Abstract
Auxin affects the shape of root systems by influencing elongation and branching. Because multidrug resistance (MDR)-like ABC transporters participate in auxin transport, they may be expected to contribute to root system development. This reverse genetic study of Arabidopsis thaliana roots shows that MDR4-mediated basipetal auxin transport did not affect root elongation or branching. However, impaired acropetal auxin transport due to mutation of the MDR1 gene caused 21% of nascent lateral roots to arrest their growth and the remainder to elongate 50% more slowly than the wild type. Reporter gene analyses indicated a severe auxin deficit in the apex of mdr1 but not mdr4 lateral roots. The mdr1 deficit was explained by 40% less acropetal auxin transport within the mdr1 lateral roots. The slow elongation of mdr1 lateral roots was rescued by auxin and phenocopied in the wild type by an inhibitor of polar auxin transport. Confocal microscopy analysis of a functional green fluorescent protein–MDR1 translational fusion showed the protein to be auxin inducible and present in the tissues responsible for acropetal transport in the primary root. The protein also accumulated in lateral root primordia and later in the tissues responsible for acropetal transport within the lateral root, fully supporting the conclusion that auxin levels established by MDR1-dependent acropetal transport control lateral root growth rate to influence root system architecture.
INTRODUCTION
Lateral root production and growth are regulated to generate an architecture that best suits the prevailing physical and chemical aspects of the soil environment (Malamy, 2005). Lateral roots originate from primordia formed by cell divisions within the pericycle at points adjacent to the protoxylem poles (Dolan et al., 1993; Malamy and Benfey, 1997; Beeckman et al., 2001). Expansion of cells produced by the dome-shaped primordium pushes the nascent organ through the cortex and epidermis until it emerges as a new lateral root. Mature lateral roots anatomically resemble the primary root, with an apical meristem, cortex, and stele, and are themselves capable of producing new lateral roots (Malamy and Benfey, 1997).
The hormone auxin influences root system architecture by promoting the production and elongation of lateral roots and by controlling growth of the primary root. Auxin stimulates lateral root production by activating pericycle cell divisions (Woodward and Bartel, 2005) through a process that depends on the NAC1 transcriptional regulator (Xie et al., 2000; Guo et al., 2005) and the ALF4 nuclear protein (DiDonato et al., 2004). By inducing expression of the SINAT5 ubiquitin ligase, which targets NAC1 for degradation (Guo et al., 2005), auxin also activates a signal attenuation mechanism to achieve a fine level of control (Xie et al., 2002). After their emergence, auxin also promotes the elongation of lateral roots (Muday and Haworth, 1994). This is opposite to the response of primary roots, which are generally inhibited by exogenous auxin. Much genetic evidence supports the connection between auxin and lateral root production. Mutants that overproduce auxin, such as sur1/alf1/rty1 (Boerjan et al., 1995; Celenza et al., 1995; King et al., 1995), sur2 (Delarue et al., 1998), and yucca (Zhao et al., 2001), produce more lateral roots, while reduced-sensitivity mutants, such as axr4, produce fewer lateral roots (Hobbie and Estelle, 1995). The arf7 arf19 double mutant is severely insensitive to auxin, and in almost completely lacking lateral roots (Wilmoth et al., 2005), it resembles the dominant solitary root allele of the IAA14 gene that negatively regulates auxin response factor activity (Fukaki et al., 2002).
The mechanism that distributes auxin throughout the plant body combines with the sensing mechanism to determine the ultimate effect of auxin on development (Friml, 2003; Woodward and Bartel, 2005; Leyser, 2006). After being produced in meristematic and other apical tissues of the plant, auxin is transported from cell to cell by a chemiosmotic mechanism (Goldsmith, 1977). Directionality of the flux through tissue is determined by the subcellular localization of PIN efflux carriers (Muday and DeLong, 2001; Friml, 2003; Teale et al., 2006). For example, in the stele of roots, concentration of PIN1 and possibly other family members in the plasma membrane on the apical ends of cells facilitates the movement of auxin toward the root tip (i.e., acropetally). This polar auxin transport through tissues is typically assayed by measuring the movement of radiolabeled indole-3-acetic acid (IAA) from a spot of application.
Upon reaching the root tip, auxin is believed to move laterally by a PIN3-mediated process before entering the basipetal stream that flows toward the base of the root through the epidermal cells. Auxin produced by the root apical meristem (Ljung et al., 2005) probably also enters the basipetal transport stream. PIN2 plays a role in basipetal transport that is analogous to the role PIN1 plays in acropetal transport. PIN2 protein is concentrated along the basal ends of the epidermal cells to facilitate efflux toward the base (Müller et al., 1998a; Swarup et al., 2005); pin2 mutants display reduced basipetal auxin transport (Chen et al., 1998).
A connection between the polar auxin transport mechanism and lateral root production has been established by pharmacological and genetic studies. Application of the auxin transport inhibitor naphthylphthalamic acid (NPA) to the root-shoot junction inhibits lateral root initiation (Reed et al., 1998). The pin1 mutant, presumably defective in root acropetal transport, initiates lateral root primordia but produces fewer mature lateral roots than the wild type (Benkova et al., 2003). Mutation of the auxin influx carrier AUX1 reduces production of lateral roots, apparently as a result of reduced auxin transport (Marchant et al., 2002). Also, the tir3 mutant, isolated on the basis of its reduced sensitivity to auxin transport inhibitors, has reduced basipetal auxin transport in stems and produces fewer lateral roots (Ruegger et al., 1997). Although impairing auxin transport clearly affects lateral roots (Reed et al., 1998), it is less clear whether basipetal or acropetal transport is more directly coupled to their production and development. One study that investigated the specific contributions of the two streams concluded that tip-produced, basipetally transported IAA is required for the initiation of lateral root primordial, while acropetally transported IAA from the shoot is required for its subsequent growth (Bhalerao et al., 2002). The work presented here addresses the different roles of acropetal and basipetal auxin transport in the root branching process by taking advantage of mutations that differentially affect the two streams.
In the past 5 years, it has been determined that polar auxin transport depends on a group of ABC transporters belonging to the multidrug resistance (MDR)-like family, also known as the P-glycoproteins (PGPs) (Sánchez-Fernández et al., 2001; Martinoia et al., 2002). Knockout mutations in the Arabidopsis thaliana MDR1 gene blocked 80% of basipetal transport in seedling hypocotyls and had a similar effect on transport down the inflorescence stem (Noh et al., 2001). The family member most closely related to MDR1, PGP1, also contributes to basipetal auxin transport in stems (Noh et al., 2001). These MDRs may directly transport IAA, as studies with protoplast preparations, cell suspension cultures, or expression in mammalian cell lines have indicated (Geisler et al., 2005; Bouchard et al., 2006; Petrášek et al., 2006), they may aid in the polar localization of PIN efflux facilitators (Noh et al., 2003), or both.
