Abstract
Organ primordia develop from founder cells into organs due to coordinated patterns of cell division. How patterned cell division is regulated during organ formation, however, is not well understood. Here, we show that the PUCHI gene, which encodes a putative APETALA2/ethylene-responsive element binding protein transcription factor, is required for the coordinated pattern of cell divisions during lateral root formation in Arabidopsis thaliana. Recessive mutations in PUCHI disturbed cell division patterns in the lateral root primordium, resulting in swelling of the proximal region of lateral roots. PUCHI expression was initially detected in all of the cells in early lateral root primordia, and later it was restricted to the proximal region of the primordia. Stable expression of PUCHI required auxin-responsive elements in its promoter region, and exogenous auxin increased the level of PUCHI mRNA accumulation. These results suggest that PUCHI acts downstream of auxin signaling and that this gene contributes to lateral root morphogenesis through affecting the pattern of cell divisions during the early stages of primordium development.
INTRODUCTION
Plasticity and adaptability are important life strategies in plants. Plant architecture is largely dependent on the formation of new organs after germination both in the shoot and the root. For example, lateral roots (LRs) are continuously formed from the primary root in the postembryonic root system. LRs are not produced directly from the parental root meristem, but instead develop from inner cells of a more mature part of the parental root.
Previous studies in Arabidopsis thaliana have detailed the development of the lateral root primordium (LRP) from pericycle cells (Malamy and Benfey, 1997; Casimiro et al., 2001; Dubrovsky et al., 2001). Initially, one or two mature pericycle cells adjacent to the xylem poles divide asymmetrically to form daughter cells, which are shorter than the flanking undivided pericycle cells. These daughter cells proliferate further with a largely fixed pattern of cell divisions to form the LR meristem, which has almost the same structure as that of the primary root meristem.
Many studies have shown that the plant hormone auxin is a key factor that controls LR formation (Smet et al., 2006; Fukaki et al., 2007). For example, application of exogenous auxin increases the number of LRs, whereas auxin transport inhibitors decrease their number (Blakely et al., 1988; Laskowski et al., 1995; Reed et al., 1998; Casimiro et al., 2001). Molecular genetic studies using Arabidopsis have identified auxin-dependent signaling processes that are important for LR initiation. Dominant or semidominant mutations in AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) genes, such as AXR5/IAA1, SHY2/IAA3, SLR/IAA14, MSG2/IAA19, and IAA28, result in a reduced number of LRs (Tian and Reed, 1999; Rogg et al., 2001; Fukaki et al., 2002; Tatematsu et al., 2004; Yang et al., 2004). This class of mutations causes stabilization of the corresponding Aux/IAA proteins, which are transcriptional repressors of auxin-responsive gene expression, and results in an inhibition of auxin signaling. For example, the slr-1 mutant, which carries a point mutation that causes stabilization of the IAA14 protein, fails to produce LRs due to the inhibition of the initial cell divisions associated with LR initiation (Fukaki et al., 2002). Another class of genes involved in LR formation includes AUXIN RESPONSE FACTOR7 (ARF7) and ARF19. These genes encode transcriptional activators that bind to the auxin-responsive element (AuxRE), a cis-regulatory sequence for auxin-responsive genes (Okushima et al., 2005; Wilmoth et al., 2005). Similar to the slr-1 mutant, LRs rarely form in the arf7 arf19 double mutant, indicating that these genes are redundantly required for LR initiation. The SLR/IAA14, ARF7, and ARF19 genes are expressed in a broad region of the root, including the pericycle, and the SLR/IAA14 protein physically interacts with ARF7 and ARF19 to block their activity (Fukaki et al., 2005). This interaction is thought to be important for the regulation of target gene activation that is required for LR initiation (Okushima et al., 2007).
Polar auxin transport, which is required for the asymmetric distribution of auxin, is also involved in LRP development. Treatment of wild-type roots with auxin transport inhibitors blocks LR initiation, whereas subsequent application of the auxin 1-naphthalene acetic acid (NAA) results in homogenous proliferation of all of the pericycle cells and formation of a highly increased number of LRs (Casimiro et al., 2001; Himanen et al., 2002). Similarly, multiple mutant combinations of PIN family genes (PINs), which encode auxin efflux carrier proteins, or in weak mutant alleles of the GNOM gene, which is required for the coordinated polar localization of PIN proteins, show homogeneous proliferation of pericycle cells in response to exogenous NAA and subsequent formation of LRPs with highly disorganized morphology (Benková et al., 2003; Geldner et al., 2004). The auxin transport pathway mediated by the PIN and GNOM proteins is thus thought to be crucial for LR initiation and subsequent primordium development. In addition, it has been reported that the auxin influx carrier AUX1 also affects LR initiation (Marchant et al., 2002).
Although it has been shown that LR initiation involves auxin accumulation controlled by the auxin transport system and the auxin signaling pathway mediated by the ARF and Aux/IAA proteins, the molecular mechanisms that control the subsequent development of the primordium remain largely unknown. Here, we identified the novel Arabidopsis gene PUCHI, which encodes a putative transcription factor of the APETALA2/ethylene-responsive element binding protein (AP2/EREBP) family. The puchi mutation disturbs cell division pattern during early LRP development and results in expansion of the proximal region of LRs. Reporter gene analyses indicate that PUCHI is expressed in the early stages of LRP development, initially in all of the primordium cells and later in the proximal region of the LR. Stable expression of PUCHI in the LRP requires the AuxREs in its promoter region and is upregulated in roots in response to exogenous auxin in an AuxRE-dependent manner. These results indicate that PUCHI is involved in the control of cell division patterns during LRP development and may act downstream of auxin signaling.
RESULTS
The puchi Mutation Affects Flower and LR Development
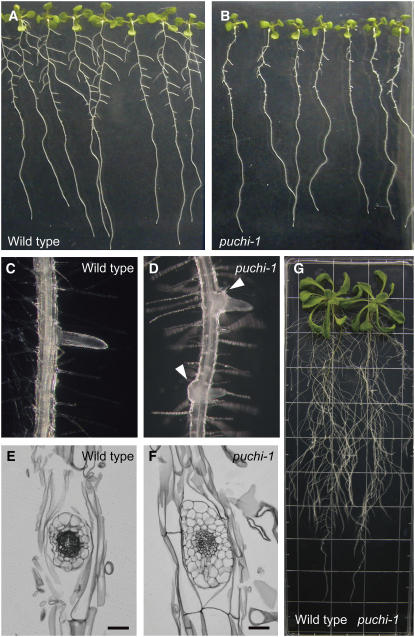
The puchi-1 mutant was initially identified by a subtle shoot phenotype, which is not described in detail in this article (see Methods). Besides this, the puchi-1 mutant showed clearly recognizable phenotypes in the root. At 9 d after germination (9 DAG), visible LRs in the puchi-1 mutant were much shorter than those of wild-type plants (Figures 1A and 1B). Mutant LRs that emerged out of the primary root surface, however, grew normally, and the root system in older plants was indistinguishable from that of wild-type plants (Figure 1G). Moreover, the proximal region of each LR in the puchi-1 mutant was significantly swollen and often bent (Figures 1C and 1D). In cross sections of mature LRs, the number of cells in this region was increased in puchi-1, especially along the apical-basal axis of the parental root (Figures 1E and 1F). In contrast with LRs, growth and morphology of the primary root of puchi was normal (Figures 1A and 1B; data not shown).
