow‐up at 6 weeks included blinded assessment of the sternal wound, and surveillance was completed at 90 days with a follow‐up by telephone. NNIS infection rates are routinely assessed by the institutional infection control team and refer to institutional data.
The combination of vancomycin and gentamicin, in a group of patients with diabetes, may compromise renal function. Renal failure was defined as the need for renal replacement (continuous venovenous haemofiltration). Renal dysfunction was defined as either renal failure or a peak postoperative creatinine >180 μmol/l with an increase of >80 μmol/l from preoperative levels during the 90‐day postoperative period.6
Economic analysis
Hospital acquisition costs for the prophylactic antibiotics were US$8.60 (£4.46, €6.56) for each patient in the control group and US$30.60 (£15.85, €23.34) for each patient in the study group. Costs of antibiotic treatments used for patients with SWI were determined by a blinded pharmacist (HL). The hospital accountant, who was also blinded, assessed the required relevant aspects of postoperative care including hospital stay and intensive therapy unit stay caused by the infection episode, the use of vacuum therapy (in days), surgical procedures including sternal wound debridement and pectoralis flap reconstruction, microbiology costs and attendance at the outpatient wound clinic. All variables analysed were entered in a prospective fashion to complete a valid dataset for each patient.
Statistical analysis
For the purpose of power calculation, the estimated SWI rate of 10%–15% at 90 days was expected to double to 20%–30% (π2 = 0.2) in a high‐risk group. Using an expected reduction of 50% (π1 = 0.1) with the study strategy, a one‐sided α of 0.05 and a power of 80% would require 157 patients in each arm. Alternatively, with a one‐sided α of 0.1, a power of 90% would require 166 patients in each arm. We proposed to recruit a total of 332 patients, with code breaking and analysis after 166 patients (power 70%) and 230 patients (power 80%). At the relevant points, specific data—SWI, sternal wound debridement and infection‐related deaths—were to be assessed (KD) and the study was terminated when two‐sided analysis showed a significant difference and this was confirmed by an independent statistician.
Data are expressed as mean (SD), median and interquartile range (25th–75th centile). Categorical variables were compared using Fisher's exact test or a χ2 test where appropriate, and continuous variables were compared using Student's t test or the Mann–Whitney U test as appropriate. p Values <0.05 indicated a significant difference. All tests were two sided.
Analysis of data was planned to be as per protocol, where prophylactic drugs were appropriately given to high‐risk patients with at least 1 week survival/enrolment of the patient. In the intention‐to‐treat approach to randomised controlled trials, data are analysed on the basis of treatment assignment, not on treatment receipt, and it is known that intention‐to‐treat analysis attenuates between‐group effects. Alternative approaches make comparisons according to the treatment received at the end of the trial (“as‐per‐protocol” analysis). In our study, we performed a sensitivity analysis and present the results of both analyses, as biased estimates of effect may occur when deviation is non‐random, when a large percentage of participants switch treatments or are lost to follow‐up, and when the method of estimating missing values accounts inadequately for the process causing loss to follow‐up.
Univariate hierarchical logistic regression analysis was used to accommodate the clustering of outcomes for individual surgeons and to calculate the unadjusted odds ratio (OR) and 95% CIs between the two study groups. Additional patient‐specific covariates were then introduced into a multivariate model to adjust for differences in the case mix between the study groups. A two‐level Bayesian logistic regression model was used to identify the independent risk factors for SWI and their interactions, as well as adjusting for the clustering of adverse outcomes within the patients of individual surgeons (appendix B).
Analysis was with the statistical software SPSS V.12.0 for Windows, Sample Power V.2.0 for power analysis calculations and MLwiN V.2.0 (University of London, London, UK) for hierarchical regression modelling.