Gene expression information indicates that MDR1 may play a major role in auxin transport in the root as it does in the shoot. Its promoter is active throughout the root (Noh et al., 2001), and expression profiling indicates that MDR1 is among the most highly expressed family members in most root tissues (Birnbaum et al., 2003). MDR4 may also contribute importantly to auxin transport in the root. It is expressed in the root cap and the epidermal cells of the root apex (Birnbaum et al., 2003; Terasaka et al., 2005). A 30% reduction in root basipetal auxin transport was measured in one allele of mdr4, referred to as pgp4-1 (Terasaka et al., 2005). This work uses mdr1 and mdr4 T-DNA insertion mutants to distinguish between the roles of acropetal and basipetal auxin transport in the branching of the root system.
RESULTS
Localization of MDR1
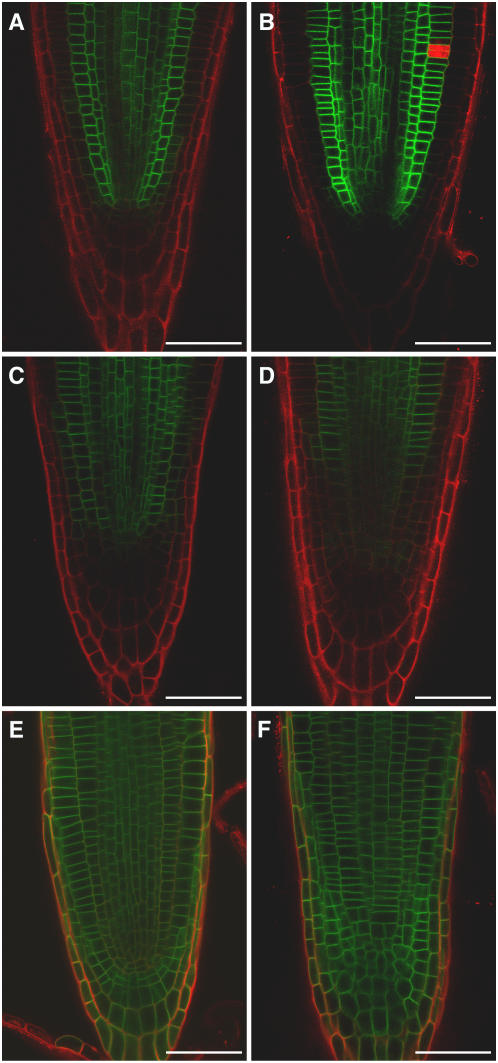
The large (80%) contribution of MDR1 to acropetal (but not basipetal) auxin transport (Lewis et al., 2007) implies that this ABC transporter functions in the central tissues of the root and not the epidermis. However, an expression profiling study that separately analyzed several root tissues at different stages of development indicated that MDR1 is expressed at reasonably high levels throughout the root apex (Birnbaum et al., 2003). Broad expression across the root apex was confirmed in an analysis of plant lines transformed with the green fluorescent protein (GFP) reporter gene under the control of the MDR1 promoter, ProMDR1:GFP (Figure 1A). The finding that the MDR1 promoter is active in all tissues of the root apex may seem at odds with a major role for MDR1 specifically in acropetal auxin transport. The apparent inconsistency was resolved by confocal microscopy analysis of mdr1 mutant plants engineered to express a GFP-MDR1 translational fusion driven by the native MDR1 promoter (ProMDR1:GFP-MDR1). The GFP signal in the root apex of these transgenic lines clearly emanated from the periphery of cells in the stele and cortex but not from epidermal or lateral root cap cells (Figures 1B to 1G). Thus, the MDR1 protein is in the tissues expected of a factor required for normal acropetal auxin transport in roots, and it is absent from tissues that transport auxin basipetally. The absence of MDR1 in the epidermis and lateral root cap, sites of basipetal transport, appears to be achieved by a posttranscriptional mechanism. This localization pattern was typical of the most apical 1 cm of the primary root. More basal regions of the root displayed less GFP-MDR1 signal in the cortex. In the most mature regions of the root, GFP-MDR1 was restricted to the stele (data not shown). At the subcellular level, the GFP signal was localized to the periphery of the expressing cells, consistent with multiple lines of evidence indicating that MDR1 is a plasma membrane protein. Unlike the polarly localized PIN1, MDR1 appears to be evenly distributed along all sides of the cells in the stele (Figure 1F). In cortical cells, the protein appears to be equally present at the apical and basal ends of cells (Figures 1C to 1E). In the cortical cells close to root meristem, the protein distribution is along all sides of the cells (Figure 1E); however, the protein is often less concentrated along the cortical/epidermal cell boundary (Figure 1C) but not always (Figure 1D).
Figure 1.
Confocal Microscopy Analysis of MDR1 Localization in the Primary Root.
(A) Expression of ProMDR1:GFP in the root tip.
(B) GFP-MDR1 localization in the root tip expressing ProMDR1:GFP-MDR1.
(C) and (D) Two examples of GFP-MDR1 in cortical (Co) and endodermal (En) cells ∼16 cortical cells basal to the meristem.
(E) GFP-MDR1 in cortical and endodermal cells approximately four cortical cells basal to the meristem.
(F) Cells in root central cylinder.
(G) GFP-MDR1 localization in a mature region of the primary root. GFP is shown in green and propidium iodide staining in red.
White bars = 50 μm; red bars = 20 μm.
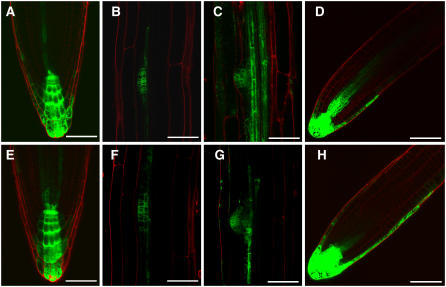
One of the earliest links between MDR proteins and auxin was the finding that MDR1 mRNA was upregulated by IAA (Noh et al., 2001). Figures 2A and 2B show that this effect of IAA translates into an increase in MDR1 protein. The upregulation by IAA occurs in the cells that normally express the gene and not in cells in which its expression is posttranscriptionally suppressed. The subcellular localization pattern was not changed by IAA treatment, but the generally higher levels accentuated the deficit in the periclinal walls of the cortical-epidermal cell boundary. Early work with MDR1 also showed that it could bind the polar auxin transport inhibitor NPA (Murphy et al., 2002). One possible mode of action for NPA would be for it to affect the membrane localization of MDR1. Treatment of roots for 3 h with 100 μM NPA did not affect the level or localization of GFP-MDR1, indicating there is no short-term effect of the blocker on MDR1 levels or localization. However, treatment for 1 d with 1 μM or especially 5 μM NPA resulted in a loss of MDR1 from the most apical portion of the root (Figures 2C and 2D). Thus, effects of long-term NPA treatment on auxin transport could be due in part to reduced levels of MDR1. Changes in auxin levels do not explain the effect of NPA on MDR1 protein levels because in the same area where MDR1 decreases, NPA causes auxin to accumulate (Figure 5K), and auxin induces higher levels of MDR1. The effect of NPA on MDR1 levels is probably not a general effect on membrane proteins because the anonymous plasma membrane marker 29-1-GFP (Cutler et al., 2000) was not affected by NPA (Figures 2E and 2F).