Figure 1.
Root Phenotypes of the puchi Mutant.
(A) and (B) Wild-type (A) and puchi-1 (B) seedlings at 9 DAG.
(C) and (D) Close-up views of young LRs in a wild-type plant (C) and a puchi-1 mutant (D). The proximal region of puchi mutant LRs is swollen (arrowheads in [D]). Note that the LR at the bottom attaches to the left side of the parental root, while its tip grows toward the right side due to bending.
(E) and (F) Cross sections of the proximal region of mature LRs from a wild-type plant (E) and a puchi-1 mutant (F). The section plane approximately corresponds to the surface of the primary root. Bars = 50 μm.
(G) A wild-type (left) and puchi-1 (right) seedling at 20 DAG. Seedlings were grown on a plate with a 2-cm grid for a scale.
To genetically test whether the phenotypes were caused by a single mutation, we crossed the puchi-1 mutant with a wild-type plant (Columbia [Col]) and examined the phenotypes in the shoot and root in subsequent generations. All F1 plants were indistinguishable from wild-type plants (n = 20). In the F2 generation, the shoot and root phenotypes cosegregated with a 3:1 ratio (25.6% mutant, n = 164), indicating that puchi-1 was a recessive mutation in a single locus. Hereafter, we focus on the characterization of LRP development in puchi-1.
LRP Development in the puchi-1 Mutant
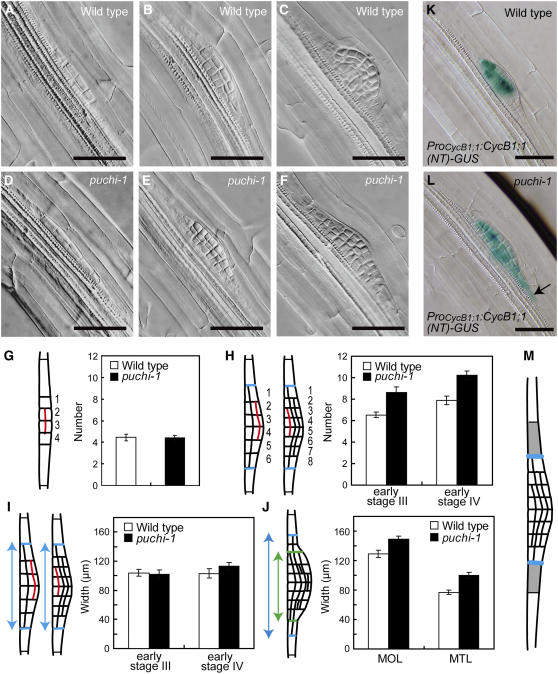
We examined LRP development in the puchi-1 mutant in detail. In the wild type, the initiation of a LR starts with anticlinal cell divisions of pericycle cells, resulting in an array of cells that are shorter than the flanking undivided cells (stage I; Malamy and Benfey, 1997; Dubrovsky et al., 2001). Subsequently, some of these short cells undergo periclinal divisions to form two cell layers (stage II; Figure 2A). Additional cell expansion and periclinal divisions result in LRP with three layers (stage III; Figure 2B) and then four layers (stage IV). From stages II to IV, anticlinal cell divisions in each layer occur and increase cell number along the radial axis of the LRP. Patterned cell divisions and expansion continue, resulting in the dome-shaped LRP (Figure 2C).
Figure 2.
LRP Development in the puchi-1 Mutant.
(A) to (F) Nomarski images of wild-type ([A] to [C]) and puchi-1 ([D] to [F]) LRPs in cleared primary roots at 7 DAG. LRPs in (A) and (B) are at stages II and III, respectively, whereas (C) represents the LRPs that have formed more than four cell layers. (D) to (F) show mutant LRPs that have formed the same number of layers as those in (A) to (C), respectively. Bars = 50 μm.
(G) The number of short cells in the outermost layer along the radial axis at early stage II, where one or two central cells have just undergone periclinal divisions (red line in the schematic diagram).
(H) The number of cells in the outermost layer of MOL at early stage III, where one to three outermost cells have undergone periclinal divisions (red line in left schematic diagram), and the early stage IV, where one to three innermost cells have undergone periclinal divisions (red line in right schematic diagram). Blue lines indicate the MOL border.
(I) Width along the innermost cell layer of MOL in LRPs at early stages III and IV. Blue lines indicate the MOL border, and arrows indicate the width of MOL.
(J) Width along the innermost cell layer of MOL and MTL in LRPs with more than four cell layers and before emergence. Blue and green lines indicate the borders of MOL and MTL, respectively. Blue and green arrows indicate the width of MOL and MTL, respectively.
(K) and (L) Expression of ProCycB1;1:CycB1;1(NT)-GUS in stage IV LRPs of wild-type (K) and of puchi-1 (L) at the corresponding stage at 9 DAG. GUS staining was for 12 h. Arrow indicates GUS staining in a cell next to the MOL border. Bars = 50 μm.
(M) Schematic diagram of the LRP. The cells next to the MOL border (blue line) are indicated in gray.
LRPs of the puchi-1 mutant were indistinguishable from that of the wild type both in terms of the overall cellular organization (Figures 2A and 2D) and the number of cells along the radial axis (Figure 2G) up to early stage II, where a few short pericycle cells at the center had undergone periclinal cell divisions. The puchi-1 mutant LRP started to deviate from the wild type from late stage II to stage III, where additional periclinal division began to form the third cell layer (Figures 2B and 2E). In these stages, the mutant LRP forms more cells than the wild-type LRP along the radial axis due to extra anticlinal divisions. To quantitatively compare the cell number, we defined an area consisting of more than one cell layer (MOL area; delimited by blue lines in Figures 2H to 2J) as a region that will presumably give rise to most part of the LR. We then counted the number of cells in the outermost layer of MOL along the radial axis. Cell number in the outermost layer of puchi-1 MOL was significantly increased compared with the wild type in primordia forming the third cell layer (Figure 2H; 6.5 ± 0.2 se, n = 13 in the wild type; 8.6 ± 0.5 se, n = 13 in puchi-1) or in the primordia forming the fourth layer (Figure 2H; 7.9 ± 0.4, n = 13 in the wild type; 10.3 ± 0.4, n = 13 in puchi-1), indicating that the frequency of anticlinal cell divisions relative to periclinal ones is increased in the puchi-1 mutant during the early stage of LRP development.
As the primordium development continued, the mutant LRP became flatter than that of the wild type (Figures 2C and 2F). The width at the most proximal region in the MOL was not significantly different between the wild type and puchi-1 LRP with three and four cell layers (Figure 2I; early stage III, 104.1 μm ± 4.4 se in the wild type [n = 13] and 102.0 μm ± 6.4 se in puchi-1 [n = 13]; early stage IV, 103.1 μm ± 6.6 se in the wild type [n = 16] and 112.8 μm ± 5.1 se in puchi-1 [n = 16]). However, the MOL of puchi-1 became ∼15% wider than that of the wild type in later stages (Figure 2J; 129.2 μm ± 5.3 se in the wild type [n = 26] and 149.0 μm ± 4.4 se in puchi-1 [n = 28]). In addition, the width of the area with more than two layers (MTL: delimited by green lines in Figure 2J) was also increased by ∼31% (Figure 2J; 76.9 μm ± 2.8 se in the wild type [n = 26] and 100.3 μm ± 4.1 se in puchi-1 [n = 28]). These results are consistent with the flatter shape of the puchi-1 mutant LRPs.