Results
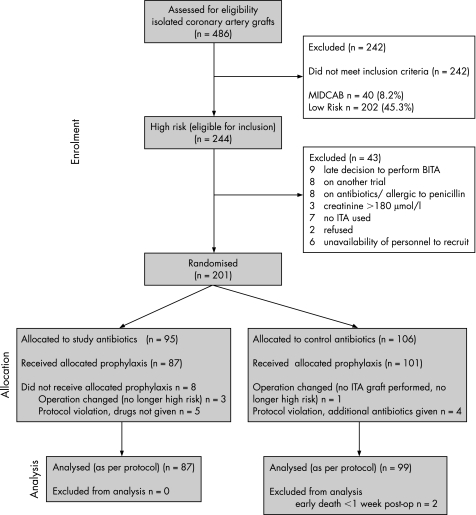
During the study period, 486 patients underwent elective primary isolated CABG, of whom 201 were recruited and 186 were analysed as per protocol (fig 1). Of the 486 patients, 37 had infective complications by NNIS criteria at 30 days, an institutional rate of 7.6%. After the initial code breaking and analysis at 166 patients, it was clear that our primary end point had been reached and once this was established no further patients were recruited. However, recruitment continued during the analysis period and therefore 20 more patients had already been recruited before the decision was made to end the trial.
Figure 1 Trial profile. BITA, bilateral internal thoracic arteries; ITA, internal thoracic artery; MIDCAB, minimally invasive direct coronary artery bypass.
Patients in the two groups were similar with respect to demographic and preoperative status (table 1). Table 2 shows the infection‐related outcome measures for both analytical methods as‐per‐protocol and intention‐to‐treat. Univariate hierarchical logistic regression analysis showed an unadjusted OR of 3.00 (95% CI 1.27 to 7.09), p = 0.01. The primary end point, SWI up to 90 days, differed significantly between the control (25.2%) and study (9.2%) groups (χ2 8.181; 1 df; p = 0.004) which translated to a risk difference of 15% with 95% CI 4% to 26%. NNIS infection rates at 30 days were 12.1% in the control group and 4.6% in the study group (p = 0.068).
Table 1 Preoperative characteristics and risk factors of patients.
| Variable | Analysis per protocol (n = 186) | Intention‐to‐treat analysis (n = 201) | ||||
|---|---|---|---|---|---|---|
| Study (n = 87) no (%) | Control (n = 99) no (%) | p Value | Study (n = 95) no (%) | Control (n = 106) no (%) | p Value | |
| Mean (SD) age (years) | 62.8 (10.1) | 65.4 (8.3) | 0.063 | 62.9 (10.2) | 65.6 (8.3) | 0.056 |
| Female | 14 (16.1) | 17 (17.2) | 0.844 | 17 (17.9) | 19 (17.9) | 0.996 |
| Inclusion criteria | ||||||
| Diabetes mellitus | 48 (55.2) | 50 (50.5) | 0.525 | 52 (54.7) | 55 (51.9) | 0.686 |
| Obesity (BMI >30) | 48 (55.2) | 49 (49.5) | 0.439 | 51 (53.7) | 51 (48.1) | 0.430 |
| BITA graft | 19 (21.8) | 22 (22.2) | 0.950 | 19 (20.0) | 23 (21.7) | 0.768 |
| Number of criteria | ||||||
| 1 factor | 62 (71.3) | 77 (77.8) | 0.308 | 69 (72.6) | 83 (78.3) | 0.350 |
| 2 factors | 22 (25.3) | 22 (22.2) | 0.624 | 23 (24.2) | 23 (21.7) | 0.672 |
| 3 factors | 3 (3.4) | 0 (0) | 0.201* | 3 (3.2) | 0 (0) | 0.207* |
| Other risk factors | ||||||
| Current smoker | 36 (41.4) | 49 (49.5) | 0.268 | 36 (37.9) | 49 (46.2) | 0.233 |
| COPD | 6 (6.9) | 6 (6.0) | 0.817 | 7 (7.4) | 7 (6.6) | 0.809 |
| Peripheral vascular disease | 5 (5.7) | 5 (5.1) | 0.834 | 5 (5.3) | 5 (4.7) | 0.859 |
| Mean (SD) duration of operation | 218.1 (53.7) | 214.2 (51.3) | 0.601 | 218.1 (54.1) | 214.2 (51.3) | 0.059 |
| Preoperative stay (days) (mean, median, (range)) | 2.6, 1 (1–61) | 2.5, 1 (0–35) | 0.776 | 2.6, 1 (1–62) | 2.5, 1 (0–35) | 0.851 |
| Re‐sternotomy for bleed | 1 (1.1) | 1 (1.0) | 1.000* | 1 (1.1) | 1 (0.9) | 1.000* |
| Postoperative ITU stay (days) (mean, median, (range)) | 2.1, 1 (1–20) | 2.4, 1 (1–17) | 0.498 | 2.0, 1 (1–20) | 2.3, 1 (1–17) | 0.557 |
| Preoperative serum creatinine level | ||||||
| >120 | 7 (8.0) | 10 (10.1) | 0.627 | 7 (7.4) | 10 (9.4) | 0.599 |
| >150 | 2 (2.3) | 2 (2) | 1.000* | 2 (2.1) | 2 (1.9) | 1.000* |
| NNIS risk prediction | ||||||
| 3.50% | 81 (93.1) | 92 (92.9) | 0.963 | 89 (93.7) | 98 (92.5) | 0.732 |
| 5.50% | 6 (6.9) | 7 (7.1) | 0.963 | 6 (6.3) | 8 (7.5) | 0.732 |
| SWIRS | 19.8% | 18.3% | 19.5% | 18.1% | ||
BITA, bilateral internal thoracic arteries; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ITU, intensive therapy unit; NNIS, National Nosocomial Infections Surveillance System; SWIRS, sternal wound infection risk score.