Figure 2.
Effect of IAA and NPA on MDR1 Localization in the Primary Root Tip.
(A) GFP-MDR1 treated as control with 0.05% DMSO.
(B) GFP-MDR1 treated with 5 μM IAA for 5 h.
(C) GFP-MDR1 treated with 1 μM NPA for 1 d.
(D) GFP-MDR1 treated with 5 μM NPA for 1d.
(E) 29-1-GFP plasma membrane marker treated as control with 0.05% DMSO.
(F) 29-1-GFP anonymous marker of the plasma membrane treated with 5 μM NPA.
GFP is shown in green and propidium iodide staining in red. Comparison of GFP signal intensity between panels is valid because all excitation and detection settings were equal. Each example is representative of at least five separate trials. Bars = 50 μm.
Figure 5.
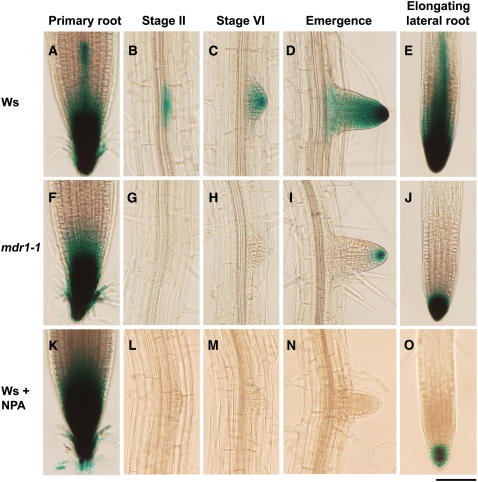
Auxin-Responsive ProDR5:GUS Expression in Primary Roots and during Lateral Root Development as Affected by mdr1 Mutation and NPA.
The 6 h GUS staining pattern in primary root apices of the wild type (A) and mdr1 (F) and in lateral root primordia in the wild type ([B] to [E]) and mdr1 ([G] to [J]). GUS staining pattern in apices of wild-type primary (K) and lateral ([L] to [O]) roots after treatment with 5 μM NPA for 2 d. Photographs are representative of at least 10 independent trials per treatment. Lateral root primordium stages were named according to Malamy and Benfey (1997): stage I ([B], [G], and [L]), stage VI, ([C], [H], and [M]), emergence, ([D], [I], and [N]); and mature lateral root, ([E], [J], and [O]). Bar = 100 μm.
Reduced Branching in the mdr1 Root System
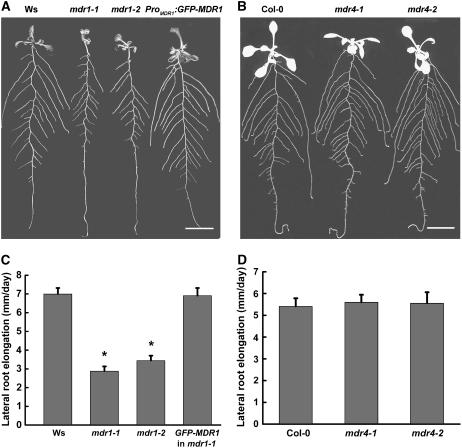
The mdr1 and mdr4 mutants were used to test the connection between each of the two antiparallel auxin transport streams and root branching. Figure 3A shows that branching of the mdr1-1 and mdr1-2 root systems was defective and the defect was completely suppressed by expression of the GFP-MDR1 molecule used for the localization studies in Figures 1 and 2, confirming that the phenotype is due to the loss of MDR1 function. Loss of MDR4 had no detectable effect on lateral root branching at this level of analysis (Figure 3B). Tests on media containing 0, 0.5, 1, or 2% sucrose did not reveal a difference in lateral root production between the wild type and either allele of mdr4 (data not shown). One explanation of the whole-plant-level observations in Figures 3A and 3B is that impaired acropetal but not basipetal auxin transport in the primary root somehow negatively affects root branching.
Figure 3.
Root System Architecture of Wild-Type, mdr1, and mdr4 Seedlings.
(A) The short lateral roots present in both alleles of mdr1 were completely rescued by ProMDR1:GFP-MDR1. Ws, Wassilewskija. Bar = 1 cm.
(B) No effect of mdr4 mutations on root system architecture. Seedlings were grown on half-strength Murashige and Skoog (MS) plates for 6 d and then transferred to a new plate to continue growth for 4 d. Roots were arranged before photographs were taken. Bar = 1 cm.
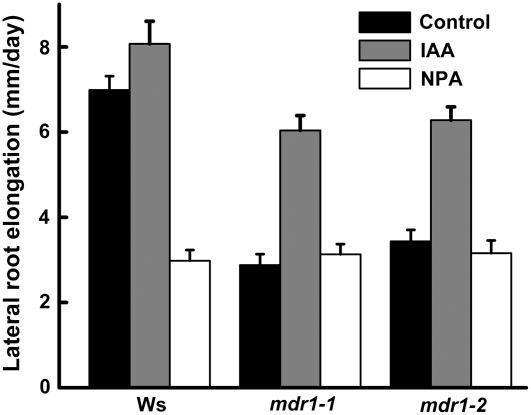
(C) Impaired lateral root growth rate in mdr1 seedlings is restored by ProMDR1:GFP-MDR1.
(D) Growth rate of lateral roots was not affected by mdr4 mutations. Data represent the average growth rates of 15 plants per genotype ± se. Growth rates statistically different (Student's t test, P > 0.05) from the wild type are indicated by asterisks.
