Abstract
Objective
To assess the long‐term effect of spinal cord stimulation (SCS) in patients with refractory cardiac syndrome X (CSX).
Methods
A prospective, controlled, long‐term follow‐up was performed of 19 patients with CSX with refractory angina who underwent SCS (SCS group, 5 men, mean (SD) age 60.9 (8.5) years); 9 comparable patients with CSX who refused SCS treatment (3 men, mean (SD) age 60.9 (8.8) years) constituted the control group. Clinical and functional status were assessed at the time of screening for SCS indication (basal evaluation) and at a median (range) follow‐up of 36 (15–82) months.
Results
The two groups at baseline did not show any difference in clinical characteristics and angina status. All indicators of angina status (angina episode frequency, duration and short‐acting nitrate use) improved significantly at follow‐up in the SCS group (p<0.001) but not in controls. Functional status, as assessed by the Seattle Angina Questionnaire and a visual analogue scale for quality of life, improved at follow‐up in the SCS group (p<0.001 for all scales) but not in controls. Exercise tolerance, exercise‐induced angina and ST segment changes also significantly improved in the SCS group but not in controls.
Conclusions
Data show that SCS can be a valid form of treatment for long‐term control of angina episodes in patients with refractory CSX.
About 20% of patients undergoing coronary angiography because of typical chest pain have normal coronary arteries.1 The causes of chest pain in these patients remain controversial and may be heterogeneous.2,3,4 A dysfunction of small coronary arterial vessels has been suggested, particularly in those with transient electrocardiographic changes during spontaneous or stress‐induced angina (cardiac syndrome X, CSX).5,6,7 An enhanced painful perception of cardiac stimuli, however, has also been reported to be a major pathophysiological component in most such patients.8,9 Although the prognosis of patients with CSX is excellent,10 a significant number present with frequent episodes of severe chest pain, refractory to maximal multidrug treatment, which may heavily limit daily activities and impair the quality of life (QoL).11,12,13 Spinal cord stimulation (SCS) has initially been proposed as a form of treatment for refractory angina pectoris in patients with obstructive coronary artery disease not suitable for percutaneous and surgical revascularisation,14,15,16 and it has recently been included as a class IIb recommendation for refractory angina in the American College of Cardiology/American Heart Association guidelines on chronic stable angina.17 Recently, some studies have shown that SCS can be applied safely and is associated with short‐term improvement of symptoms and QoL in patients with angina and normal coronary arteries.18,19,20 However, the long‐term effects of SCS in patients with CSX with refractory angina have not yet been assessed.
Methods
Study protocol
This study was designed as a prospective, long‐term comparison between a group of patients with CSX with refractory angina episodes who underwent SCS (SCS group) and a group of patients with CSX eligible for SCS because of refractory angina episodes who refused this form of treatment (controls).
Clinical and functional assessment for the study protocol was performed at the time of screening for SCS indication (basal evaluation) and at a long‐term follow‐up visit (FU evaluation) in all patients. Additionally, in the SCS group, a clinical assessment was also performed 6 months after device implantation to evaluate short‐term effects of SCS treatment. All clinical and diagnostic investigations were performed using the same methods.
The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board. All patients gave informed written consent for participation in the study.
Patients
A total of 30 consecutive patients with CSX (8 men, 22 women, mean (SD) age 60.9 (8.6) years) were proposed to undergo SCS device implantation because of refractory angina pectoris at our institute (Istituto di Cardiologia, Università Cattolica del Sacro Cuore, Rome, Italy) from March 1998 to May 2004. All patients fulfilled the following inclusion criteria: (1) a clinical diagnosis of CSX (ie, predominant effort angina, evidence of myocardial ischaemia according to ST segment depression during exercise stress test and/or reversible perfusion defects on stress myocardial scintigraphy and totally smooth coronary arteries at angiography); (2) no evidence of coronary artery spasm according to clinical history and electrocardiographic findings (ie, no angina at rest, no ST‐segment elevation during effort angina) and, in those reporting angina at rest, also according to ergonovine test results; (3) coronary angiography performed ⩽12 months before enrolment; and (4) no other cardiac (eg, valvular heart disease or cardiomyopathy) or systemic diseases, as assessed by careful clinical and diagnostic investigation. Patients with hypertension, however, were not excluded from this study after blood pressure was effectively controlled (<140/90 mm Hg;) by drug treatment and left ventricular hypertrophy was excluded by echocardiography.4
Eligibility for SCS also required: (1) recurrent episodes of angina pectoris which, in the view of the patient, significantly limited his/her activities and QoL; (2) persistence of angina episodes despite maximal tolerated anti‐ischaemic drug treatment, variably including anti‐ischaemic drugs (β‐blockers, calcium‐channel blockers and nitrates) and alternative drugs (xanthine derivatives, ACE inhibitors, statins and imipramine); (3) no contraindications to SCS; (4) written informed consent to undergo SCS device implantation after being informed of the technical aspects, clinical implications and possible side effects and complications.
Exclusion criteria for SCS treatment included bleeding disorders and severe disease of the vertebral column, which could harm insertion of the epidural electrode in the correct position.