*χ2 test with Yates correction.
Table 2 Postoperative outcomes.
| Outcome | Analysis per protocol (n = 186) | Intention‐to‐treat analysis (n = 201) | ||||
|---|---|---|---|---|---|---|
| Study group (n = 87), no (%) | Control group (n = 99), no (%) | p Value | Study group (n = 95), no (%) | Control group (n = 106), no (%) | p Value | |
| NNIS 30‐day infection | 4 (4.6) | 12 (12.1%) | 0.068 | 4 (4.21) | 12 (11.32) | 0.063 |
| Sternal wound infection (90‐day infection) | 8 (9.2) | 25 (25.2) | 0.004 | 8 (8) | 25 (23.5) | 0.004 |
| Superficial | 4 (4.6) | 11 (11.1) | 0.104 | 4 (4.20) | 11 (10.38) | 0.097 |
| Deep | 2 (2.3) | 8 (8.1) | 0.156* | 2 (2.1) | 8 (7.55) | 0.148* |
| Organ space | 2 (2.3) | 6 (6.1) | 0.368* | 2 (2.1) | 6 (5.66) | 0.355* |
| Deep + organ space | 4 (4.6) | 14 (14.1) | 0.028 | 4 (4.2) | 14 (13.21) | 0.026 |
| Sternal surgical debridement | 4 (4.5) | 19 (19.2) | 0.003 | 4 (4.2) | 19 (17.92) | 0.002 |
| Harvest site infection | 4 (4.6) | 7 (7.1) | 0.476 | 5 (5.2) | 7 (6.6) | 0.689 |
| Infection‐related mortality | 0 (0) | 2 (2.0) | 0.535* | 0 (0) | 2 (2.0) | 0.526* |
| All‐cause mortality | 1 (1.1) | 2 (2.0) | 1.000* | 1 (1.1) | 4 (3.8) | 0.630* |
| Postoperative renal dysfunction | 11 (12.6) | 18 (18.2) | 0.299 | 12 | 18 (17) | 0.388 |
| Postoperative renal replacement | 1 (1.1) | 5 (5.1) | 0.277* | 2 (2.1) | 5 (4.7) | 0.533* |
| Postoperative hospital stay (days) (mean, median (range)) | 9.1,7,(4–73) | 12.0,7,(4–69) | 0.063† | 9.5,7,(4–73) | 11.7,7,(4–69) | 0.131† |
| Cost of antibiotic used (US$) (mean, median (range)) | 358, 31 (31–12714) | 454, 9 (9–21316) | <0.001 | NA | ||
| Hospital cost (US$1000) (mean, median (range)) | 14.8, 8 (5.7–126.8) | 18.6, 10, (5.7–97.3) | 0.04 | |||
N/A, not assessed as excluded from analysis; NNIS, National Nosocomial Infections Surveillance System.
*χ2 test with Yates correction
†Mann–Whitney U test.
The reduction in infection (control versus study group) was seen across all grades of infection in both the superficial (11.1% and 4.6%, respectively; p = 0.104) and deep categories (8.1% and 2.3%; p = 0.156), being significant in combined deep or organ‐space category (14.1% and 4.6%; p = 0.028). This was reflected in the significant reduction in the need for surgical intervention (debridement, vacuum therapy or pectoralis flap construction), from 19.2% to 4.5% (p = 0.003), and a marginally significant reduction in readmissions due to SWI after hospital discharge, from 11.1% to 3.4% (χ2 = 3.907, p = 0.048).