Initiation and Growth of Lateral Roots
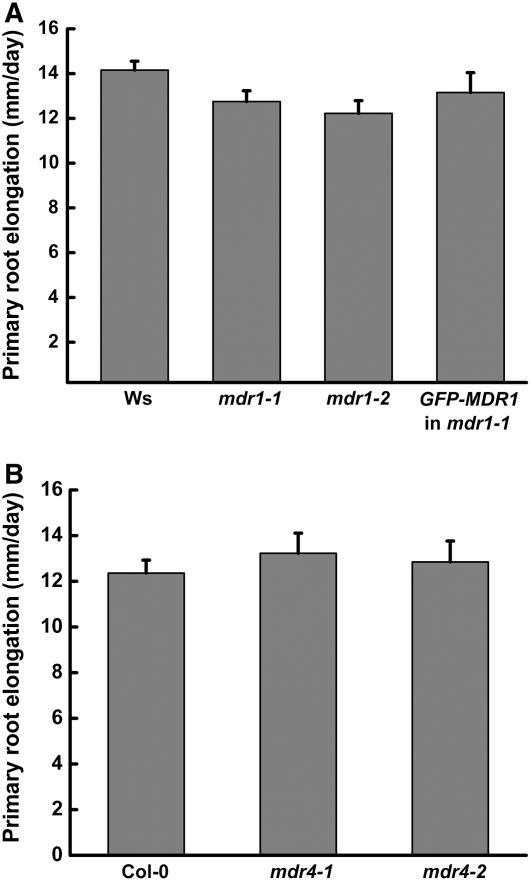
The reduced root branching in mdr1 may have been due to a reduced rate of primordium formation. To investigate this possibility, the observable primordia in 8-d-old seedlings were counted and classified using the methods and nomenclature of Malamy and Benfey (1997). The results in Table 1 show that mdr1 roots contained as many lateral root primordia as the wild type and that these primordia were distributed across the different developmental stages similarly to the wild type. This result implies that the earliest events in lateral root production, such as cell cycle activation within the pericycle, are not affected by a decrease in acropetal auxin transport, and the visible mdr1 phenotype (Figure 3A) is probably due to reduced postemergence growth of the lateral roots. This interpretation was supported by the finding that 21% of lateral roots in mdr1 did not enter an elongation phase after emergence, compared with only 1% that arrested in the wild type. Furthermore, the elongation rate of those mdr1 lateral roots that grew after emerging was ∼50% slower than the wild type (Figure 3C). Again, the slower growth was completely corrected by the ProMDR1:GFP-MDR1 transgene. The mdr1 mutation had no significant effect on the rate of primary root elongation (Figure 4A). Loss of MDR4, which reduced basipetal auxin transport by ∼50%, did not affect the growth of lateral roots at any stage of development or elongation of the primary root (Table 1, Figures 3D and 4B).
Table 1.
Mutations in MDR1and MDR4 Do Not Affect Lateral Root Initiation
| Lateral Root Developmental Stage
|
||||
|---|---|---|---|---|
| Genotype | Stages II to IV | Stages V to VII | Emergence | Total |
| Ws | 10.1 ± 1.9 | 6.1 ± 0.7 | 19.0 ± 2.2 | 35.3 ± 3.4 |
| mdr1-1 | 8.3 ± 1.1 | 6.0 ± 1.0 | 17.5 ± 1.6 | 31.8 ± 2.0 |
| mdr1-2 | 8.4 ± 1.1 | 5.0 ± 1.6 | 21.2 ± 1.8 | 34.6 ± 3.0 |
| ProMDR1:GFP-MDR1 | 8.6 ± 1.2 | 6.0 ± 1.0 | 17.6 ± 3.2 | 32.2 ± 5.0 |
| Col-0 | 8.1 ± 0.4 | 8.1 ± 0.6 | 16.1 ± 1.0 | 32.4 ± 1.4 |
| mdr4-1 | 8.5 ± 0.7 | 8.3 ± 0.6 | 17.5 ± 1.5 | 34.3 ± 1.5 |
| mdr4-2 | 7.9 ± 0.4 | 8.4 ± 0.4 | 17.6 ± 1.3 | 33.9 ± 1.5 |
Seedlings were grown vertically on plates for 6 d under white light and then transferred to new plates. At day 8, the lateral root primordia were visualized with a light microscope, counted, and scored according to stage. Results shown are the mean ± se (n > 7).
Figure 4.
Primary Root Elongation Rate in the Wild Type and mdr Mutants.
(A) Primary roots of wild-type (Ws) and mdr1 seedlings elongated at similar rates.
(B) Primary roots of wild-type (Col-0) and mdr4 seedlings elongated at similar rates.
Values shown are mean ± se of 15 plants per genotype.
Auxin Distribution, Acropetal Transport, and MDR1 Localization in Lateral Roots
Histochemical analysis of the ProDR5:β-glucuronidase (GUS) auxin reporter in wild-type and mdr1 root systems was performed to determine if reduced acropetal auxin transport altered auxin distribution in a way that could explain the impaired lateral root growth. The representative results in Figure 5 show a severe auxin deficit in mdr1 lateral root primordia from early stages through postemergence development. In the primary root apex, the mdr1 mutation resulted only in a small reduction in ProDR5:GUS signal in a region of the stele just basal to the apical maximum (Figures 5A and 5F). Blocking polar auxin transport in wild-type plants by transferring them to media containing NPA produced an auxin deficit in lateral root primordia essentially like that of mdr1 (Figures 5L to 5O). With longer staining times (3 d), 11% of mdr1 lateral root primordia showed some GUS signal, indicating that a very weak auxin gradient persists in mdr1 primordia, but NPA treatment completely eliminated the GUS signal (see Supplemental Figure 1 online).
In the primary root apex, NPA caused an increase in the ProDR5:GUS signal (Figure 5K), as has been observed before (Casimiro et al., 2001; Lin and Wang, 2005). If this NPA-induced increase is due to blocked efflux of auxin synthesized at the root apex, then the opposite response of lateral root apices may be evidence that they produce less auxin than the primary root apex. An mdr4-1 line transformed with ProDR5:GFP was constructed for use in the Lewis et al. (2007) studies on gravitropism. It was used here to examine the influence of MDR4 on auxin distribution in developing lateral roots. Consistent with the lack of effect of the mdr4 mutations on lateral root growth and development, auxin distribution appeared normal in these mutants (Figure 6).
Figure 6.
Auxin-Responsive ProDR5:GFP Expression in Primary Root Tips and Developing Lateral Roots of the Wild Type and the mdr4-1 Mutant.
ProDR5:GFP localization pattern in primary root apices of the wild type (A) and mdr4-1 (E) and in lateral root primordia in the wild type ([B] to [D]) and mdr4-1 ([F] to [H]). Bars = 50 μm.
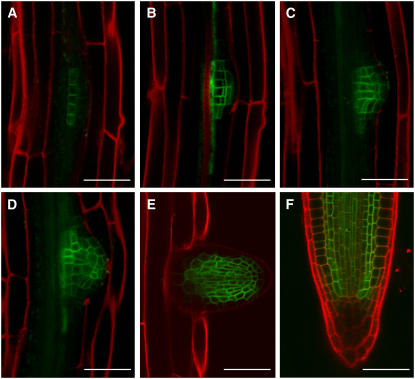
Confocal analysis of the functional GFP-MDR1 molecule showed it distinctly accumulated in pericycle derivatives beginning at an early stage of primordium development, and later, as in the primary root, it was restricted to the central cylinder and cortical cells in which acropetal auxin transport occurs (Figure 7). The GFP-MDR1 localization and ProDR5:GUS patterns indicate that MDR1 is required for acropetal auxin transport within the lateral root. The results of a 3H-IAA–based transport assay supported this hypothesis (Lewis et al., 2007). As shown in Figure 8, acropetal transport within the lateral root was reduced by ∼60% in mdr1 mutants, and, like the effect of the mutations on growth rate, the transport defect was rescued by the GFP-MDR1 molecule.
Figure 7.
Localization of MDR1 During Lateral Root Development.
