Abstract
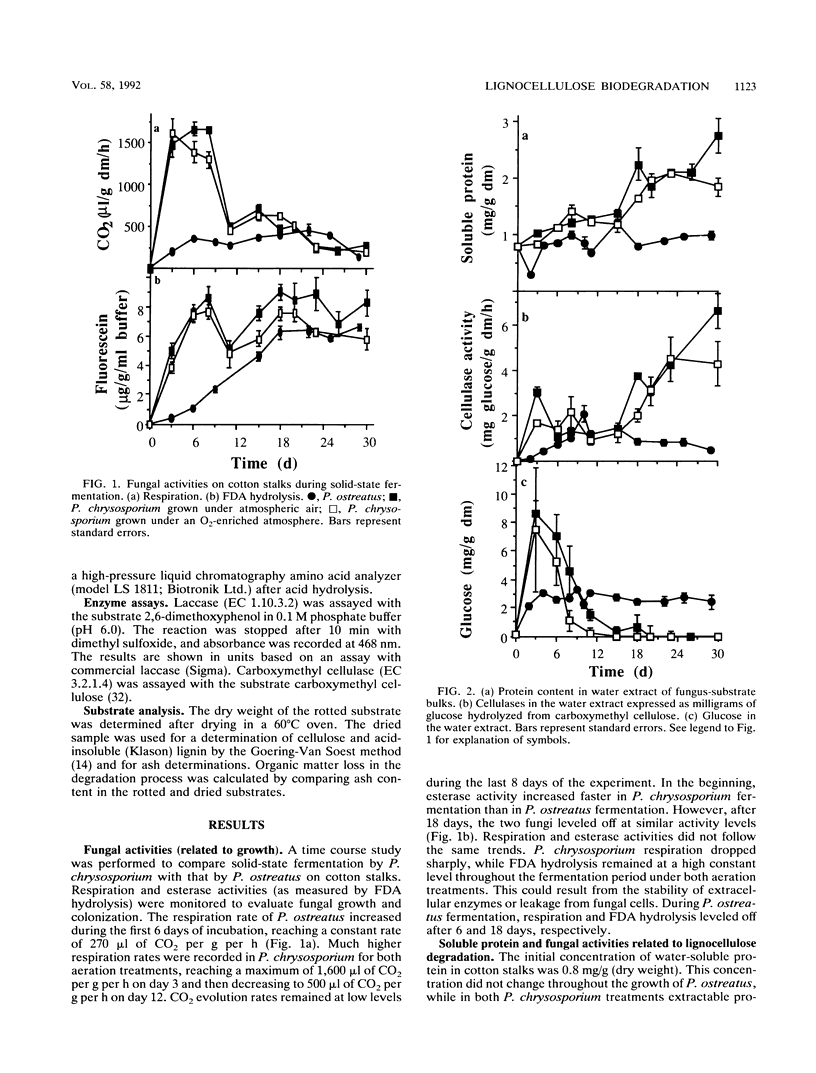
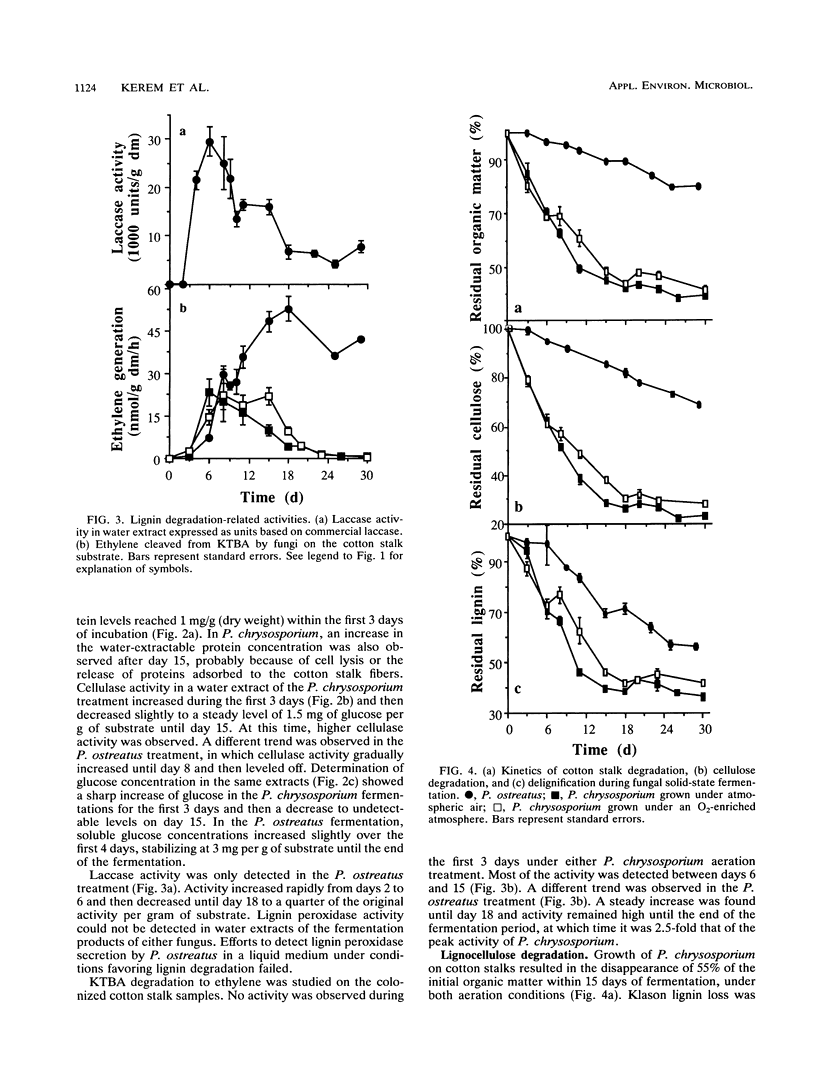
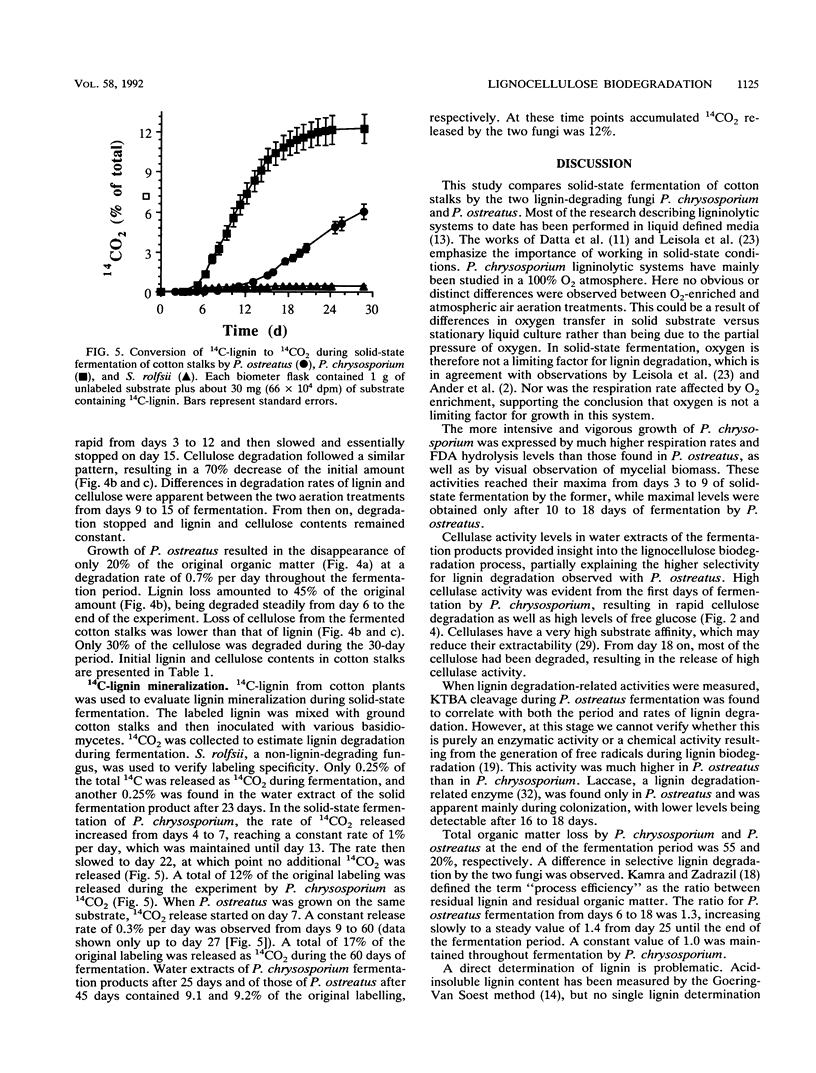
Lignocellulose degradation and activities related to lignin degradation were studied in the solid-state fermentation of cotton stalks by comparing two white rot fungi, Pleurotus ostreatus and Phanerochaete chrysosporium. P. chrysosporium grew vigorously, resulting in rapid, nonselective degradation of 55% of the organic components of the cotton stalks within 15 days. In contrast, P. ostreatus grew more slowly with obvious selectivity for lignin degradation and resulting in the degradation of only 20% of the organic matter after 30 days of incubation. The kinetics of 14C-lignin mineralization exhibited similar differences. In cultures of P. chrysosporium, mineralization ceased after 18 days, resulting in the release of 12% of the total radioactivity as 14CO2. In P. ostreatus, on the other hand, 17% of the total radioactivity was released in a steady rate throughout a period of 60 days of incubation. Laccase activity was only detected in water extracts of the P. ostreatus fermentation. No lignin peroxidase activity was detected in either the water extract or liquid cultures of this fungus. 2-Keto-4-thiomethyl butyric acid cleavage to ethylene correlated to lignin degradation in both fungi. A study of fungal activity under solid-state conditions, in contrast to those done under defined liquid culture, may help to better understand the mechanisms involved in lignocellulose degradation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colberg P. J., Young L. Y. Anaerobic degradation of soluble fractions of [C-lignin]lignocellulose. Appl Environ Microbiol. 