Table 3 reports the distribution of microbial isolates in the as‐per‐protocol analysis. Of the 25 patients with SWI in the control group, cultures from 6 were negative while those from 10 grew Gram‐positive organisms, 15 grew Gram‐negative organisms and 1 grew Candida from the sternum, with 10 of these patients having multiple organisms. Of cultures from the 8 patients with SWI in the study group, 1 had no growth, 4 grew Gram‐positive organisms, 7 grew Gram‐negative organisms and 5 grew multiple organisms.
Table 3 Microbial isolates from sternal wound (per‐protocol analysis).
| No. of patients | Control group (n = 99) | Study group (n = 87) |
|---|---|---|
| With SWI | 25 | 8 |
| With positive growth | 19 | 7 |
| With multiple organisms | 10 | 5 |
| With Gram‐positive bacteria* | 10† | 4† |
| Staphylococcus aureus | 4 | 2 |
| Enterococcus faecalis | 8 | 2 |
| Number of Gram‐positive organisms resistant to cefuroxime | 2 | 2 |
| Number of Gram‐positive organisms resistant to rifampicin | 4 | 1 |
| Number of Gram‐positive organisms resistant to vancomycin | 0 | 0 |
| With Gram‐negative bacteria | 15† | 7† |
| Pseudomonas aeruginosa | 2 | 3 |
| Bacteroides fragilis | 2 | 1 |
| Enterobacteriaceae | 14 | 7 |
| Enterobacter cloacae | 1 | 1 |
| Escherichia coli | 12 | 5 |
| Proteus mirabilis | 1 | 1 |
| Klebsiella pneumoniae | 0 | 2 |
| Serratia marcescens | 0 | 1 |
| With other organisms | ||
| Candida albicans | 1 | 0 |
SWI, sternal wound infection.
*Flucloxacillin was often used as the preferred treatment, blind, and may have contributed to the low number of isolates.
†Patients may have more than one organism.
Total antibiotic costs (prophylactic and treatment) were significantly lower (−21.2%, US$96 (£49.7, €73.2) in the study group, and, similarly, hospital costs were also significantly lower (−20.6%, US$3800 (£1967.26, €2896.9)). The NNIS risk score failed to discriminate this group as a high‐risk group and predicted an infection rate of 3.5% (table 1), where SWIRS predicted a 20% rate. Of the 202 patients not included in the study because they were low risk (fig 1), 6 (2.9%) patients developed SWI by 30 days (institutional data).
The study group showed no increase in renal dysfunction (risk difference of 5.6%, with 95% CI of –16% to 5%) or renal replacement (risk difference of –4%, with 95% CI of –9% to 1%) and all‐cause mortality (risk difference of –0.9%, with 95% CI of –4% to 3%; table 2).
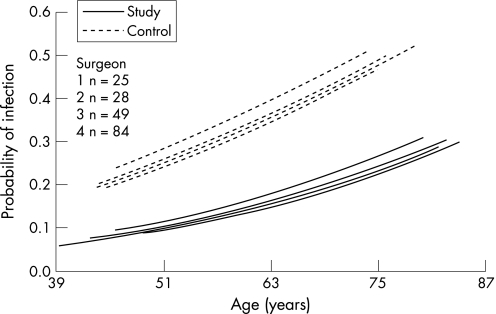
Using multilevel hierarchical regression analysis15,16 to evaluate the effect of the surgeon on the incidence of infection, we calculated the σμ12 (variance between surgeons) to be 0.094 (χ2 = 0.027, p = 0.86), which shows that no difference between surgeons was identified (fig 2).
Figure 2 Difference in probability of infection between the study and control groups, taking the age of the patient and performance of four different surgeons into consideration.
Discussion
This prospective randomised double‐blind controlled clinical trial shows that longer and broader‐spectrum antibiotic prophylaxis significantly decreases the incidence of SWI in high‐risk patients. Furthermore, it shows a significant economic benefit with the study regimen of US$3896 (£2017.16, €2971.56) per patient, which does not include the lost‐opportunity cost of the extended hospital stay, re‐admission or stay in the intensive therapy unit.