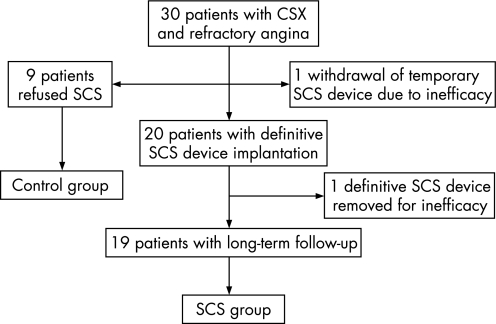
Of the 30 patients who were proposed to undergo SCS treatment, 9 refused the treatment and form the control group of this study. Of the 21 patients who underwent SCS, 1 successively refused definitive neurostimulator implantation because of ineffectiveness of SCS during the trial period with the temporary device. The SCS device was removed in another patient 12 months after the definitive implantation owing to loss of effectiveness. Thus, the SCS group eventually included 19 patients with complete long‐term FU (fig 1). The two patients in whom SCS was withdrawn due to inefficacy were excluded from the analyses. However, they were considered as failures of treatment when assessing the global efficacy of SCS.
Figure 1 Schematic diagram of the study protocol. CSX, cardiac syndrome X; SCS, spinal cord stimulation.
SCS device implantation
Details of SCS device implantation at our hospital have been described elsewhere.19 Briefly, all patients first underwent temporary SCS implantation for 2–3 weeks. Under sterile conditions and local anaesthesia, the epidural space was punctured at the level of T6 and a quadripolar electrode catheter was introduced and advanced under x ray control into the epidural space. A suitable position for the electrode catheter was sought, corresponding to the site where a prickling sensation (paresthesia) was felt covering the area of radiation of angina under neurostimulation. The ideal stimulation parameters were then found for the patient, and the electrode catheter was connected to an external portable pulse generator. In patients who reported significant symptom relief during 2–3 weeks of temporary SCS, a permanent implantation was undertaken. The decision to proceed with definitive SCS treatment was left at the total discretion of the patient. A quadripolar Itrel 2 or Itrel 3 internal pulse generator (Medtronic Italia, Milano, Italy) was placed in a subcutaneous abdominal or gluteal pocket. An extension lead was connected to the electrode by subcutaneous tunnelling. A pattern of continuous SCS stimulation was advised. However, the patients were left free to turn the device on and off and change the level of stimulation as desired using an external magnet or remote control.
Clinical and functional assessment
During the basal and FU evaluations, all patients were asked to characterise their angina episodes by defining precisely the circumstance in which chest pain occurred, the frequency of angina episodes, their duration and the frequency of sublingual short‐acting nitrate use in the past 4 weeks.
For purposes of analyses, with regard to frequency of angina attacks, patients were classified as having <1 episode/week, 2–3 episodes/week, 4–6 episodes/week, 1–3 episodes/day and ⩾4 episodes/day. A similar classification was used with regard to the number of short‐acting nitrates usually taken to relieve angina episodes (<1 tablet/week, 2–3 tablets/week, 4–6 tablets/week, 1–3 tablets/day and ⩾4 tablets/day). With regard to the duration of angina episodes, patients were classified as having attacks usually lasting <5 min, 5–9 min, 10–19 min and ⩾20 min.
The Seattle Angina Questionnaire (SAQ) was given to the patients. SAQ consists of 19 multiple‐choice items resulting in 5 scales that quantify physical limitation, angina stability, angina frequency, treatment satisfaction and disease perception. For each scale, a score from 0 to 100 is obtained, with higher values indicating better angina status.21
Finally, patients were asked to score their QoL using the EuroQoL Visual Analogue Scale (VAS), graduated from 0 (worst imaginable condition) to 100 (best imaginable condition).22
Exercise stress test
Symptom‐limited treadmill exercise stress tests were performed according to the Bruce protocol. Unless potentially serious abnormalities appeared, such as clinically significant arrhythmias, hypotensive (>40 mm Hg reduction in systolic blood pressure compared with a previous measurement) or hypertensive (systolic blood pressure >240 mm Hg or diastolic blood pressure >140 mm Hg) response to exertion,23 the test was stopped only because of muscular fatigue or crescendo angina. Three ECG leads (II, V2 and V5) were continuously monitored during the test. In all, 12‐lead ECG was printed and brachial artery cuff blood pressure was recorded before exercise, at the end of each stage during the effort and at 1‐min intervals during recovery. ST segment depression was considered to be significant when either horizontal or downsloping and ⩾1 mm at 0.08 s from the J point.
Statistical analysis
Comparisons of basal characteristics between the two groups were performed by the unpaired t test for continuous variables and by the Fisher exact test for proportions. Baseline and FU data of continuous functional and exercise variables of the two groups were compared by 2‐way analysis of variance. In case of global statistical significance, multiple between‐group and within‐group comparisons were performed by the paired t test with Bonferroni correction.
Ordinal clinical variables (eg, angina frequency and duration, short‐acting nitrate use) were compared independently at the basal evaluation and at FU using the Mann–Whitney U test. In case of statistical differences between the two groups at FU, within‐group changes from baseline were assessed in each group using the paired Wilcoxon test, with Bonferroni correction for multiple comparisons.