The increased width of the primordium observed in the puchi mutant LRPs may indicate that cell divisions occur in a wider area in the mutant primordium than in the wild type. To test this possibility, we examined expression of the ProCycB1;1:CycB1;1(NT)-GUS reporter, which specifically marks cells in the late G2 and M phases (Colón-Carmona et al., 1999). The intensity and pattern of β-glucuronidase (GUS) staining appeared normal in puchi-1 LRPs, suggesting that the mutation did not affect overall frequency or duration of G2/M (Figures 2K and 2L). However, the frequency of staining in cells at the periphery was significantly higher in puchi-1 mutant primordia than in the wild type (Figures 2K and 2L, Table 1; P < 0.01, Fisher's exact test). These results indicate that PUCHI restricts the area of cell proliferation.
Table 1.
Frequency of ProCycB1;1:CycB1;1(NT)-GUS Positive Cells
Number of LRPs in which either or both of the cells next to the MOL border (Figure 2M, gray cells) are stained with GUS.
LRPs with three or four cell layers.
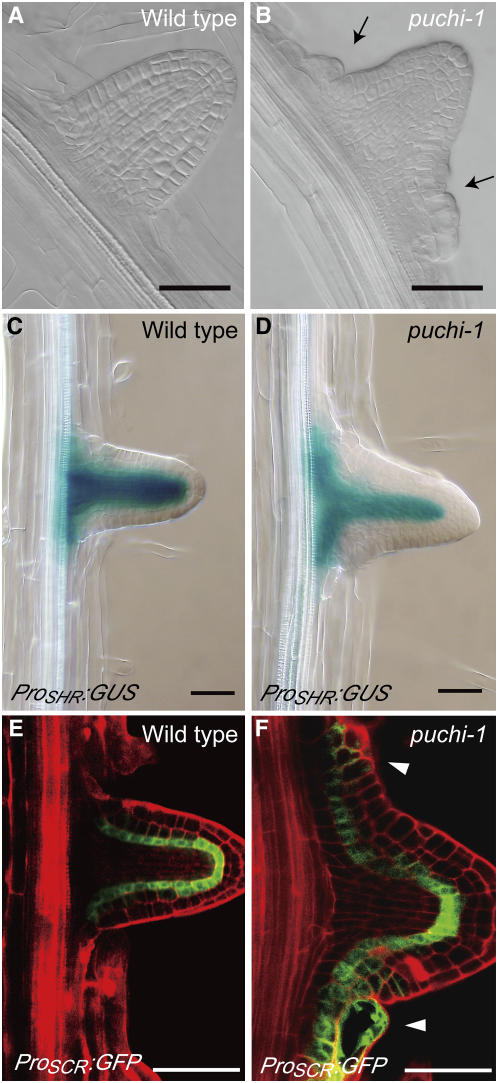
Later, when the LR meristem emerges out of the parental root, the puchi-1 mutant LR forms extra tissue consisting of highly enlarged cells at the periphery of the most proximal region, whereas no such tissue is observed in the corresponding region of wild-type LR (Figures 3A and 3B). The formation of such extra tissue is consistent with a wider area of cell divisions in earlier stages.
Figure 3.
Morphology and the Radial Pattern in a Young LR.
(A) and (B) Nomarski images of a young LR in the wild type (A) and puchi-1 (B) with similar length. The LR of puchi-1 contains ectopic tissue (arrows), resulting in the expansion of the organ.
(C) and (D) ProSHR:GUS expression in a young LR from a wild-type plant (C) and a puchi-1 mutant (D).
(E) and (F) ProSCR:GFP expression in a young LR from a wild-type plant (E) and a puchi-1 mutant (F). Arrowheads indicate surface cells ectopically expressing GFP.
Bars = 50 μm.
We next examined expression of the radial pattern markers ProSHR:GUS and ProSCR:green fluorescent protein (GFP) in the expanded region of puchi-1 mutant LRs. Expression of ProSHR:GUS, which was detected in the stele of the wild-type root (Helariutta et al., 2000), was more expanded toward the periphery than that in the wild type (Figures 3C and 3D). Similarly, expression of ProSCR:GFP, which was normally detected in the endodermis (Di Laurenzio et al., 1996), was expanded in puchi-1 (Figures 3E and 3F). In addition, although ProSCR:GFP expression was restricted to a single cell layer in the proximal region of wild-type LRs (Figure 3E), the GFP signal was detected in more than one cell layer, including the primordia surface, in puchi LRs (Figure 3F, arrowheads). Besides these abnormalities, the relative position of the ProSHR:GUS and ProSCR:GFP expression domains was essentially the same as that of the wild type in that the SCR domain surrounds the SHR domain. This observation suggests that the radial pattern in the proximal region of the puchi mutant LR was largely maintained.
Although the puchi mutation strongly affected cell division patterns during early stages of LRP development, the mutant developed an LR meristem similar to that of the wild-type LRP when the primordium emerged out of the parental root, except that the cellular organization and expression pattern of ProSCR:GFP around the quiescent center (QC) was slightly disturbed (see Supplemental Figures 1A to 1F online). These subtle phenotypes, however, were eventually rescued in mature LRs (see Supplemental Figures 1G and 1H online), which was consistent with normal growth of mature LRs (Figure 1G). These results indicate that PUCHI is strictly required for proper morphogenesis in early stages of LRP development but not for the establishment or maintenance of the LR meristem.
Cloning of the PUCHI Gene
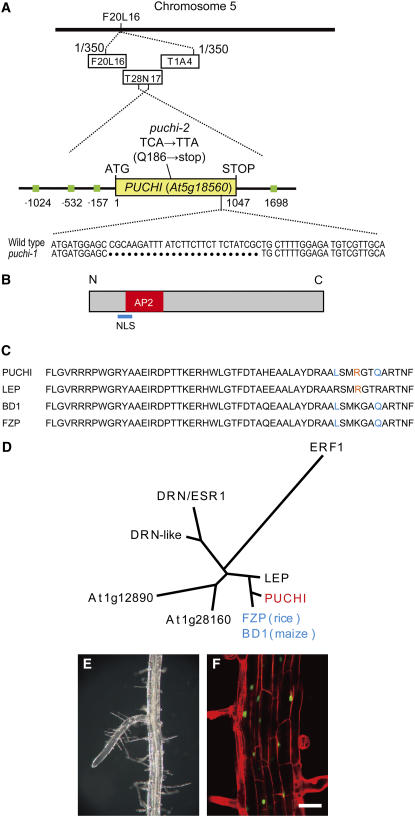
To clone the PUCHI gene, we mapped the mutated locus in puchi-1 based on the expanded LR phenotype. The mutation was located in a region between two polymorphic markers on the BAC clones F20L16 and T1A4 from chromosome 5 (Figure 4A). Among the 26 predicted genes in this region of the puchi-1 mutant, we found a 28-bp deletion that caused a frame shift in the predicted open reading frame (ORF) of the annotated gene At5g18560 (Figure 4A). We then obtained a TILLING line that carried a nonsense mutation in the same gene (line 172F1; Henikoff et al., 2004). Homozygous mutants isolated from this line showed identical phenotypes to those of the puchi-1 mutant both in the shoot and the root. In addition, a crossing experiment showed that the two mutants were allelic (data not shown). We thus designated the mutation in 172F1 as puchi-2 (Figure 4A). Next, we cloned a 6.7-kb genomic fragment that spanned the 3.9-kb upstream and 1.7-kb downstream sequences of the At5g18560 coding region and transformed it into the puchi-1 mutant. Most transgenic plants from the T1 generation showed wild-type phenotypes both in the shoot and the root (five of six; Figure 4E; data not shown). We therefore concluded that At5g18560 was the PUCHI gene.