The overall incidence of the NNIS SWI at our institution (7.6%) conforms to the incidence previously reported in several studies,5,9,17,18 which varies from 1% to 10% depending on the definition of SWI and the duration of follow‐up. Large published case series have also reported an incidence of superficial infection up to 90 days postoperatively of up to 10%.3,4 By targeting a high‐risk group, defined on the basis of obesity and diabetes (patient‐related) and the use of a bilateral internal thoracic artery graft (procedure‐related),5 we expected to see an incidence of 20%–30% and our calculated SWIRS predicted an incidence of 20%.
Of the 446 patients who underwent median sternotomy (fig 1), <10% had operations of >5 h duration (a high‐risk group indicated by the NNIS), and this group did not show a significant increase in infection (institutional data). On the other hand, the NNIS risk score of the recruited 186 patients in the trial was 3.5% and considering the results, the score failed to discriminate this group as a high‐risk group. The use of the NNIS seems to be ineffective in cardiac surgery owing to the lack of specificity to this type of surgery, in which there are additional risk factors that predispose to wound infection that are not considered by the NNIS.10 However, comparisons are still being made between institutions using the NNIS, which may disadvantage those institutions that operate on more patients with diabetes or obesity or centres that use bilateral internal thoracic arteries as a routine grafting strategy.8,11
The infection rate at 30 days in the 202 low‐risk patients was 2.9%, which was within the NNIS expectation. The trial was conducted after a significant institutional response to a perceived high incidence of SWI based on NNIS risk (data not presented), that did not take into account patient‐related and procedure‐related risks. Procedure and plant factors had been strenuously addressed, with both internal and external reviews, over a period of 1 year. Following these thorough institutional improvements, the infection rate of 25% (in the control group) was in line with the study assumption of a 90‐day infection rate,4 and relates to the high‐risk nature of these patients. SWI at 90 days was still nearly double that at 30 days (NNIS), and reflects the experience of previous studies.4,18 The study regimen had a significant impact, reducing SWI by 64%.
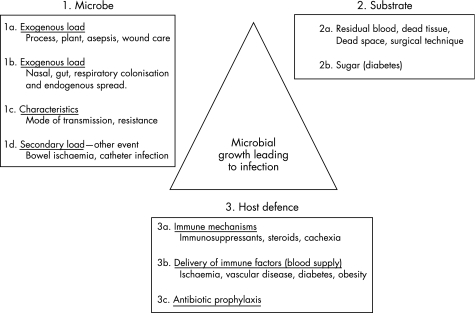
SWI is multifactorial in origin, and, as fig 3 illustrates, antibiotic prophylaxis is only one component of the SWI prevention triad. Two patients in the study group, both women with large breasts, developed mediastinitis. Large breast size is a recognised risk factor of SWI because of mechanical traction on the sternotomy wound.19 More probably, the extra demand for vascular supply by the breasts leaves the sternum more ischaemic. One of the two patients developed acute abdominal distress on the fifth postoperative day and required a laparotomy for a perforated duodenal ulcer. Presumably, a secondary endogenous bacteraemia was inevitable, which explains why her wounds grew Proteus and Escherichia coli. Jakob et al20 showed, by DNA analysis, that endogenous infection is a major source of infection around the time of surgery and therefore significant non‐cardiac events in the immediate postoperative period may need appropriate cover. The other patient was an obese woman with diabetes who had been dependent on insulin for the past 20 years; because she was diagnosed preoperatively as having an occluded left internal thoracic artery, her right one was used instead. She was discharged home but returned with a necrotic sternum infected with Pseudomonas. On the other hand, in the control group, six patients (three males) developed mediastinitis and two of them died from infection‐related causes (33%).
Figure 3 The sternal would infection (SWI) prevention triad.
Epidemiology of the infective organisms in sternotomy wounds has shown that 70%–80% are caused by Gram‐positive cocci.5,21 Spelman et al,14 in a retrospective study, compared a change of antibiotics, and argued for a combination of rifampicin and vancomycin, on which we based our antibiotic prophylaxis.14 Rifampicin has good oral absorption, with inhibitory tissue concentration that lasts from 2 to 8 h, and the combination of rifampicin with vancomycin may reduce the development of resistance.14 As we limited our study to high‐risk patients, which included obese patients, we preferred a weight‐based regimen. Farber et al22 have shown that a 15 mg/kg dose of vancomycin is necessary to ensure that adequate tissue and bone concentrations are reached.