Stage I (A), stage IV (B), stage VI (C), stage VII (D), emerged (E), and mature lateral root tip (F). GFP is in green, and propidium iodide counterstaining is in red. Bars = 50 μm.
Figure 8.
Acropetal Auxin Transport in Lateral Roots.
3H-IAA was applied at the base of the lateral root and radioactivity was later quantified in an apical portion of the lateral root. Values are mean ± se from three independent trials with 10 lateral roots per genotype per trial. Values that are statistically different (Student's t test, P > 0.05) from the wild type are indicated by asterisks.
Control of Lateral Root Growth Rate by Auxin Transport within the Lateral Root
The following experiments addressed the relationships between MDR1-controlled auxin levels in the lateral root and the control of lateral root elongation. Exogenous IAA (0.05 μM) approximately doubled mdr1 lateral root growth rate, almost completely restoring it to wild-type levels (Figure 9). NPA, which phenocopied the ProDR5:GUS pattern in mdr1, reduced the growth rate of wild-type lateral roots to the same level as mdr1. NPA did not further reduce mdr1 lateral root growth rate (Figure 9). These data indicate that an auxin deficit arising from impaired acropetal transport within the lateral root limited the elongation rate of mdr1 lateral roots.
Figure 9.
The mdr1 Lateral Root Elongation Phenotype Is Partially Rescued by IAA and Phenocopied in the Wild Type by NPA.
Seedlings were transferred to plates supplemented with 0.05 μM IAA or 1 μM NPA 6 d after germination and then lateral root elongation between days 8 and 10 was measured to determine growth rate. Values are mean ± se from three independent trials with five plants per genotype per trial.
DISCUSSION
By responding to signals such as water and mineral availability, lateral root development adapts the root system to the prevailing soil environment. Studies employing auxin application, pharmacological inhibitors, or removal of auxin sources by surgery or mutation have shown key roles for auxin and its distribution mechanisms in lateral root development (Reed et al., 1998; Casimiro et al., 2001; Bhalerao et al., 2002). This study is unique in using mutations that selectively impaired basipetal or acropetal transport as tools to examine the impact each auxin stream has on root growth and development. The mdr1 and mdr4 mutations are particularly well suited to this sort of genetic dissection of the polar auxin transport streams because they do not display major pleiotropic effects, such as altered anatomy, distorted growth, or insensitivity to auxin or gravity. A number of insights into the connection between auxin transport and root system development emerge from study of these two ABC transporter mutants. The auxin deficit that significantly impaired mdr1 lateral root elongation did not detectably affect early primordium development at the resolution level of the light microscope. While this result is surprising given the compelling evidence that primordium initiation is strongly auxin dependent, Casimiro et al. (2001) also found no significant difference in lateral root number when they used the stm1 mutation to reduce shoot-derived auxin transported to the root. It may be that the lower auxin levels in mdr1 are sufficient for primordia production but suboptimal for postemergence elongation. The existence of two phases in lateral root development, one dependent on MDR1 (postemergence elongation) and one not (early primordium development), may coincide with the distinction Laskowski et al. (1995) made between the early stages of primordium formation and the later phase in which the nascent lateral formed its own apical meristem proper.
MDR1 protein accumulated in the lateral root primordium (Figure 7) consistent with a genomics study that found its gene to be upregulated within hours of primordium formation (Vanneste et al., 2005). MDR1 and its closest homolog, PGP1, are the only two (of 22) in this family to be associated with lateral root initiation by induction during primordium formation. Also, treatment with high nitrate or the transient overexpression of the ANR1 transcription factor, either of which increases lateral root growth rate, was shown to increase MDR1 expression according to GENEVESTIGATOR (https://www.genevestigator.ethz.ch/; Zimmermann et al., 2004). The absence of MDR4 mRNA in the primordia-enriched samples profiled by Vanneste et al. (2005) is consistent with the lack of lateral root phenotype found in this study of mdr4 mutants (Figure 3, Table 1). A previous study of mdr4 (pgp4) reported ∼15% more lateral roots in the mutant when grown in half-strength MS medium containing 2% sucrose (Santelia et al., 2005). The mdr4 lines used in this study when grown under conditions described by Santelia et al. (2005) also displayed a small increase in lateral root number, but the difference was not statistically significant. This aspect of these two studies can be considered in agreement.
There are two previously published studies with which these results do not fully agree. First, reduced primary root growth rate in mdr4 reported by Terasaka et al. (2005) was not observed (Figure 4B) regardless of sucrose concentration (data not shown), even when roots were studied with high-resolution computer vision techniques (Lewis et al., 2007). Santelia et al. (2005) also reported no difference in primary root growth of mdr4 seedlings. More importantly, the reduced number of lateral roots in mdr4 plants reported by Terasaka et al. (2005) is inconsistent with the detailed studies of two independent mdr4 alleles presented here (Table 1, Figure 3) and the 15% increase in lateral roots reported by Santelia et al. (2005). The other point of disagreement between these results and previous publications concerns the localization of MDR1 at the tissue and cellular levels. Figures 1, 2, and 7 show that a functional GFP-MDR1 fusion protein driven by its native promoter localizes to the cortex, endodermis, and central cylinder of roots and is absent from the epidermis and lateral root cap. This expression pattern is wholly consistent with the demonstrated role in acropetal auxin transport and lack thereof in basipetal transport (Lewis et al., 2007). However, a recent immunolocalization study showed MDR1 uniformly present in all root tissues, including the epidermis (Blakeslee et al., 2007). The discrepancy may be due to insufficient specificity of the polyclonal antibody used by Blakeslee et al. (2007), which was raised against a portion of the protein (amino acids 334 to 620) that includes the highly conserved ATP binding cassette and ABC transporter signature motif characteristic of the ABC superfamily (Sánchez-Fernández et al., 2001). The acropetal auxin transport function ascribed to MDR1 by Lewis et al. (2007) and in Figure 8 is more consistent with the localizations shown in Figures 1, 2, and 7 than the whole-mount immunolocalizations reported by Blakeslee et al. (2007).
The MDR1-dependent transport process that concentrates auxin in early lateral root primordia (Figure 5) and which later sustains lateral root growth may acquire directionality from PIN proteins. PIN1 is localized asymmetrically in a manner consistent with it directing auxin toward the primordium apex, and loss of multiple PIN family members impaired primordium formation (Benkova et al., 2003). MDR1 is mostly symmetrically distributed around the cell surface (Figures 1, 2, and 7) but is also clearly required for polar auxin transport and lateral root growth. Perhaps MDR1 creates a physiological condition in the lateral root primordium that permits PIN-directed auxin transport to proceed efficiently. Because MDR1 mRNA (Noh et al., 2001) and protein (Figure 2) increase in response to auxin, any permissiveness it engenders may feed forward to create more. The more auxin is focused into the developing primordium or expanding lateral root, the more MDR1 is produced, enhancing PIN-directed efflux toward the growing zone and apex. This hypothetical MDR1-engendered permissiveness could involve cellular ionic relations, as PIN-mediated auxin efflux should require a charge-compensating ionic current across the plasma membrane, and mammalian MDR1 may affect ion fluxes (Valverde et al., 1992; Hoffman and Roepe, 1997; Howard and Roepe, 2003).