Figure 4.
Molecular Characterization of PUCHI.
(A) Location of the puchi mutations. The PUCHI locus was mapped between two polymorphic markers located on the F20L16 and T1A4 BACs on chromosome 5. The number of recombination events obtained between each marker and PUCHI among the F2 plants is indicated. The yellow box indicates the predicted ORF, whereas the green boxes denote the AuxREs. The numbers below the ORF indicate the relative nucleotide positions from the start codon (ATG).
(B) The structure of the predicted PUCHI protein. PUCHI has a putative nuclear localization signal (NLS; blue bar) and an AP2 DNA binding domain (red box) in the N-terminal half of the protein.
(C) Alignment of the predicted amino acid sequences of the AP2 domains from PUCHI, LEP, BD1, and FZP. Amino acids conserved among PUCHI, BD1, and FZP but not in LEP are indicated in blue, whereas the amino acid conserved only between PUCHI and LEP is denoted in orange.
(D) A phylogenic tree of PUCHI and related Arabidopsis AP2/EREBP family members (black), maize BD1, and rice FZP (blue) constructed based on the amino acid sequences of the AP2 domains. Among the Arabidopsis AP2/EREBP proteins, PUCHI belongs to the same subgroup as LEP and DRN/ESR1, which are involved in the development of shoot organs.
(E) The LR of a puchi-1 mutant transformed with the 6.7-kb genomic fragment containing PUCHI.
(F) Subcellular localization of the PUCHI-GFP fusion protein expressed under the control of the 35S promoter.
Bar = 50 μm.
The PUCHI gene has a single exon and encodes a protein containing 348 amino acids with a putative nuclear localization signal and an AP2 DNA binding domain in the N-terminal half of the protein (Figure 4B). The PUCHI protein has been classified as a member of the AP2/EREBP family, which are plant-specific transcription factors (Riechmann and Meyerowitz, 1998; Alonso et al., 2003). Phylogenic studies have indicated that PUCHI belongs to the same subgroup as the Arabidopsis LEAFY PETIOLE (LEP), DORNRÖSCHEN/ENHANCER OF SHOOT REGENERATION1 (DRN/ESR1), and DRN-LIKE (DRL/ESR2) proteins, which have been reported to affect shoot development or embryo patterning (van der Graaff et al., 2000; Banno et al., 2001; Alonso et al., 2003; Kirch et al., 2003; Chandler et al., 2007). The closest homolog of PUCHI in Arabidopsis is LEP, which shares 95% amino acid identity in the AP2 domain (Figures 4C and 4D). The sequence of the 3′ region of the PUCHI ORF, however, did not show any significant similarities with any other ORFs of the Arabidopsis genome, suggesting that PUCHI is a unique gene in this species. The AP2 domain of PUCHI is highly homologous with that of the maize (Zea mays) protein BRANCHED SILKLESS1 (BD1; Chuck et al., 2002) and the rice (Oryza sativa) protein FRIZZY PANICLE (FZP; Komatsu et al., 2003; Figures 4C and 4D), both of which affect the inflorescence architecture.
To investigate the subcellular localization of the PUCHI protein, we expressed a PUCHI-GFP fusion protein under the control of the cauliflower mosaic virus 35S promoter. Pro35S:PUCHI-GFP rescued the LR phenotype of the puchi-1 mutant (data not shown), indicating that the fusion protein was functional. A strong fluorescent signal was detected in the nuclei of root epidermal cells, whereas a weaker signal was observed in the cytoplasm (Figure 4F), which was consistent with the proposed function of PUCHI as a transcription factor.
Expression Analysis of PUCHI
We next investigated the pattern of PUCHI expression. In situ hybridizations in root tissue using a PUCHI-specific probe failed to produce a detectable signal (data not shown), suggesting that the expression level of PUCHI is low. We therefore used reporter genes to analyze PUCHI expression. We inserted the coding sequence of GFP into the 6.7-kb genomic fragment so that it was fused in frame to the 5′ end of the PUCHI ORF (genomic GFP-PUCHI). When this construct was introduced into the puchi-1 background, the root phenotype was rescued in all of the T1 plants (n = 21), indicating that the construct was able to drive the expression of the GFP-PUCHI fusion protein in cells that require PUCHI function for normal LR development.
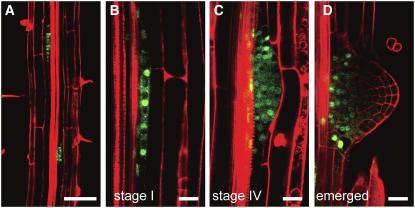
In cells of the rescued transgenic plants, the GFP-PUCHI signal was detected mainly in the nucleus, although a weak signal was observed in the cytoplasm. At a low magnification, GFP-positive cells were observed in the pericycle layer of the primary root (Figure 5A). These signals marked all of the short pericycle cells in LRPs at stage I (Figure 5B) but not the flanking pericycle cells. The GFP signal was detected in all of the LRP cells until the primordia formed the fourth cell layer (Figure 5C). After this stage, the signal was excluded from the tip of each primordium, whereas it remained at the proximal region (Figure 5D). Thus, the expression of the GFP-PUCHI fusion protein driven by a native cis-regulatory region of PUCHI marked the early stages of LRP formation, which was consistent with the function of PUCHI deduced from the mutant phenotype.
Figure 5.
Expression Analysis of GFP-PUCHI in the puchi-1 Mutant.
(A) Expression of genomic GFP-PUCHI in the primary root. Bar = 100 μm.
(B) to (D) Expression of genomic GFP-PUCHI in LRPs at stage I (B), stage IV (C), and stage VI (D). Bars = 20 μm.
PUCHI Acts Downstream of Auxin Signaling
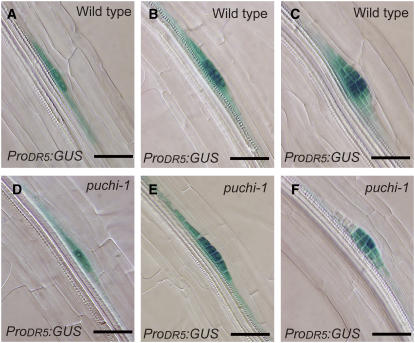
Local auxin gradient is important for LRP initiation (Benková et al., 2003). To investigate whether PUCHI influences the pattern of auxin accumulation in the early LRP, we examined expression of the auxin-responsive DR5 reporter (ProDR5:GUS; Sabatini et al., 1999; Benková et al., 2003). In the wild type, the strongest level of GUS expression was detected at the central region, and the expression level decreased gradually toward the periphery of the LRP (Figures 6A to 6C; Benková et al., 2003). When we observed puchi-1 mutant LRPs, the expression pattern of ProDR5:GUS was essentially the same as that of the wild type at least from stage I to the formation of the fourth cell layer (Figures 6D to 6F), indicating that PUCHI did not affect the pattern of auxin distribution or the primary transcriptional response to auxin in early stages of LRP development.