Additionally, 20%–30% of infections are caused by Gram‐negative organisms.2,5,21 Despite this, four studies have chosen to compare cephalosporins with vancomycin alone. Maki et al23 showed a benefit of vancomycin (15 mg/kg). By contrast, Finkelstein et al24 compared vancomycin 1 g three times daily for 24 h with cefazolin 1 g three times daily for 24 h,24 Salminen et al25 compared ceftriaxone 2 g with vancomycin 500 mg four times a day for 48 h and Vuorisalo et al26 compared vancomycin 500 mg four times a day with cefazolin for 48 h, and all the latter three studies failed to show any difference. Spelman et al,14 despite showing an overall reduction in infection, had seen an increase in Gram‐negative infections after the use of narrow‐spectrum prophylaxis. Therefore, we added Gram‐negative cover with gentamicin 2 mg/kg as a single dose on induction.
Maher et al13 found a benefit in continuing antibiotics for 48 h after removal of the chest tubes; therefore, we elected to continue antibiotics for this duration. This satisfied our criteria of effective cover against recognised pathogens with a strategy aiming to minimise resistance.
There was a significant benefit—statistical, clinical and economic—using the study regimen in the high‐risk group of patients, which was effective in reducing superficial, deep and organ‐space infections. There was also a significant reduction in the need for readmission and surgical intervention in the study group, whereas there were two infection‐related deaths in the control group. Despite the recognised renal effects of the antibiotic regimen used in the study, there was no difference in postoperative renal dysfunction between the two groups.
Surprisingly, the microbial isolates of the wounds showed a lower incidence of Gram‐positive infection in the control group despite the use of vancomycin and rifampicin in the study group (table 3). This could be attributed to the use of flucloxacillin, which was often the preferred treatment for wound infection and was started by the treating physicians empirically and before obtaining the microbiology results. The greater proportion of negative cultures in the control group (6/25) compared with the study group (1/8) would support this. The predominant organisms in the study group wounds were Gram‐negative organisms sensitive to gentamicin, and it is possible that 48 h Gram‐negative cover would have provided a more complete cover.
In most guidelines for antibiotic prophylaxis, patient‐related and procedure‐related factors are generally disregarded.27 Although our regimen was effective in this group of high‐risk patients, other agents may be necessary in other high‐risk groups (eg, chronic lung disease, immunosuppression), where the antibiotic prophylaxis should be tailored to the patient's susceptibility and risks. Guidelines that disregard individual patient characteristics or specific procedure‐related factors may be inappropriate for all patients. The implications of such a policy for antibiotic resistance may be significant, but this may require other solutions such as rotation of antibiotics and their combinations rather than denying patients effective antibiotic prophylaxis. It is important to report that there was no increase in the institutional incidence of vancomycin‐resistant Enterococcus or methicillin‐resistant Staphylococcus aureus during or after the trial.
Randomisation and implementation were carried out by the same individual (KD or MC) and therefore we have compared our preoperative variables to show comparability between the groups (fig 1). A limitation of the study was the inability to completely blind all carers to the two arms of the study. This was primarily because we anticipated that the use of rifampicin, with its orange colouration of urine, would have unmasked blinding on urinary catheterisation. We therefore accepted this level of blinding at the immediate perioperative stage by the principal surgeon. The onset of wound infection is, however, remote from the operation, and active surveillance by hospital personnel was routine and included all surgical patients. This process and the management of wound infection had been standardised. It was possible for them to unmask the randomisation, but this would have required a determined study of the medical notes. The primary care physicians, pharmacist and hospital accountant were more effectively blinded, as they had no access to the relevant medical notes. Although this may have biased the diagnosis of superficial infection, they are less likely to influence “harder” data such as sternal wound debridement (where management was protocolised) and diagnosis of deep and organ‐space infection.
The study regimen varied three important aspects of antibiotic prophylaxis for CABG: broad spectrum, weight based and continued for 48 h, compared with the control regimen in this group of high‐risk patients. The relative contribution of each of these factors is not assessable by our study. We recognise the limitation of being a single‐centre trial, but we offer an alternative management strategy for patients at high risk of SWI. We reinforce the need to stratify patients before comparison, using an appropriate risk‐stratification system. This group of patients is not recognised as high risk by the NNIS definition, and institutions with a large proportion of these patients may be disadvantaged if NNIS criteria are used as the basis of risk stratification.