In the SCS group, repeated measure analysis of variance and the Friedman test were applied to compare the changes in continuous and ordinal variables, respectively, at 6‐month FU and at the last FU in the SCS group, compared with the basal evaluation. The paired t test and Wilcoxon test with Bonferroni correction were used for multiple comparisons, as appropriate.
Data are reported as mean (SD), unless differently indicated. A p value of <0.05 was considered significant.
Results
General characteristics of patients
The two groups of patients were similar with regard to the main baseline clinical characteristics (table 1). The duration of angina before screening for SCS ranged from 1 to 10 years. Owing to persistent or worsening symptoms, coronary angiography had been repeated at least once in eight patients, confirming the presence of smooth epicardial coronary arteries. Initially the patients had predominant effort angina, but over time the pain developed at rest as frequently as following exercise. All patients had undergone at least one hospitalisation because of ungovernable angina during the 3 months preceding enrolment in the study.
Table 1 Main clinical characteristics of both groups of patients at the time of evaluation for spinal cord stimulation.
| SCS (n = 19), % | Controls (n = 9), % | p Value | |
|---|---|---|---|
| Mean (SD) age (years) | 60.9 (8.5) | 60.9 (8.8) | 1.0 |
| Sex (M:F) | 5:14 | 3:6 | 0.78 |
| Cardiovascular risk factors | |||
| Hypertension | 12 (63.2) | 5 (55.6) | 0.85 |
| Dyslipidaemia | 11 (57.9) | 7 (77.8) | 0.75 |
| Smoking history | 2 (10.5) | 3 (33.3) | 0.33 |
| Family history of CVD | 8 (42.1) | 6 (66.7) | 0.52 |
| Drug treatment | |||
| β‐blockers | 9 (47.4) | 6 (66.7) | 0.74 |
| Calcium‐channel blockers | 5 (26.3) | 5 (55.6) | 0.45 |
| Nitrates | 4 (21.1) | 2 (22.2) | 0.95 |
| ACE‐inhibitors | 7 (36.8) | 2 (22.2) | 0.69 |
| Aldosterone receptor blockers | 3 (15.8) | 1 (11.1) | 0.77 |
| HMG‐CoA‐reductase inhibitors | 6 (31.6) | 4 (44.4) | 0.71 |
CVD, cardiovascular disease, HMG‐CoA, 3‐hydroxy‐3‐methylglutaryl coenzyme A.
Values are represented as n (%) unless otherwise denoted.
Evidence of ST‐segment depression during the exercise stress test was present in 28 of the 30 patients enrolled in the study. In one patient, ST segment changes could not be assessed during exercise because of left bundle branch block; in the other patient a reliable symptom‐limited exercise test could not be performed because of limited physical activity related to hip osteoarthritis. According to inclusion criteria, dipyridamole radionuclide stress test documented reversible myocardial perfusion defects in both these patients.
SCS at follow‐up
Patients in the SCS group were followed up for a median (range) period of 36 (15–82) months. The FU period was 12–24 months in five patients, 25–36 months in five patients, 36–48 months in two patients, 48–60 months in three patients and >60 months in four patients. The duration of FU of the control group was similar to that of the SCS group (median (range) 34 (17–72) months, p = 0.92).
At the last FU, 12 (63%) patients with SCS were using a continuous pattern of neurostimulation, while 7 (37%) were using an intermittent pattern of stimulation. After SCS device implantation, stimulation parameters during FU were modified in six patients, with stimulus intensity being raised in four patients (who had lost perception of chest paresthesias) and lowered in two patients (who began to feel paresthaesias as bothersome).
Characteristics of angina episodes
The two groups did not show any difference in the characteristics of angina episodes at the basal evaluation (table 2). However at FU, the frequency of angina (p = 0.005) and the use of short‐acting nitrates (p = 0.028) were significantly lower in the SCS group than in controls, as a result of a significant improvement at FU in the SCS group (p<0.001 for each characteristic of angina) but not in controls. Of note, at FU, angina at rest was reported by all controls but by only 21% of patients with SCS (p<0.001), whereas there were no differences at baseline.
Table 2 Characteristics of angina episodes at basal evaluation and at follow‐up evaluation in patients treated with spinal cord stimulation and control patients.