Figure 6.
Expression Patterns of ProDR5:GUS in the LRP.
(A) to (C) Expression of ProDR5:GUS in wild-type LRPs at stage I (A), stage II (B), and stage IV (C).
(D) to (F) Expression of ProDR5:GUS in puchi LRPs at stages corresponding to (A) to (C), respectively. GUS staining was for 2 h.
Bars = 50 μm.
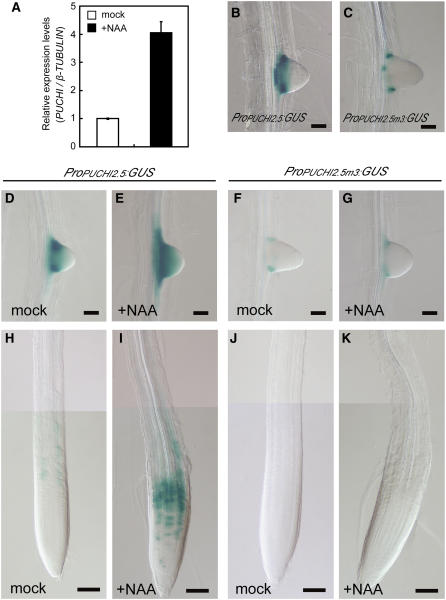
Next, we investigated whether the expression of PUCHI is controlled by auxin. To this end, 7-DAG wild-type seedlings were treated with 1 μM NAA for 90 min, and the level of PUCHI mRNA was analyzed using quantitative RT-PCR analysis. The results showed a clear induction of PUCHI gene expression after a 90-min treatment with NAA (Figure 7A).
Figure 7.
Effects of Auxin on PUCHI Expression.
(A) Results of quantitative RT-PCR analysis showing the expression level of PUCHI in 7-DAG wild-type seedlings incubated in liquid Murashige and Skoog (MS) medium supplemented with (+NAA) or without (mock) 1 μM NAA for 90 min. PUCHI expression is significantly induced by NAA. The levels of PUCHI expression were normalized to β-TUBULIN. Data shown are the average of five biological replicates, with error bars representing se.
(B) and (C) Expression of ProPUCHI2.5:GUS (B) and ProPUCHI2.5m3:GUS (C). GUS staining was performed for 3 h (B) or 72 h (C).
(D) to (K) Effects of auxin treatment. 7-DAG seedlings carrying ProPUCHI2.5:GUS ([D], [E], [H], and [I]) or ProPUCHI2.5m3:GUS ([F], [G], [J], and [K]) were placed onto MS medium containing 5 μM NAA ([E], [G], [I], and [K]) or onto mock MS medium ([D], [F], [H], and [J]) for 12 h. Young LRs are shown in (D) to (G). The distal region of the primary roots is shown in (H) to (K).
Bars = 50 μm in (B) to (G) and 100 μm in (H) to (K).
AuxREs containing the TGTCxC motif have been identified in a number of promoters of primary auxin response genes, and ARF proteins bind to AuxREs to regulate the transcription of these genes (Ulmasov et al., 1999). We identified three AuxREs in the region upstream of the PUCHI ORF (two of them are inverted GxGACA sequences) and another one in the region downstream of the PUCHI ORF (Figure 4A), raising the possibility that PUCHI transcription is regulated by auxin through these AuxREs. To test this possibility, we fused the 2.5-kb promoter region that contains all three upstream AuxREs to the GUS reporter gene (ProPUCHI2.5:GUS). All of the T1 transgenic plants carrying this reporter showed similar GUS activity in the LRPs (eight of eight). In these plants, GUS staining was detected at the periphery of LRPs at stage II (see Supplemental Figure 2A online). Shortly thereafter, the domain of GUS expression formed a ring that marked the proximal region of the LRP (see Supplemental Figures 2B and 2C online; Figure 7B). This expression pattern was similar to that observed in genomic GFP-PUCHI lines, except that the ProPUCHI2.5:GUS lines lacked GUS activity in the central part of the LRP at early stages of development. In addition to the expression in the LRP, ProPUCHI2.5:GUS lines occasionally show weak and patchy expression in a distal region of the primary root (data not shown).
We then introduced single nucleotide substitutions into all three AuxREs simultaneously so that the sequences changed from TGTCxC to TATCxC (ProPUCHI2.5m3:GUS) and transformed this construct into wild-type plants. We failed to detect GUS activity in the LRPs of more than half of the transgenic plants in the T1 generation (7 of 10; data not shown). Among the other three T1 plants, one line showed weak and patchy staining within the expression domain of the reporter gene with the wild-type promoter (cf. Figures 7B and 7C). The two other lines showed staining patterns that were identical to that of the wild-type reporter (data not shown). These results demonstrate that at least one of the three upstream AuxREs is required for the stable activity of the PUCHI promoter. The three transgenic lines with detectable GUS activity did not show altered spatial patterns of staining (Figure 7C), suggesting that the spatial control of PUCHI expression does not require these three AuxREs.
We next examined the effects of exogenous auxin on expression driven by the PUCHI promoter. When 5 μM NAA was applied to ProPUCHI2.5:GUS lines, GUS activity was significantly induced in the distal region of the primary root (Figures 7H and 7I), indicating that the elevated auxin level activated the PUCHI promoter in this region. By contrast, the pattern and intensity of GUS activity in LRPs were not affected by NAA application (Figures 7D and 7E). These results suggest that the activity of the promoter in cells within LRPs was saturated and not responsive to the application of exogenous auxin. We then examined the effects of the AuxREs on the auxin responsiveness of the PUCHI promoter using the ProPUCHI2.5m3:GUS line with weak basal GUS activity. No induction was observed in the distal part of the primary root (Figures 7J and 7K) or in the LRPs (Figures 7F and 7G), indicating that the induction of the PUCHI promoter activity by exogenous auxin requires at least one of the three upstream AuxREs.
DISCUSSION
The PUCHI Gene Encodes a Putative AP2/EREBP Transcription Factor That Controls LR Development
We identified the PUCHI gene as a novel regulator of LRP development in Arabidopsis. The predicted PUCHI protein is a member of the AP2/EREBP family of transcription factors and belongs to a subfamily that includes the Arabidopsis LEP, DRN/ESR1, and DRL/ESR2 proteins, which affect leaf petiole formation, the maintenance of shoot meristem, or embryo patterning (van der Graaff et al., 2000; Kirch et al., 2003; Ikeda et al., 2006; Chandler et al., 2007). No phenotypes associated with LR formation, however, have been reported for these genes. The PUCHI protein is also highly homologous with the maize BD1 protein and the rice FZP protein, which have been shown to function in the establishment of floral meristem identity. Because the puchi mutant developed normal flowers except for the ectopic pin-shaped protrusions, PUCHI seems to have a function that is different from those of BD1 and FZP. This notion is supported by the fact that the abnormalities in the underground portions of bd1 and fzp mutants have not been reported and by the observation that BD1 is not expressed in the root (Chuck et al., 2002). Therefore, our analysis has identified a novel factor that controls LR development.