1985 Feb;49(2):345–349. doi: 10.1128/aem.49.2.345-349.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Crawford R. L. Microbial degradation of lignocellulose: the lignin component. Appl Environ Microbiol. 1976 May;31(5):714–717. doi: 10.1128/aem.31.5.714-717.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Floyd S., Pometto A. L., 3rd, Crawford R. L. Degradation of natural and Kraft lignins by the microflora of soil and water. Can J Microbiol. 1977 Apr;23(4):434–440. doi: 10.1139/m77-064. [DOI] [PubMed] [Google Scholar]
- Datta A., Bettermann A., Kirk T. K. Identification of a specific manganese peroxidase among ligninolytic enzymes secreted by Phanerochaete chrysosporium during wood decay. Appl Environ Microbiol. 1991 May;57(5):1453–1460. doi: 10.1128/aem.57.5.1453-1460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Relationship Between Lignin Degradation and Production of Reduced Oxygen Species by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Nov;46(5):1140–1145. doi: 10.1128/aem.46.5.1140-1145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Asada Y., Kuwahara M. Screening of basidiomycetes for lignin peroxidase genes using a DNA probe. Appl Microbiol Biotechnol. 1990 Jan;32(4):436–442. doi: 10.1007/BF00903779. [DOI] [PubMed] [Google Scholar]
- Kutsuki H., Gold M. H. Generation of hydroxyl radical and its involvement in lignin degradation by Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1982 Nov 30;109(2):320–327. doi: 10.1016/0006-291x(82)91723-5. [DOI] [PubMed] [Google Scholar]