In conclusion, this single‐centre randomised study has shown a significant reduction in SWI, in a high‐risk group of patients undergoing CABG, with clinical and economic benefits of broad‐spectrum and prolonged (48 h) antibiotic prophylaxis.
Acknowledgements
We thank all the surgical and anaesthetic teams at Harefield Hospital for their support during the study.
Abbreviations
CABG - coronary artery bypass grafting
CDCP - Centers for Disease Control and Prevention
LR - likelihood ratio
NNIS - National Nosocomial Infections Surveillance System
OR - odds ratio
SWI - sternal wound infection
SWIRS - sternal wound infection risk score
Appendix A
By adopting a background infection rate of 5% (prior odds), the probability of infection (posterior probability) was calculated for each patient using the equations (1a and 1b) which utilised the following likelihood ratios (LRs) derived from the existing literature: left internal thoracic artery versus no left internal thoracic artery, LR = 1.5; bilateral internal thoracic arteries versus no internal thoracic artery, LR = 4.5; obesity versus no obesity, LR = 2; and diabetes versus no diabetes LR = 2.5.5
Posterior odds = prior odds×likelihood ratio
Posterior probability = posterior odds/(1+posterior odds)
Appendix B
The initial model estimates were derived using a second‐order penalised quasi‐likelihood estimation. Subsequently, a Bayesian approach was used, utilising diffuse priors (γ(ε,ε) prior distribution, where ε was set to 0.001) and the Gibb's re‐sampling method with 50 000 iterations to calculate confidence limits and to correct bias in the parameter estimation (44,45). The probability of wound infection − logityij of patient i operated on by surgeon j was calculated with the following equation:
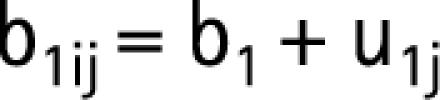 |
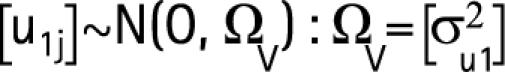 |
where subscript i takes the value of 1 to the number of patients operated by surgeon j, b1 is the constant for level 1 (fixed effect), b2 the regression coefficient for every unit increase in the patient's age and b3 the fixed estimates for patients in the study group versus the control group. The term u1j refers to the random departures or “residuals” at the surgeon level. This allows the j'th surgeon's line to differ from the average regression line. u1j is the level 2 random coefficient for surgeon j, or “surgeon‐effect”, which assumes a normal distribution with a mean of zero and variance σμ12.
Footnotes
Competing interests: None.
References
- 1.Milton H. Mediastinal surgery. Lancet 1897I872 [Google Scholar]
- 2.Kreter B, Woods M. Antibiotic prophylaxis for cardiothoracic operations. Meta‐analysis of thirty years of clinical trials. J Thorac Cardiovasc Surg 1992104590–599. [PubMed] [Google Scholar]
- 3.Ridderstolpe L, Gill H, Granfeldt H. Superficial and deep sternal wound complications: incidence, risk factors, and mortality. Eur J Cardiothorac Surg 2001201168–1175. [DOI] [PubMed] [Google Scholar]
- 4.Jonkers D, Elenbaas T, Terporten P.et al Prevalence of 90‐days postoperative wound infections after cardiac surgery. Eur J Cardiothorac Surg 20032397–102. [DOI] [PubMed] [Google Scholar]
- 5.Gummert J F, Barten M J, Hans C. Mediastinitis and cardiac surgery—an updated risk factor analysis in 10,373 consecutive adult patients. Thorac Cardiovasc Surg 20025087–91. [DOI] [PubMed] [Google Scholar]
- 6.Antunes P E, Prieto D, Ferrao de Oliveira J.et al Renal dysfunction after myocardial revascularization. Eur J Cardiothorac Surg 200425597–604. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis W R. Benchmarking for prevention: the Centers for Disease Control and Prevention's National Nosocomial Infections Surveillance (NNIS) system experience. Infection 200331(Suppl 2)44–48. [PubMed] [Google Scholar]
- 8. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, issued August 2003. Am J Infect Control 200331481–498. [DOI] [PubMed] [Google Scholar]