| Basal evaluation | p Value | Follow‐up | p Value | |||
|---|---|---|---|---|---|---|
| SCS, n (%) | Controls, n (%) | SCS, n (%) | Controls, n (%) | |||
| Frequency of angina episodes | ||||||
| <1/week | 0 (0) | 0 (0) | 6 (31.6) | 1 (11.1) | ||
| 2–3/week | 1 (5.3) | 1 (11.1) | 6 (31.6) | 0 (0) | ||
| 4–6/week | 5 (26.3) | 4 (44.4) | 0.105 | 7 (36.8) | 4 (44.4) | 0.005 |
| 1–3/day | 7 (36.8) | 3 (33.3) | 0 (0) | 4 (44.4) | ||
| ⩾4/day | 6 (31.6) | 1 (11.1) | 0 (0) | 0 (0) | ||
| Duration of angina episodes | ||||||
| <5 min | 1 (5.3) | 0 (0) | 8 (42.1) | 2 (22.2) | ||
| 5–9 min | 5 (26.3) | 4 (44.4) | 0.498 | 9 (47.4) | 2 (22.2) | 0.095 |
| 10–19 min | 5 (26.3) | 2 (22.2) | 2 (10.5) | 3 (33.3) | ||
| ⩾20 min | 8 (42.1) | 3 (33.3) | 0 (0) | 2 (22.2) | ||
| Sublingual nitrates use | ||||||
| <1/week | 1 (5.3) | 3 (33.3) | 14 (73.7) | 3 (33.3) | ||
| 2–3/week | 6 (31.6) | 0 (0) | 5 (26.3) | 2 (22.2) | ||
| 4–6/week | 3 (15.8) | 4 (44.4) | 0.263 | 0 (0) | 2 (22.2) | 0.028 |
| 1–3/day | 7 (36.8) | 1 (11.1) | 0 (0) | 2 (22.2) | ||
| ⩾4/day | 2 (10.5) | 1 (11.1) | 0 (0) | 0 (0) | ||
| Angina episodes trigger | ||||||
| Exercise | 16 (84.2) | 7 (77.8) | 0.678 | 17 (89.5) | 8 (88.9) | 0.963 |
| Stress | 6 (31.6) | 3 (33.3) | 0.926 | 4 (21.1) | 2 (22.2) | 0.944 |
| Rest | 14 (73.7) | 8 (88.9) | 0.360 | 4 (21.1) | 9 (100) | <0.001 |
SCS, spinal cord stimulation.
Values are represented as mean (SD).
Functional angina status and quality of life
Angina functional status and QoL, as assessed by SAQ and VAS, were similar at baseline in the two groups. However at FU, all SAQ scores and VAS‐assessed QoL were significantly higher in the SCS group than in controls, due to a significant improvement of scores in the SCS group (p<0.001 for all scales), whereas no significant changes were observed in controls (table 3).
Table 3 Seattle Angina Questionnaire results and Quality of Life rating at basal evaluation and at follow‐up evaluation in patients treated with SCS and control patients.
| SCS (n = 19) | Controls (n = 9) | p Value | |
|---|---|---|---|
| Physical limitation scale | |||
| Basal evaluation | 31.1 (13.4) | 38.9 (9.7) | <0.001 |
| Follow‐up evaluation | 57.2 (10.3)* | 35.0 (9.0) | |
| Angina stability scale | |||
| Basal evaluation | 9.5 (15.4) | 13.3 (14.1) | <0.001 |
| Follow‐up evaluation | 73.7 (13.4)* | 26.7 (26.5) | |
| Angina frequency scale | |||
| Basal evaluation | 27.9 (15.1) | 27.2 (15.2) | <0.01 |
| Follow‐up evaluation | 63.2 (15.0)* | 42.2 (17.9) | |
| Treatment satisfaction scale | |||
| Basal evaluation | 38.5 (12.2) | 42.9 (10.1) | <0.01 |
| Follow‐up evaluation | 59.6 (10.1)* | 47.1 (8.4) | |
| Disease perception scale | |||
| Basal evaluation | 29.5 (11.2) | 38.5 (15.2) | <0.001 |
| Follow‐up evaluation | 57.9 (13.3)* | 39.3 (15.1) | |
| Visual analogical scale | |||
| Basal evaluation | 35.0 (13.4) | 38.3 (15.4) | <0.001 |
| Follow‐up evaluation | 69.2 (12.7)* | 41.7 (13.7) |
SCS, spinal cord stimulation.
Values are represented as n (%).
*p<0.001 versus basal evaluation.
Exercise stress test results
Both patients in whom exercise stress test could not be assessed belonged to the SCS group. Thus exercise test results were available for 17 patients of the SCS group and for all nine controls. Exercise stress test results were similar in the two groups at baseline (table 4). At FU, patients in the SCS group showed less exercise‐induced angina and ⩾1 mm ST‐segment depression than in controls, although the differences did not achieve statistical significance. Yet, time and rate‐pressure product at angina, at 1 mm ST‐segment depression and at peak exercise were significantly better at FU in the SCS group than in controls, as a result of a significant improvement in the SCS group but not in controls.
Table 4 Exercise test results at basal evaluation and at follow‐up evaluation in patients treated with spinal cord stimulation and control patients.