PUCHI Is Required for Proper Pattern of Cell Divisions in Early Stages of LRP Development
After the first round of anticlinal divisions, pericycle cells that will eventually form the LRP divide periclinally to adapt their growth orientation to the new axis. As LRP development proceeds, cell divisions become restricted to the central part of the primordium, resulting in a dome-shaped structure. The increase in cell number and the width along the radial axis and the frequency of ProCycB1;1:CycB1;1(NT)-GUS in the puchi-1 mutant indicate that PUCHI is required for restricting the zone of cell proliferation in the LRP at early stages.
Although the defect in overall shape of the puchi mutant LRPs was restricted to the peripheral region, excess of anticlinal cell divisions did occur in the central region of the puchi mutant LRP, showing that PUCHI is also required for the proper cell division pattern in the entire region of the early primordium. Consistently, the expression of genomic GFP-PUCHI was initially detected in all of the primordium cells, and this construct was able to fully complement the LR phenotype. These results indicate that PUCHI controls overall morphology of the LRP in combination with other position-specific factors.
Expression of genomic GFP-PUCHI becomes restricted to the proximal region after the primordium have formed the fourth cell layer, indicating that PUCHI function is continuously required for morphogenesis of this region in later stages of LRP formation. On the other hand, the exclusion of genomic GFP-PUCHI expression from the distal region is consistent with the fact that PUCHI is not strictly required for the establishment or maintenance of the LR meristem. Interestingly, the puchi mutant displays a subtle and transient defect in the cellular organization around the QC in the young LR meristem. Whether this phenotype reflects direct involvement of PUCHI in QC organization or a secondary effect of defects in early cell division pattern remains to be determined.
PUCHI Expression Is Controlled by Auxin Signaling
We showed that expression of PUCHI is induced by exogenous auxin. In addition, AuxREs in the upstream promoter sequence are required for PUCHI expression in LRPs as well as for the induction of expression in the distal root region in response to auxin treatment. These results suggest that the expression of PUCHI is regulated by auxin through ARF transcription factors during the early stages of LRP development (Figure 8). Among the known ARF proteins, ARF7 and ARF19 are key regulators of LR initiation, and their activity is negatively regulated by the IAA protein SLR/IAA14 (Fukaki et al., 2005; Okushima et al., 2005). In addition, ectopic expression of a stabilized mutant IAA14 protein in early LRPs results in the formation of disorganized primordia, suggesting that the normal auxin response mediated by Aux/IAA signaling is required for proper patterning of LRP (Fukaki et al., 2005). Microarray analyses have indicated that the induction of PUCHI expression by auxin does not occur in the slr-1 or arf7 arf19 mutant background (Okushima et al., 2005; Vanneste et al., 2005). Although it is not known whether the ARF7 and ARF19 proteins are involved not only in LR initiation but also in subsequent morphogenesis of the LRP, it is possible that PUCHI expression may be directly regulated by these ARF proteins. Alternatively, expression of PUCHI may be regulated by other unknown ARF proteins that are activated by auxin during early LRP development.
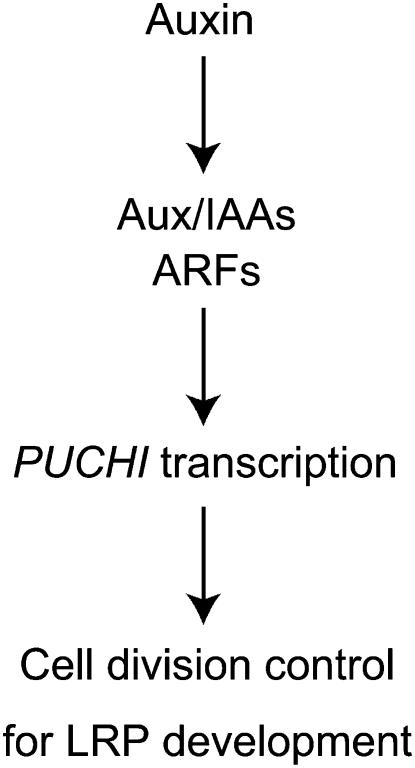
Figure 8.
Model for the PUCHI-Dependent Auxin Signaling in Early LRP Development.
Auxin promotes the transcription of PUCHI, possibly through affecting Aux/IAA and ARF protein functions. PUCHI then controls the cell division for LRP development as a transcriptional regulator.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana accession Col was used as the wild-type strain. The puchi-1 mutant was isolated from a T-DNA insertion line (SALK_046393; Alonso et al., 2003) based on a subtle phenotype in the shoot, namely, the formation of small pin-shaped protrusions at the base of pedicels (see Supplemental Figure 3 online). The mutant name puchi was derived from a Japanese mimetic word describing a small round object or projection. Detailed analysis of the shoot phenotype in puchi is in progress. The T-DNA insertion, which was not linked to the puchi phenotype, was removed before detailed analysis by backcrossing the mutant three times to Col. The puchi-2 mutant (172F1) was obtained from the collection of Arabidopsis mutants obtained using the Targeting Induced Local Lesions in Genomes (TILLING) method (Henikoff et al., 2004). Seeds of the ProDR5:GUS, ProCycB1;1:CycB1;1(NT)-GUS, and ProSHR:GUS lines were kindly provided by T. Guilfoyle (University of Missouri), Peter Doerner (University of Edinburgh), and Philip N. Benfey (Duke University), respectively. The ProSCR:GFP line has been described previously (pspt 3-6; Saito et al., 2005). For analysis of seedling phenotypes, seeds were surface sterilized and sown on the MS plates as described by Fukaki et al. (1996). After incubation for at least 2 d at 4°C in darkness, plates were incubated in a growth chamber at 23°C under constant white light.
Auxin Treatments
Auxin treatment for quantitative RT-PCR analysis was performed by preincubating seedlings in the liquid MS medium for 30 min and then incubating them in the medium supplemented with 0.001% DMSO and 1 μM NAA for 90 min. For auxin treatment of ProPUCHI2.5:GUS and ProPUCHI2.5m3:GUS plants, seedlings were transferred onto MS plates supplemented with 0.05% DMSO and 5 μM NAA and incubated for 12 h. Both treatments were performed at 23°C under constant white light. For mock treatments, medium without NAA but with an equivalent amount of DMSO was used.
Map-Based Cloning of PUCHI
The puchi-1 mutant was crossed to the accession Landsberg erecta, and F2 seedlings with the puchi LR phenotype were examined for recombination events between the mutation and PCR-based polymorphic markers. The genomic sequence of the PUCHI locus was amplified by PCR using ExTaq DNA polymerase (TaKaRa). The resulting PCR products were directly sequenced using a BigDye Terminator v3.1 cycle sequencing kit and an ABI PRISM 3100 sequencer (Applied Biosystems).
Complementation of the puchi Mutant
For complementation analysis, the 6.7-kb PUCHI genomic fragment, including 3.9 kb of upstream sequence and 1.7 kb of downstream sequence, was amplified with PCR from the T28N17 BAC clone, subcloned into pUC19 (gPUCHI6.7), and then inserted into pBIN19AN, a binary vector modified from pBIN19. This construct was transformed into the Agrobacterium tumefaciens strain GV3101 (pMP90) and was then introduced into puchi-1 plants. T1 plants were selected for resistance to kanamycin (30 μg/mL).