- 9.Sharma M, Berriel‐Cass D, Baran J., Jr Sternal surgical‐site infection following coronary artery bypass graft: prevalence, microbiology, and complications during a 42‐month period. Infect Control Hosp Epidemiol 200425468–471. [DOI] [PubMed] [Google Scholar]
- 10.Roy M C, Herwaldt L A, Embrey R.et al Does the Centers for Disease Control's NNIS system risk index stratify patients undergoing cardiothoracic operations by their risk of surgical‐site infection? Infect Control Hosp Epidemiol 200021186–190. [DOI] [PubMed] [Google Scholar]
- 11.Surgical site infection surveillance in England CDR Weekly. 2004;14:1–5. [Google Scholar]
- 12.McConkey S J, L'Ecuyer P B, Murphy D M.et al Results of a comprehensive infection control program for reducing surgical‐site infections in coronary artery bypass surgery. Infect Control Hosp Epidemiol 199920533–538. [DOI] [PubMed] [Google Scholar]
- 13.Maher K O, VanDerElzen K, Bove E L.et al A retrospective review of three antibiotic prophylaxis regimens for pediatric cardiac surgical patients. Ann Thorac Surg 2002741195–1200. [DOI] [PubMed] [Google Scholar]
- 14.Spelman D, Harrington G, Russo P.et al Clinical, microbiological, and economic benefit of a change in antibiotic prophylaxis for cardiac surgery. Infect Control Hosp Epidemiol 200223402–404. [DOI] [PubMed] [Google Scholar]
- 15.Spiegelhalter D J, Aylin P, Best N G.et al Commissioned analysis of surgical performance using routine data: lessons from the Bristol inquiry. J R Stat Soc A 2002165191–221. [Google Scholar]
- 16.Rasbash J, Browne W, Goldstein H.A users guide to MLwiN. London: University of London, 2001
- 17.Harrington G, Russo P, Spelman D.et al Surgical‐site infection rates and risk factor analysis in coronary artery bypass graft surgery. Infect Control Hosp Epidemiol 200425472–476. [DOI] [PubMed] [Google Scholar]
- 18.Swenne C L, Lindholm C, Borowiec J.et al Surgical‐site infections within 60 days of coronary artery by‐pass graft surgery. J Hosp Infect 20045714–24. [DOI] [PubMed] [Google Scholar]
- 19.Copeland M, Senkowski C, Ulcickas M.et al Breast size as a risk factor for sternal wound complications following cardiac surgery. Arch Surg 1994129757–759. [DOI] [PubMed] [Google Scholar]
- 20.Jakob H G, Borneff‐Lipp M, Bach A.et al The endogenous pathway is a major route for deep sternal wound infection. Eur J Cardiothorac Surg 200017154–160. [DOI] [PubMed] [Google Scholar]
- 21.Gardlund B, Bitkover C Y, Vaage J. Postoperative mediastinitis in cardiac surgery—microbiology and pathogenesis. Eur J Cardiothorac Surg 200221825–830. [DOI] [PubMed] [Google Scholar]
- 22.Farber B F, Karchmer A W, Buckley M J.et al Vancomycin prophylaxis in cardiac operations: determination of an optimal dosage regimen. J Thorac Cardiovasc Surg 198385933–935. [PubMed] [Google Scholar]
- 23.Maki D G, Bohn M J, Stolz S M.et al Comparative study of cefazolin, cefamandole, and vancomycin for surgical prophylaxis in cardiac and vascular operations. A double‐blind randomized trial. J Thorac Cardiovasc Surg 19921041423–1434. [PubMed] [Google Scholar]
- 24.Finkelstein R, Rabino G, Mashiah T.et al Vancomycin versus Cefazolin prophylaxis for cardiac surgery in the setting of a high prevalence of methicillin‐resistant staphylococcal infections. J Thorac Cardiovasc Surg 2002123326–332. [DOI] [PubMed] [Google Scholar]
- 25.Salminen U S, Viljanen T U, Valtonen V V.et al Ceftriaxone versus vancomycin prophylaxis in cardiovascular surgery. J Antimicrob Chemother 199944287–290. [DOI] [PubMed] [Google Scholar]
- 26.Vuorisalo S, Pokela R, Syrjala H. Comparison of vancomycin and cefuroxime for infection prophylaxis in coronary artery bypass surgery. Infect Control Hosp Epidemiol 199819234–239. [DOI] [PubMed] [Google Scholar]
- 27.Bratzler D W, Houck P M. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis 2004381706–1715. [DOI] [PubMed] [Google Scholar]