| SCS (n = 17) | Controls (n = 9) | p Value | |
|---|---|---|---|
| Peak exercise | |||
| Test duration (s) | |||
| Basal | 362 (126) | 313 (72) | <0.05 |
| Follow‐up | 428 (101)* | 310 (86) | |
| Heart rate (bpm) | |||
| Basal | 123 (25) | 117 (22) | 0.15 |
| Follow‐up | 132 (21) | 116 (16) | |
| Systolic blood pressure (mm Hg) | |||
| Basal | 138 (11) | 146 (13) | <0.005 |
| Follow‐up | 160 (15)† | 147 (10) | |
| Rate‐pressure product (bpm x mm Hg) | |||
| Basal | 17002 (3848) | 17138 (3887) | <0.005 |
| Follow‐up | 21156 (4066)† | 17082 (2914) | |
| 1 mm ST segment depression | |||
| No of patients | |||
| Basal | 14 (82%) | 7 (78%) | 0.58 |
| Follow‐up | 8 (47%) | 7 (78%) | 0.085 |
| Time (s) | |||
| Basal | 274 (148) | 262 (99) | <0.05 |
| Follow‐up | 365 (131)‡ | 268 (60) | |
| Heart rate (bpm) | |||
| Basal | 107 (17) | 104 (14) | <0.01 |
| Follow‐up | 124 (21)‡ | 103 (13) | |
| Systolic blood pressure (mm Hg) | |||
| Basal | 135 (11) | 136 (11) | <0.05 |
| Follow‐up | 155 (16)‡ | 138 (7) | |
| Rate‐pressure product (bpm x mm Hg) | |||
| Basal | 14571 (3076) | 14127 (2138) | <0.005 |
| Follow‐up | 19432 (4543)‡ | 13984 (1972) | |
| Angina | |||
| No of patients | |||
| Basal | 10 (59%) | 6 (67%) | 0.52 |
| Follow‐up | 9 (53%) | 6 (67%) | 0.40 |
| Time (s) | |||
| Basal | 236 (84) | 254 (54) | <0.05 |
| Follow‐up | 350 (101)‡ | 262 (92) | |
| Heart rate (bpm) | |||
| Basal | 99 (15) | 101 (8) | <0.01 |
| Follow‐up | 115 (17)* | 99 (8) | |
| Systolic blood pressure (mm Hg) | |||
| Basal | 132 (6) | 135 (8) | <0.05 |
| Follow‐up | 146 (12)‡ | 137 (12) | |
| Rate‐pressure product (bpm x mm Hg) | |||
| Basal | 13073 (2177) | 13702 (1553) | <0.01 |
| Follow‐up | 16845 (3580)‡ | 13628 (2131) | |
SCS, spinal cord stimulation.
Values are represented as mean (SD).
*p<0.01 versus basal evaluation.
†p<0.05 versus basal evaluation.
‡p<0.005 versus basal evaluation.
Global efficacy of SCS
A significant improvement of angina symptoms at FU, defined as a reduction of at least two of the five classes of angina frequency, was reported by 13 of all 21 patients (62%) who underwent SCS (including the two patients who had undergone withdrawal of SCS because of inefficacy), but by none of the controls (p = 0.003).
Similarly, reduction of at least two of the five classes of short‐acting nitrate consumption was reported by 11 of all 21 patients (52%) who underwent SCS, but by none of the controls (p = 0.01).
Medium‐term and long‐term effect of SCS
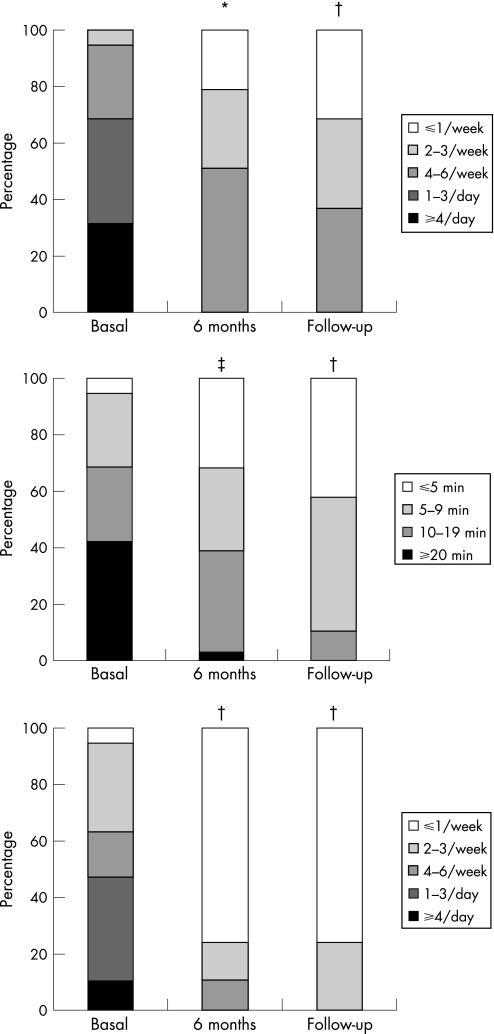
Figure 2 shows the main characteristics of angina episodes before SCS treatment, after 6 months of SCS treatment and at the last FU in the SCS group, whereas table 5 summarises the SAQ and VAS‐assessed QoL scores and the main exercise stress test results at the same time points. When compared with the basal assessment, all analysed parameters showed a significant improvement after 6 months of SCS treatment, which remained substantially unmodified at the long‐term FU.
Figure 2 Proportion of patients of the spinal cord stimulation (SCS) group in the different classes of frequency (upper panel) and duration (mid panel) of angina episodes, and of consumption of sublingual nitrate tablets (bottom panel) before SCS treatment (basal assessment), after 6 months of SCS treatment and the last follow‐up (average 36 months). *p = 0.005, †p = 0.001, ‡p<0.001.