Because loss of MDR1 function impairs auxin transport down both the shoot (Noh et al., 2001) and root (Lewis et al., 2007), considerably less shoot-derived auxin may be expected in mdr1 roots. However, the auxin maximum at the tip of mdr1 roots as visualized by ProDR5:GUS was very similar to the wild type (Figures 5A and 5F) consistent with a previous study (Lin and Wang, 2005). Apparently, a major reduction in transport toward the root tip does not affect the steady state level of auxin in the area of the concentration maximum. Perhaps de novo synthesis (Müller et al., 1998b; Ljung et al., 2005) and recirculation of auxin within the primary root apex (Blilou et al., 2005) generate a stable apical auxin maximum that provides sufficient auxin for cell expansion in the elongation zones without a substantial contribution from the shoot via MDR1-mediated acropetal transport. That scenario would explain the lack of an mdr1 effect on primary root growth. It may also explain why NPA does not reduce the ProDR5:GUS signal at the primary root apex (Figure 5K).
Auxin levels in the lateral root, by contrast, tell a very different story. Beginning with the primordium, the ProDR5:GUS signal was very much reduced in mdr1 compared with the wild type (Figures 5A to 5J). Ljung et al. (2005) found that lateral root apices eventually develop the capacity to synthesize auxin, but apparently the apical auxin maximum in the lateral roots of these young seedlings results largely from MDR1-dependent acropetal transport. The low amount of auxin in mdr1 primordia is apparently sufficient to sustain their development (Table 1), which is known to be auxin dependent (Benkova et al., 2003), whereas the greater deficit caused by NPA treatment (see Supplemental Figure 1 online) is apparently not (Casimiro et al., 2001). Once the lateral root emerges, however, loss of MDR1 or NPA treatment slows or even stops lateral root elongation. In apparent contrast with primary roots, auxin status and growth control in lateral roots depends on MDR1-mediated acropetal transport.
It is surprising that development of the primary root was not more severely affected by major impairments of acropetal or basipetal transport and that the mdr1 mdr4 double mutant was not more severely affected than either single mutant. Pharmacological agents, such as NPA, can block acropetal transport to a similar extent as the mdr1 mutations, yet they cause major deformations and inhibitions of root development (Ruegger et al., 1997; Reed et al., 1998) not seen in mdr1, mdr4, or mdr1 mdr4 double mutants. The major disruptions to growth and development caused by NPA and related compounds may reflect the compounding effects of simultaneously blocking the lateral pathways and reflux loops in addition to the major axial pathways. Perhaps other MDR family members not affected by mdr1 and mdr4 mutations mediate the additional fluxes NPA disrupts.
This study used mutations that selectively disrupt acropetal and basipetal auxin transport to demonstrate that MDR1-mediated concentration of auxin in the primordium is not as important to lateral root growth as is subsequent MDR1-mediated acropetal transport of auxin within the emerged lateral root (Figure 10). The results also demonstrate that basipetal transport can be substantially reduced without detectably affecting root system architecture, consistent with normal lateral root development in pin2 mutants (Benkova et al., 2003). These insights into auxin flow and root development were made possible by the discrete effects of two different mdr mutants. Auxin transport mediated by MDR-like transporters probably controls many aspects of root growth and development and responses to environment in addition to those examined here and by Lewis et al. (2007). Further study of mdr1, mdr4, and possibly other MDR family members will be useful in dissecting the specific roles of acropetal and basipetal auxin transport in plant growth and development.
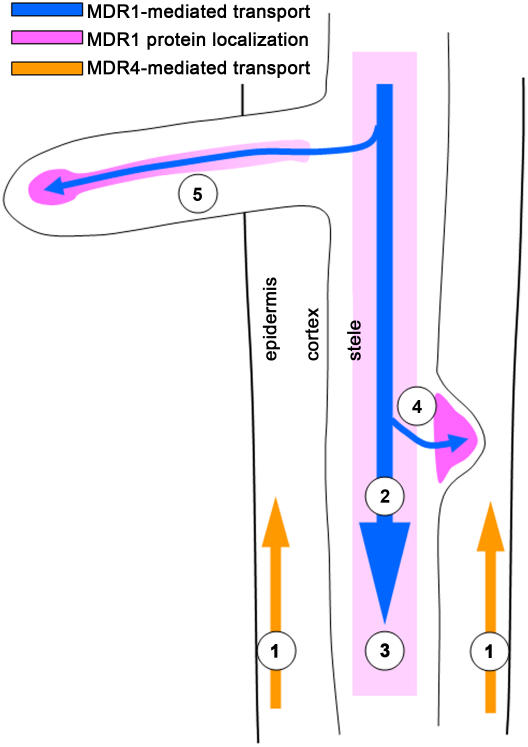
Figure 10.
Model Summarizing Key Conclusions about Polar Auxin Transport Streams and Lateral Root Growth.
Basipetal auxin transport mediated by MDR4 (1) does not play a detectable role in lateral root growth and development. In the mature region of the root where lateral roots are produced, MDR1 expression is restricted to the stele (2) where it mediates acropetal auxin transport (3). MDR1 expression in the early primordium (4) is responsible for concentrating auxin there. However, even in the absence of the MDR1-mediated auxin accumulation, lateral root primordia progress through the early stages of development apparently normally. MDR1-mediated acropetal auxin transport within the lateral root (5) is necessary for normal elongation of the lateral root and also for producing the local auxin maximum at the apex of the lateral root.
METHODS
Plant Material and Growth Conditions
Seeds were surface-sterilized and plated on half-strength MS agar plates (2.15 g/L MS salts [Sigma-Aldrich], and 0.8% bacto-agar, pH 5.8) containing 1% sucrose or 0.5% sucrose in the case of plants used for auxin transport assays. The plates were maintained in darkness at 4°C for 2 to 3 d and then oriented vertically under 80 to 100 μmol m−2 s−1 constant white light at 22°C.
Histochemical Staining of ProDR5:GUS Activity
The ProDR5:GUS reporter gene was introduced into the mdr1-1 mutant by genetic crossing, and doubly homozygous individuals were isolated from the F3 generation. Roots from 8-d-old seedlings were stained for GUS activity in 1 mL of a solution containing 50 mM phosphate buffer, 0.5% Triton X-100, 500 μM K3Fe(CN)6, and 1 μM X-Glu (Gold BioTechnology) at 37°C. In the case of NPA treatment, 6-d-old plants were transferred to agar plates containing the indicated concentration and maintained in this condition for 2 d before staining commenced. All samples were observed using a ×20 UPlanFl objective on an Olympus BX60 microscope equipped with an Olympus DP70 camera.