- Lee B., Pometto A. L., Fratzke A., Bailey T. B. Biodegradation of degradable plastic polyethylene by phanerochaete and streptomyces species. Appl Environ Microbiol. 1991 Mar;57(3):678–685. doi: 10.1128/aem.57.3.678-685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I. M. A semi-micro method for the determination of lignin and its use in predicting the digestibility of forage crops. J Sci Food Agric. 1972 Apr;23(4):455–463. doi: 10.1002/jsfa.2740230405. [DOI] [PubMed] [Google Scholar]
- Perez J., Jeffries T. W. Mineralization of C-Ring-Labeled Synthetic Lignin Correlates with the Production of Lignin Peroxidase, not of Manganese Peroxidase or Laccase. Appl Environ Microbiol. 1990 Jun;56(6):1806–1812. doi: 10.1128/aem.56.6.1806-1812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor W. A., Tang R. H. Ethylene formation from methional. Biochem Biophys Res Commun. 1978 Mar 30;81(2):498–503. doi: 10.1016/0006-291x(78)91562-0. [DOI] [PubMed] [Google Scholar]
- Périé F. H., Gold M. H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991 Aug;57(8):2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannia G., Limongi P., Cocca E., Buonocore F., Nitti G., Giardina P. Purification and characterization of a veratryl alcohol oxidase enzyme from the lignin degrading basidiomycete Pleurotus ostreatus. Biochim Biophys Acta. 1991 Jan 23;1073(1):114–119. doi: 10.1016/0304-4165(91)90190-r. [DOI] [PubMed] [Google Scholar]
- Schnürer J., Rosswall T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol. 1982 Jun;43(6):1256–1261. doi: 10.1128/aem.43.6.1256-1261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]