Table 5 Seattle Angina Questionnaire, Quality of Life and main exercise test results at basal evaluation and at short‐term and long‐term evaluation in patients treated with spinal cord stimulation.
| Baseline | 6 months | Follow‐up | p | |
|---|---|---|---|---|
| Seattle Angina Questionnaire | ||||
| Physical limitation scale | 31.4 (14.1) | 59.3 (11.9)* | 59.1 (10.1)* | <0.001 |
| Angina stability scale | 8.8 (14.5) | 68.1 (16.0)* | 73.8 (14.1)* | <0.001 |
| Angina frequency scale | 30.6 (14.4) | 64.6 (10.8)* | 67.7 (11.3)* | <0.001 |
| Treatment satisfaction scale | 37.5 (12.5) | 63.0 (9.7)* | 61.0 (9.9)* | <0.001 |
| Disease perception scale | 29.6 (11.7) | 59.8 (9.9)* | 60.0 (11.9)* | <0.001 |
| Quality of life | ||||
| Visual analogue scale | 35.3 (14.5) | 72.5 (11.4)* | 71.6 (12.3) | <0.001 |
| Exercise stress test | ||||
| Test duration (s) | 362 (126) | 384 (116)† | 428 (101)† | 0.001 |
| Peak RPP (bpm x mm Hg) | 17002 (3848) | 19044 (3802)† | 21156 (4066)* | <0.001 |
| Time to 1 mm ↓ST (s) | 274 (148) | 360 (143)† | 365 (131)* | <0.001 |
| 1 mm ↓ST RPP (bpm × mm Hg) | 14571 (3076) | 18256 (2216)† | 19432 (4543)‡ | 0.01 |
| Time to angina (s) | 236 (84) | 334 (125)‡ | 350 (101)‡ | <0.005 |
| Angina RPP (bpm x mm Hg) | 13073 (2177) | 16072 (2506)‡ | 16845 (3580)‡ | <0.001 |
RPP, rate‐pressure product; ↓ST, ST segment depression.
Values are represented as mean (SD).
*p<0.001 versus basal evaluation.
†p<0.05 versus basal evaluation.
‡p<0.005 versus basal evaluation.
Adverse events
There were no major complications related to SCS device implantation and long‐term treatment. Skin infection at the level of puncture, for screening electrode catheter introduction into the epidural space, occurred in one patient, requiring removal of the device and replacement with another system after controlling the infection. Abdominal neurostimulator bulging occurred in another patient and was treated with translocation of the device in the right gluteal region. Three patients during FU required replacement of the neurostimulator because of battery failure, and an electrode dislocation requiring repositioning occurred in three other patients.
Discussion
In this prospective study we show that the early beneficial effects of SCS in patients with CSX and angina episodes refractory to maximal multidrug treatment are maintained at 3 year FU. To the best of our knowledge, our study is the first to show long‐term efficacy of SCS in patients with CSX afflicted by severe anginal symptoms using a controlled protocol.
SCS in refractory CSX
Patients with CSX have an excellent prognosis,10 but a significant number have a poor QoL because of frequent, recurrent anginal pain episodes refractory to maximally tolerated multidrug treatment that heavily limit daily activities.11,12,13
Several studies have shown that SCS may be an effective and safe treatment for refractory angina pectoris in patients with obstructive coronary artery stenoses,14,15,16 and it has been included as a class IIb recommendation for this form of disease in the American College of Cardiology/American Heart Association guidelines on chronic stable angina.17
A few small, uncontrolled studies have suggested that SCS may also represent an effective treatment for refractory angina in patients with CSX. Eliasson et al18 first reported favourable effects of SCS on exercise stress test results, and Chauhan et al24 found a significant improvement of coronary blood flow during transcutaneous electrical nerve stimulation, a therapeutic technique believed to have effects similar to SCS. Furthermore, SCS was shown to be associated with short‐term improvement of symptoms and QoL in patients with angina and normal coronary arteries or patients with typical CSX.19,25 In a crossover study, we also documented favourable effects of SCS on angina episodes, QoL and SAQ scores, as well as on episodes of ST‐segment depression on Holter monitoring and on both symptoms and ST‐segment changes induced by the dobutamine stress test in a small group of patients with CSX treated with SCS.20 Finally, long‐term persistence of the beneficial effects of neuromodulatory techniques (transcutaneous electrical nerve stimulation or SCS) has recently been suggested in an observational uncontrolled trial.26
In this study on a larger and well characterised population of patients affected by CSX, we show, using a prospective, controlled protocol, that SCS is persistently effective over a long period (average 3 years), as shown by substantial improvement of all indexes used to characterise the angina/ischaemic status of the patients. Importantly, we found an improvement of subjective clinical variables as well as of stress‐induced ischaemic ECG changes, in agreement with previous short‐term data.18,19,20 No significant improvement was found at FU, both for clinical and exercise variables, in the control group of patients with CSX and refractory angina who refused to undergo SCS treatment.