Radioactive Auxin Transport Assays
The measurements of acropetal auxin transport in the lateral roots were made on 12-d-old vertically grown seedlings with >20-mm-long lateral roots. Blocks containing 5 μL of 3 μM 3H-IAA in 1% agar were placed at the base of the lateral root. After 3 h, eight lateral roots were collected by cutting 10 mm from the base of each and placing it into 2.5 mL of scintillation fluid, and the amount of radioactivity within each sample was determined as described above.
Determination of Primary Root and Lateral Root Elongation Rate
To quantify primary root growth rate, images were obtained with a flatbed scanner at days 6 and 7 and the difference in length determined. Growth rates of the 4th, 5th, and 6th emerged lateral roots, provided they were at least 5 mm long, were determined from the increases in length between days 8 and 10. In studies of the effects of treatments on the growth rate of lateral roots, 6-d-old seedlings were transferred to agar plates containing the indicated concentration of NPA (Chem Service) or IAA (Sigma-Aldrich) and then their elongation between days 8 and 10 was determined.
Transgene Constructs
To generate the chimeric fusion construct GFP-MDR1, the complete MDR1 coding sequence was amplified by PCR from the Arabidopsis thaliana MDR1 cDNA clone (Noh et al., 2001) using two oligonucleotide primers, 5′-GCGGATCCATGTCGGAAACTAACA-3′ and 5′-GCGGATCCATCGTTATAGTCCATAGAAATC-3′. The underlined regions denote BamHI sites that were introduced to both primers to facilitate the cloning of amplified DNA. After digestion with BamHI, the fragment was fused in frame to the C-terminal end of eGFP in pEGAD vector. To replace the 35S promoter, a 4.3-kb fragment of 5′ untranslated region containing the native promoter of MDR1 was amplified by PCR using primers 5′-GTCCGATCGATTGTTAAGAGATAGACGAGATAGCTG-3′ and 5′-GCGACCGGTGGTTTTTTGTAGATCCGAGTTAAAGAAAG-3′ (PvuI and AgeI sites are underlined, respectively) and digested. The 35S promoter in the resulting construct was removed by PacI and AgeI and replaced by the PCR fragment through ligation. The resulting vector was introduced into Agrobacterium tumefaciens GV3101 and used to transform mutant mdr1-1 using the floral dip method (Clough and Bent, 1998). To generate an MDR1 promoter-driven GFP construct (ProMDR1:GFP), the MDR1 cDNA was removed using BamHI and the resulting construct was religated. The vector was introduced through the same way into wild-type Ws plants. Plants carrying the transgene were isolated through basta selection. The homozygous lines for the transgene were obtained in the T3 generation.
Confocal Fluorescence Microscopy Analysis
Confocal microscopy was performed on a Zeiss LSM 510 laser scanning confocal microscope equipped with a Meta detector. Propidium iodide was used as a cell wall stain to delineate the root structure. Optics employed were a C-Apochromat ×40 water immersion lens or a plan-Apochromat ×63 oil immersion lens. The sample was excited with the 488-nm laser line from a 30-mW argon gas laser. The fluorescence was captured in 10-nm bandwidths and then linear unmixing was performed to isolate the GFP signal from the stained background. Pixels that were determined to be GFP and propidium iodide were false-colored green and red, respectively.
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are At3g28860 (MDR1) and At2g47000 (MDR4).
Supplemental Data
The following material is available in the online version of this article.
Supplemental Figure 1. Auxin-Responsive ProDR5:GUS Expression in Lateral Root Primordia of mdr1 and NPA-Treated Wild-Type Seedlings.
Supplementary Material
Acknowledgments
We thank the Salk Institute and ABRC for providing mdr4-1 and mdr4-2 alleles. This work was supported by National Science Foundation Grant IOB-0517350 and DBI-0421266 to E.P.S.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Edgar P. Spalding (spalding@wisc.edu).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Beeckman, T., Burssens, S., and Inzé, D. (2001). The peri-cell-cycle in Arabidopsis. J. Exp. Bot. 52 403–411. [DOI] [PubMed] [Google Scholar]
- Benkova, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertova, D., Jürgens, G., and Friml, J. (2003). Local, eflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602. [DOI] [PubMed] [Google Scholar]
- Bhalerao, R.P., Eklof, J., Ljung, K., Marchant, A., Bennett, M., and Sandberg, G. (2002). Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 29 325–332. [DOI] [PubMed] [Google Scholar]
- Birnbaum, K., Shasha, D.E., Wang, J.Y., Jung, J.W., Lambert, G.M., Galbraith, D.W., and Benfey, P.N. (2003). A gene expression map of the Arabidopsis root. Science 302 1956–1960. [DOI] [PubMed] [Google Scholar]
- Blakeslee, J.J., et al. (2007). Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou, I., Xu, J., Wildwater, M., Willemsen, V., Paponov, I., Friml, J., Heidstra, R., Aida, M., Palme, K., and Scheres, B. (2005). The PIN auxin efflux facilitator network controls growth patterning in Arabidopsis roots. Nature 433 39–44. [DOI] [PubMed] [Google Scholar]
- Boerjan, W., Cervera, M.T., Delarue, M., Beeckman, T., Dewitte, W., Bellini, C., Caboche, M., Vanonckelen, H., Vanmontagu, M., and Inzé, D. (1995). Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7 1405–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard, R., Bailly, A., Blakeslee, J.J., Oehring, S.C., Vincenzetti, V., Lee, O.R., Paponov, I., Palme, K., Mancuso, S., Murphy, A.S., Schulz, B., and Geisler, M. (2006). Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P-glycoproteins. J. Biol. Chem. 281 30603–30612. [DOI] [PubMed] [Google Scholar]
- Casimiro, I., Marchant, A., Bhalerao, R.P., Beeckman, T., Dhooge, S., Swarup, R., Graham, N., Inzé, D., Sandberg, G., Casero, P.J., and Bennet, M. (2001). Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza, J.L., Grisafi, P.L., and Fink, G.R. (1995). A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 9 2131–2142. [DOI] [PubMed] [Google Scholar]
- Chen, R., Hilson, P., Sedbrook, J., Rosen, E., Caspar, T., and Masson, P.H. (1998). The Arabidopsis thaliana AGRAVITROPICA 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc. Natl. Acad. Sci. USA 95 15112–15117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Cutler, S.R., Ehrhardt, D.W., Griffitts, J.S., and Somerville, C.R. (2000). Random GFP∷cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA 97 3718–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue, M., Prinsen, E., Van Onckelen, H., Caboche, M., and Bellini, C. (1998). Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J. 14 603–611. [DOI] [PubMed] [Google Scholar]
- DiDonato, R.J., Arbuckle, E., Buker, S., Sheets, J., Tobar, J., Totong, R., Grisafi, P., Fink, G.R., and Celenza, J.L. (2004). Arabidopsis ALF4 encodes a nuclear-localized protein required for lateral root formation. Plant J. 37 340–353. [DOI] [PubMed] [Google Scholar]