Plasmid Construction for Expression Analysis of PUCHI
A full-length cDNA sequence representing PUCHI has been reported by Genoscope (GenBank accession number BX832365). For Pro35S:PUCHI-GFP, the PUCHI ORF deduced from the cDNA sequence was introduced into the pDONR221 vector using the Gateway BP Clonase enzyme mix (Invitrogen) and transferred from the pDONR221 vector to the pGWB5 vector (GWB vectors are kind gifts from Tsuyoshi Nakagawa) using Gateway LR recombination reactions (Invitrogen). The 2.5-kb 5′ sequence, including the region immediately upstream of the start codon of PUCHI, was amplified from the T28N17 BAC using the PCR primers pPUCHI-XbaI_F (5′-GGCTCTAGATTTAGAACTCTATGTAACATCCGG-3′) and pPUCHI-XbaI_R (5′-GGCTCTAGAGATGATGAAGAAATGGTTTTTTTG-3′), digested with XbaI, and subcloned into pBluescript II. The promoter fragment was then inserted upstream of the GUS gene in the binary vector pBI101 to generate ProPUCHI2.5:GUS. The mutated promoter fragment for ProPUCHI2.5m3:GUS was made on a 4.4-kb PUCHI genomic fragment, including 2.5 kb of upstream sequence and 0.9 kb of downstream sequence, which was subcloned into pBluescript II (gPUCHI4.4) using a three-step approach as follows (see Supplemental Figures 4A to 4C online). In Step I, the point mutation was introduced into the AuxRE closest to the start codon in gPUCHI4.4 using the QuikChange protocol (Wang and Malcolm, 1999) and the following mutagenic primer pair for the PCR: pPUCHI156_F (5′-CAAGCTCATTaTCTCTCTATTTATAAC-3′) and pPUCHI156_R (5′-GTTATAAATAGAGAGAtAATGAGCTTG-3′). Next (Step II), a 500-bp PCR fragment that contained the point mutation in the two other AuxREs was amplified from the wild-type 2.5-kb promoter using the following mutagenic primer pair: pPUCHI1020_F (5′-CAAGGTGAtAAAATTGTTCTCATTTC-3′) and pPUCHI528_R (5′-GCAAAATCTGGAATaTCACAGTAACC-3′). Finally (Step III), the triple mutant 2.5-kb promoter was generated using the mutated product from Step I as a template and the 500-bp PCR fragment as a mutagenic mega primer pair according to the method described by Kirsch and Joly (1998). The mutant 2.5-kb fragment was then inserted upstream of the GUS gene in the binary vector pBI101 (ProPUCHI2.5m3:GUS). These constructs were transformed into Col by Agrobacterium-mediated transformation. For genomic GFP-PUCHI, PCR amplification of the 6.7-kb PUCHI genomic fragment, including pUC19, was performed using the QuikChange protocol and the following primer pair: PUCHI N_F (5′-ATGTCAACCTCCAAAACCCTAGACCATAATAAACC-3′) and PUCHI N_R (5′-GATGATGAAGAAATGGTTTTTTTGAAAGGAGGTTTC-3′). A GFP-encoding fragment excised from pUC19_gggGFPgggp using SmaI (Morita et al., 2002) was ligated with this product. The ligated fragment was then inserted into pBIN19AN, and the construct was transformed into the puchi-1 mutant by Agrobacterium-mediated transformation.
RNA Isolation and Quantitative PCR
Total RNA was isolated from plant tissues using the RNeasy kit (Qiagen). First-strand cDNA was synthesized from 2 μg of total RNA with an oligo(dT)24 primer and SuperScriptII reverse transcriptase (Invitrogen). Transcripts were quantified by real-time PCR analyses using 1/60th of the resulting cDNA as template. β-TUBULIN was used as an internal standard (Lorrain et al., 2004). Real-time PCR was performed with the LightCycler system (Roche) with SYBR Premix Ex Taq (TaKaRa). The following primers were used for the amplification of the gene-specific region of each gene: PUCHI LCL (5′-ACGGCTCGTTATCTTCTTCACT-3′) and PUCHI LCR (5′-TGGACTTATTATGTTCTTCGCTTG-3′); and β-TUBULIN_F (5′-GAGGGAGCCATTGACAACATCTT-3′) and β-TUBULIN_R (5′-GCGAACAGTTCACAGCTATGTTCA-3′).
Microscopy
For whole-mount visualization, the seedlings were cleared and mounted according to Malamy and Benfey (1997) and observed under the Eclipse E800 Nomarski microscope (Nikon). For GUS staining, tissues were incubated in 50 mM sodium phosphate, pH 7.2, 0.5 mM ferricyanide, 0.5 mM ferrocyanide, and 2 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid at 37°C. After incubation, samples were cleared and observed under a Nomarski microscope as described above. For detecting the expression of GFP-PUCHI, root samples were counterstained with 50 μg/mL of propidium iodide (Sigma-Aldrich), and fluorescence images were obtained using the FV1000 confocal laser scanning microscope (Olympus). GFP fluorescence was detected with the 490- to 540-nm spectral settings for emission and 488 nm for excitation. The fluorescence of propidium iodide was detected with the 560- to 660-nm spectral settings for emission and 543 nm for excitation. Scanning electron microscopy was preformed as described previously (Aida et al., 1999).
Accession Number
The Genoscope GenBank accession number and Arabidopsis Genome Initiative code for PUCHI are BX832365 and At5g18560, respectively.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The Structure of the LR Meristem in the puchi-1 Mutant.
Supplemental Figure 2. The Expression of ProPUCHI2.5:GUS in Wild-Type LRPs.
Supplemental Figure 3. Flower Phenotypes of the puchi Mutant.
Supplemental Figure 4. A Schematic Diagram of the Construction of ProPUCHI2.5m3:GUS.
Supplementary Material
Acknowledgments
We thank the ABRC for providing mutant seeds and BAC clones. We also thank T. Guilfoyle, P. Doerner, P.N. Benfey, and T. Nakagawa for providing materials and N. Fujihara for technical assistance. This work was partly supported by a Grant-in-Aid for Scientific Research on Priority Areas (14036222) to M.T., a Grant-in-Aid for Young Scientists (12740439) to M.A. and (17770035) to T.K., and a Grant-in-Aid for Scientific Research on Priority Areas (17027019) to H.F.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Masao Tasaka (m-tasaka@bs.naist.jp).
Online version contains Web-only data.