Mechanisms of SCS in CSX
Coronary microvascular dysfunction and increased cardiac pain perception are the major pathophysological mechanisms of CSX4,5,6,7,8,9 and both could be related, at least in part, to alterations in cardiac adrenergic function.27,28 SCS probably exerts its beneficial effects by acting on both these pathophysiological components. Recent reports have shown that SCS modulates cardiac autonomic function, which may lead to improved microcirculatory function.29,30 Furthermore, studies with positron emission tomography have shown that neuromodulation may favour redistribution of coronary blood flow towards myocardial ischaemic areas.24 Moreover, as suggested by a more gradual increase in heart rate and blood pressure during exercise,18,19 SCS might limit the increase of myocardial oxygen consumption during physical or mental stress.
The direct modulatory effect on pain transmission of SCS is also well demonstrated. The analgesic effect of SCS seems to be mainly mediated by stimulation of inhibitory neurons in the posterior horns of the spinal cord,31,32 but may also include the release of anti‐algogenic substances in the central33 and peripheral34 nervous system. A recent study in patients with CSX has demonstrated the lack of habituation to pain stimuli and an enhanced cortical activity facilitating the transmission to the cortex of pain stimuli.35 Hence, in these patients, SCS might normalise cardiac pain perception through modulation of an abnormal central pain processing of cardiac stimuli.
Limitations of the study
Some limitations of our study should be acknowledged. First, the study was not randomised; thus some selection bias for SCS treatment cannot be excluded. However, basal clinical characteristics and the FU time of the two groups were similar, suggesting that they could be appropriately compared.
Second, a role for a placebo effect of SCS treatment cannot be completely excluded in our patients due to the lack of a “placebo group”; however, this could not be included as perception by the patient of chest paraesthesia during SCS in the area of referred angina has been until now believed important for effective neurostimulation. The long‐lasting improvement of symptoms and the improvement of exercise‐induced ECG alterations, however, make it unlikely that the beneficial effects of SCS observed in this study could be exclusively related to a mere placebo effect.
Third, the FU time of patients was very variable, resulting in only a few patients at each time point, which precluded time‐based analyses; however, patients with CSX needing SCS are rare and the dilution of enrolment over a long time was inevitable; yet, the FU time of the two groups was similar, thus making unlikely an influence of time on our results.
Finally, reproducibility of exercise stress test results was not required for inclusion of patients in the study; however, it is unlikely that an improvement of exercise results occurred accidentally in patients treated with SCS but not in control patients who presented similar exercise findings at the basal test.
Conclusions
Our data show that SCS can be a valid form of treatment for long‐term control of angina episodes in patients with refractory CSX. In order to achieve stronger evidence and characterisation of the clinical effects of SCS in these patients, appropriate randomised trials, possibly also including some form of “sham” neurostimulation, are warranted.
Abbreviations
CSX - cardiac syndrome X
FU - follow up
QoL - quality of life
SAQ - Seattle Angina Questionnaire
SCS - spinal cord stimulation
VAS - visual analogue scale
Footnotes
Competing interests: None declared.
References
- 1.Phibbs B, Fleming T, Ewy G A.et al Frequency of normal coronary arteriograms in three academic medical centers and one community hospital. Am J Cardiol 198862472–474. [DOI] [PubMed] [Google Scholar]
- 2.Maseri A, Crea F, Kaski J C.et al Mechanisms of angina pectoris in syndrome X. J Am Coll Cardiol 199117499–506. [DOI] [PubMed] [Google Scholar]
- 3.Cannon R O, Camici P G, Epstein S E. Pathophysiological dilemma of syndrome X. Circulation 199285883–892. [DOI] [PubMed] [Google Scholar]
- 4.Lanza G A. Cardiac syndrome X: a critical overview and future directions. Heart 200793159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opherk D, Zebe H, Weihe E.et al Reduced coronary dilatory capacity and ultrastructural changes of the myocardium in patients with angina pectoris but normal coronary arteriograms. Circulation 198163817–825. [DOI] [PubMed] [Google Scholar]
- 6.Cannon R O, Epstein S E. Microvascular angina as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol 1988611338–1343. [DOI] [PubMed] [Google Scholar]
- 7.Chauhan A, Mullins P A, Taylor M.et al Both endothelium‐dependent and endothelium‐independent function is impaired in patients with angina pectoris and normal coronary angiograms. Eur Heart J 19971860–68. [DOI] [PubMed] [Google Scholar]
- 8.Cannon R O, 3rd, Quyyumi A A, Schenke W H.et al Abnormal cardiac sensitivity in patients with chest pain and normal coronary arteries. J Am Coll Cardiol 1990161359–1366. [DOI] [PubMed] [Google Scholar]
- 9.Pasceri V, Lanza G A, Buffon A.et al Role of abnormal pain sensitivity and behavioral factors in determining chest pain in syndrome X. J Am Coll Cardiol 19983162–66. [DOI] [PubMed] [Google Scholar]