References
- Aida, M., Ishida, T., and Tasaka, M. (1999). Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: Interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126 1563–1570. [DOI] [PubMed] [Google Scholar]
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Banno, H., Ikeda, Y., Niu, Q.W., and Chua, N.H. (2001). Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell 13 2609–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertova, D., Jurgens, G., and Friml, J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602. [DOI] [PubMed] [Google Scholar]
- Blakely, L.M., Blakely, R.M., Colowit, P.M., and Elliott, D.S. (1988). Experimental studies on lateral root formation in radish seedling roots: II. Analysis of the dose–response to exogenous auxin. Plant Physiol. 87 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro, I., Marchant, A., Bhalerao, R.P., Beeckman, T., Dhooge, S., Swarup, R., Graham, N., Inze, D., Sandberg, G., Casero, P.J., and Bennett, M. (2001). Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler, J.W., Cole, M., Flier, A., Grewe, B., and Werr, W. (2007). The AP2 transcription factors DORNRÖSCHEN and DORNRÖSCHEN-LIKE redundantly control Arabidopsis embryo patterning via interaction with PHAVOLUTA. Development 134 1653–1662. [DOI] [PubMed] [Google Scholar]
- Chuck, G., Muszynski, M., Kellogg, E., Hake, S., and Schmidt, R.J. (2002). The control of spikelet meristem identity by the branched silkless1 gene in maize. Science 298 1238–1241. [DOI] [PubMed] [Google Scholar]
- Colón-Carmona, A., You, R., Haimovitch-Gal, T., and Doerner, P. (1999). Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 20 503–508. [DOI] [PubMed] [Google Scholar]
- Di Laurenzio, L., Wysocka-Diller, J., Malamy, J.E., Pysh, L., Helariutta, Y., Freshour, G., Hahn, M.G., Feldmann, K.A., and Benfey, P.N. (1996). The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86 423–433. [DOI] [PubMed] [Google Scholar]
- Dubrovsky, J.G., Rost, T.L., Colon-Carmona, A., and Doerner, P. (2001). Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta 214 30–36. [DOI] [PubMed] [Google Scholar]
- Fukaki, H., Fujisawa, H., and Tasaka, M. (1996). SGR1, SGR2, SGR3: Novel genetic loci involved in shoot gravitropism in Arabidopsis thaliana. Plant Physiol. 110 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki, H., Nakao, Y., Okushima, Y., Theologis, A., and Tasaka, M. (2005). Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J. 44 382–395. [DOI] [PubMed] [Google Scholar]
- Fukaki, H., Okushima, Y., and Tasaka, M. (2007). Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 256 111–137. [DOI] [PubMed] [Google Scholar]
- Fukaki, H., Tameda, S., Masuda, H., and Tasaka, M. (2002). Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 29 153–168. [DOI] [PubMed] [Google Scholar]
- Geldner, N., Richter, S., Vieten, A., Marquardt, S., Torres-Ruiz, R.A., Mayer, U., and Jürgens, G. (2004). Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 131 389–400. [DOI] [PubMed] [Google Scholar]
- Helariutta, Y., Fukaki, H., Wysocka-Diller, J., Nakajima, K., Jung, J., Sena, G., Hauser, M.T., and Benfey, P.N. (2000). The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101 555–567. [DOI] [PubMed] [Google Scholar]
- Henikoff, S., Till, B.J., and Comai, L. (2004). TILLING. Traditional mutagenesis meets functional genomics. Plant Physiol. 135 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen, K., Boucheron, E., Vanneste, S., de Almeida Engler, J., Inze, D., and Beeckman, T. (2002). Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14 2339–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, Y., Banno, H., Niu, Q.W., Howell, S.H., and Chua, N.H. (2006). The ENHANCER OF SHOOT REGENERATION 2 gene in Arabidopsis regulates CUP-SHAPED COTYLEDON 1 at the transcriptional level and controls cotyledon development. Plant Cell Physiol. 47 1443–1456. [DOI] [PubMed] [Google Scholar]
- Kirch, T., Simon, R., Grunewald, M., and Werr, W. (2003). The DORNRÖSCHEN/ENHANCER OF SHOOT REGENERATION1 gene of Arabidopsis acts in the control of meristem cell fate and lateral organ development. Plant Cell 15 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, R.D., and Joly, E. (1998). An improved PCR-mutagenesis strategy for two-site mutagenesis or sequence swapping between related genes. Nucleic Acids Res. 26 1848–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu, M., Chujo, A., Nagato, Y., Shimamoto, K., and Kyozuka, J. (2003). FRIZZY PANICLE is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development 130 3841–3850. [DOI] [PubMed] [Google Scholar]
- Laskowski, M.J., Williams, M.E., Nusbaum, H.C., and Sussex, I.M. (1995). Formation of lateral root meristems is a two-stage process. Development 121 3303–3310. [DOI] [PubMed] [Google Scholar]
- Lorrain, S., Lin, B., Auriac, M.C., Kroj, T., Saindrenan, P., Nicole, M., Balague, C., and Roby, D. (2004). VASCULAR ASSOCIATED DEATH1, a novel GRAM domain-containing protein, is a regulator of cell death and defense responses in vascular tissues. Plant Cell 16 2217–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy, J.E., and Benfey, P.N. (1997). Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124 33–44. [DOI] [PubMed] [Google Scholar]
- Marchant, A., Bhalerao, R., Casimiro, I., Eklof, J., Casero, P.J., Bennett, M., and Sandberg, G. (2002). AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, M.T., Kato, T., Nagafusa, K., Saito, C., Ueda, T., Nakano, A., and Tasaka, M. (2002). Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell 14 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima, Y., Fukaki, H., Onoda, M., Theologis, A., and Tasaka, M. (2007). ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima, Y., et al. (2005). Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: Unique and overlapping functions of ARF7 and ARF19. Plant Cell 17 444–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, R.C., Brady, S.R., and Muday, G.K. (1998). Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 118 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann, J.L., and Meyerowitz, E.M. (1998). The AP2/EREBP family of plant transcription factors. Biol. Chem. 379 633–646. [DOI] [PubMed] [Google Scholar]
- Rogg, L.E., Lasswell, J., and Bartel, B. (2001). A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell 13 465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini, S., Beis, D., Wolkenfelt, H., Murfett, J., Guilfoyle, T., Malamy, J., Benfey, P., Leyser, O., Bechtold, N., Weisbeek, P., and Scheres, B. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99 463–472. [DOI] [PubMed] [Google Scholar]
- Saito, C., Morita, M.T., Kato, T., and Tasaka, M. (2005). Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell 17 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet, I.D., Vanneste, S., Inze, D., and Beeckman, T. (2006). Lateral root initiation or the birth of a new meristem. Plant Mol. Biol. 60 871–887. [DOI] [PubMed] [Google Scholar]
- Tatematsu, K., Kumagai, S., Muto, H., Sato, A., Watahiki, M.K., Harper, R.M., Liscum, E., and Yamamoto, K.T. (2004). MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Q., and Reed, J.W. (1999). Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126 711–721. [DOI] [PubMed] [Google Scholar]
- Ulmasov, T., Hagen, G., and Guilfoyle, T.J. (1999). Activation and repression of transcription by auxin-response factors. Proc. Natl. Acad. Sci. USA 96 5844–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff, E., Dulk-Ras, A.D., Hooykaas, P.J., and Keller, B. (2000). Activation tagging of the LEAFY PETIOLE gene affects leaf petiole development in Arabidopsis thaliana. Development 127 4971–4980. [DOI] [PubMed] [Google Scholar]
- Vanneste, S., et al. (2005). Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17 3035–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., and Malcolm, B.A. (1999). Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. Biotechniques 26 680–682. [DOI] [PubMed] [Google Scholar]
- Wilmoth, J.C., Wang, S., Tiwari, S.B., Joshi, A.D., Hagen, G., Guilfoyle, T.J., Alonso, J.M., Ecker, J.R., and Reed, J.W. (2005). NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 43 118–130. [DOI] [PubMed] [Google Scholar]
- Yang, X., Lee, S., So, J.H., Dharmasiri, S., Dharmasiri, N., Ge, L., Jensen, C., Hangarter, R., Hobbie, L., and Estelle, M. (2004). The IAA1 protein is encoded by AXR5 and is a substrate of SCF (TIR1). Plant J. 40 772–782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.