- 10.Kaski J C, Rosano G M C, Collins P.et al Cardiac syndrome X: clinical characteristics and left ventricular function. Long‐term follow‐up study. J Am Coll Cardiol 199525807–814. [DOI] [PubMed] [Google Scholar]
- 11.Lavey E B, Winkle R A. Continuing disability of patients with chest pain and normal coronary arteriograms. J Chron Dis 197932191–196. [DOI] [PubMed] [Google Scholar]
- 12.Pasternak R C, Thibault G E, Savoia M.et al Chest pain with angiographically insignificant coronary arterial obstruction: clinical presentation and long‐term follow‐up. Am J Med 198068813–817. [DOI] [PubMed] [Google Scholar]
- 13.Ockene I S, Shay M J, Alpert J S.et al Unexplained chest pain in patients with normal coronary arteriograms: a follow‐up study of functional status. N Engl J Med 19803031249–1252. [DOI] [PubMed] [Google Scholar]
- 14.TenVaarwerk I A, Jessurun G A, DeJongste M J.et al Clinical outcome of patients treated with spinal cord stimulation for therapeutically refractory angina pectoris. The Working Group on Neurocardiology. Heart 19998282–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray S, Carson K G S, Ewings P D.et al Spinal cord stimulation significantly decreases the need for acute hospital admission for chest pain in patients with refractory angina pectoris. Heart 19998289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannheimer C, Eliasson T, Augustinsson L E.et al Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris: the ESBY study. Circulation 1998971157–1163. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons R J, Abrams J, Chatterjee K, American College of Cardiology, American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) et al ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients with Chronic Stable Angina). J Am Coll Cardiol 200341159–168. [DOI] [PubMed] [Google Scholar]
- 18.Eliasson T, Albertsson P, Hardhammar P.et al Spinal cord stimulation in angina pectoris with normal coronary arteriograms. Coron Artery Dis 19934819–827. [DOI] [PubMed] [Google Scholar]
- 19.Lanza G A, Sestito A, Sandric S.et al Spinal cord stimulation in patients with refractory anginal pain and normal coronary arteries. Ital Heart J 2001225–30. [PubMed] [Google Scholar]
- 20.Lanza G A, Sestito A, Sgueglia G A.et al Effect of spinal cord stimulation on spontaneous and stress‐induced angina and ‘ischemia‐like' ST‐segment depression in patients with cardiac syndrome X. Eur Heart J 200526983–989. [DOI] [PubMed] [Google Scholar]
- 21.Spertus J A, Winder J A, Dewhurst T A.et al Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 199525333–341. [DOI] [PubMed] [Google Scholar]
- 22.EuroQol Group EuroQol—a new facility for the measurement of health related quality of life. Health Policy 199016199–208. [DOI] [PubMed] [Google Scholar]
- 23. Gibbons RJ, Balady GJ, Bricker JT, et al, American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article, a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 20021061883–1892. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan A, Mullins P A, Thuraisingham S I.et al Effect of transcutaneous electrical nerve stimulation on coronary blood flow. Circulation 199489694–702. [DOI] [PubMed] [Google Scholar]
- 25.Jessurun G A, Hautvast R W, Tio R A.et al Electrical neuromodulation improves myocardial perfusion and ameliorates refractory angina pectoris in patients with syndrome X: fad or future? Eur J Pain 20037507–512. [DOI] [PubMed] [Google Scholar]
- 26.de Vries J, Dejongste M J, Durenkamp A.et al The sustained benefits of long‐term neurostimulation in patients with refractory chest pain and normal coronary arteries. Eur J Pain 200711360–365. [DOI] [PubMed] [Google Scholar]
- 27.Rosano G M, Ponikowski P, Adamopoulos S.et al Abnormal autonomic control of the cardiovascular system in syndrome X. Am J Cardiol 1994731174–1179. [DOI] [PubMed] [Google Scholar]
- 28.Lanza G A, Giordano A, Pristipino C.et al Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I]metaiodobenzylguanidine myocardial scintigraphy. Circulation 199796821–826. [DOI] [PubMed] [Google Scholar]
- 29.Foreman R D, Linderoth B, Ardell J L.et al Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res 200047367–375. [DOI] [PubMed] [Google Scholar]
- 30.Olgin J E, Takahashi T, Wilson E.et al Effects of thoracic spinal cord stimulation on cardiac autonomic regulation of the sinus and atrioventricular nodes. J Cardiovasc Electrophysiol 200213475–481. [DOI] [PubMed] [Google Scholar]
- 31.Eliasson T, Augustinsson L E, Mannheimer C. Spinal cord stimulation in severe angina pectoris‐presentation of current studies, indications and clinical experience. Pain 199665169–179. [DOI] [PubMed] [Google Scholar]
- 32.Jessurum G A J, DeJongste M J L, Blanksma P K. Current views on neurostimulation in the treatment of cardiac ischemic syndromes. Pain 199666109–116. [DOI] [PubMed] [Google Scholar]
- 33.Tonelli L, Setti T, Falasca A.et al Investigation on cerebrospinal fluid opioids and neurotransmitters related to spinal cord stimulation. Appl Neurophysiol 198851324–332. [DOI] [PubMed] [Google Scholar]
- 34.Eliasson T, Mannheimer C, Waagstein F.et al Myocardial turnover of endogenous opioids and calcitonin gene‐related peptide in the human heart and the effects of spinal cord stimulation on pacing‐induced angina pectoris. Cardiology 199889170–177. [DOI] [PubMed] [Google Scholar]
- 35.Valeriani M, Sestito A, Le Pera D.et al Abnormal cortical pain processing in patients with cardiac syndrome X. Eur Heart J 200526975–982. [DOI] [PubMed] [Google Scholar]