- Dolan, L., Janmaat, K., Willemsen, V., Linstead, P., Poethig, S., Roberts, K., and Scheres, B. (1993). Cellular organisation of the Arabidopsis thaliana root. Development 119 71–84. [DOI] [PubMed] [Google Scholar]
- Friml, J. (2003). Auxin transport - Shaping the plant. Curr. Opin. Plant Biol. 6 7–12. [DOI] [PubMed] [Google Scholar]
- Fukaki, H., Tameda, S., Masuda, H., and Tasaka, M. (2002). Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 29 153–168. [DOI] [PubMed] [Google Scholar]
- Geisler, M., et al. (2005). Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 44 179–194. [DOI] [PubMed] [Google Scholar]
- Goldsmith, M.H.M. (1977). The polar transport of auxin. Annu. Rev. Plant Physiol. 28 439–478. [Google Scholar]
- Guo, H.S., Xie, Q., Fei, J.F., and Chua, N.H. (2005). MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell 17 1376–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie, L., and Estelle, M. (1995). The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 7 211–220. [DOI] [PubMed] [Google Scholar]
- Hoffman, M.M., and Roepe, P.D. (1997). Analysis of ion transport perturbations caused by hu MDR 1 protein overexpression. Biochemistry 36 11153–11168. [DOI] [PubMed] [Google Scholar]
- Howard, E.M., and Roepe, P.D. (2003). Purified human MDR 1 modulates membrane potential in reconstituted proteoliposomes. Biochemistry 42 3544–3555. [DOI] [PubMed] [Google Scholar]
- Laskowski, M.J., Williams, M.E., Nusbaum, H.C., and Sussex, I.M. (1995). Formation of lateral root meristems is a two-stage process. Development 121 3303–3310. [DOI] [PubMed] [Google Scholar]
- Lewis, D.R., Miller, N.D., Splitt, B.L., Wu, G., and Spalding, E.P. (2007). Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis Multidrug Resistance-Like ABC transporter genes. Plant Cell 19 1838–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser, O. (2006). Dynamic integration of auxin transport and signalling. Curr. Biol. 16 R424–R433. [DOI] [PubMed] [Google Scholar]
- Lin, R., and Wang, H. (2005). Two homologous ATP-binding cassette transporter proteins, AtMDR1 and AtPGP1, regulate Arabidopsis photomorphogenesis and root development by mediating polar auxin transport. Plant Physiol. 138 949–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung, K., Hull, A.K., Celenza, J., Yamada, M., Estelle, M., Normanly, J., and Sandberg, G. (2005). Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17 1090–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, J.J., Stimart, D.P., Fisher, R.H., and Bleecker, A.B. (1995). A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell 7 2023–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy, J.E. (2005). Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 28 67–77. [DOI] [PubMed] [Google Scholar]
- Malamy, J.E., and Benfey, P.N. (1997). Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124 33–44. [DOI] [PubMed] [Google Scholar]
- Marchant, A., Bhalerao, R., Casimiro, I., Eklof, J., Casero, P.J., Bennett, M., and Sandberg, G. (2002). AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia, E., Klein, M., Geisler, M., Bovet, L., Forestier, C., Kolukisaoglu, U., Muller-Rober, B., and Schulz, B. (2002). Multifunctionality of plant ABC transporters–More than just detoxifiers. Planta 214 345–355. [DOI] [PubMed] [Google Scholar]
- Muday, G.K., and DeLong, A. (2001). Polar auxin transport: Controlling where and how much. Trends Plant Sci. 6 535–542. [DOI] [PubMed] [Google Scholar]
- Muday, G.K., and Haworth, P. (1994). Tomato root-growth, gravitropism, and lateral development - Correlation with auxin transport. Plant Physiol. Biochem. 32 193–203. [PubMed] [Google Scholar]
- Müller, A., Guan, C.H., Gälweiler, L., Tanzler, P., Huijser, P., Marchant, A., Parry, G., Bennett, M., Wisman, E., and Palme, K. (1998. a). AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 17 6903–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, A., Hillebrand, H., and Weiler, E.W. (1998. b). Indole-3-acetic acid is synthesized from L-tryptophan in roots of Arabidopsis thaliana. Planta 206 362–369. [DOI] [PubMed] [Google Scholar]
- Murphy, A.S., Hoogner, K.R., Peer, W.A., and Taiz, L. (2002). Identification, purification, and molecular cloning of N-1-naphthylphthalamic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiol. 128 935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh, B., Bandyopadhyay, A., Peer, W.A., Spalding, E.P., and Murphy, A.S. (2003). Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 424 999–1002. [DOI] [PubMed] [Google Scholar]
- Noh, B., Murphy, A.S., and Spalding, E.P. (2001). Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13 2441–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášek, J., et al. (2006). PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312 914–918. [DOI] [PubMed] [Google Scholar]
- Reed, R.C., Brady, S.R., and Muday, G.K. (1998). Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 118 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger, M., Dewey, E., Hobbie, L., Brown, D., Bernasconi, P., Turner, J., Muday, G., and Estelle, M. (1997). Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Fernández, R., Davies, T.G.E., Coleman, J.O.D., and Rea, P.A. (2001). The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J. Biol. Chem. 276 30231–30244. [DOI] [PubMed] [Google Scholar]
- Santelia, D., Vincenzetti, V., Azzarello, E., Bovet, L., Fukao, Y., Duchtig, P., Mancuso, S., Martinoia, E., and Geisler, M. (2005). MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett. 579 5399–5406. [DOI] [PubMed] [Google Scholar]
- Swarup, R., Kramer, E.M., Perry, P., Knox, K., Leyser, H.M.O., Haseloff, J., Beemster, G.T.S., Bhalerao, R., and Bennett, M.J. (2005). Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat. Cell Biol. 7 1057–1065. [DOI] [PubMed] [Google Scholar]
- Teale, W.D., Paponov, I.A., and Palme, K. (2006). Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 7 847–859. [DOI] [PubMed] [Google Scholar]
- Terasaka, K., Blakeslee, J.J., Titapiwatanakun, B., Peer, W.A., Bandyopadhyay, A., Makam, S.N., Lee, O.R., Richards, E.L., Murphy, A.S., Sato, F., and Yazaki, K. (2005). PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 17 2922–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde, M.A., Díaz, M., Sepúlveda, F.V., Gill, D.R., Hyde, S.C., and Higgins, C.F. (1992). Volume-regulated chloride channels associated with the human multi-drug resistance P-glycoprotein. Nature 355 830–833. [DOI] [PubMed] [Google Scholar]
- Vanneste, S., et al. (2005). Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17 3035–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmoth, J.C., Wang, S., Tiwari, S.B., Joshi, A.D., Hagen, G., Guilfoyle, T.J., Alonso, J.M., Ecker, J.R., and Reed, J.W. (2005). NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 43 118–130. [DOI] [PubMed] [Google Scholar]
- Woodward, A.W., and Bartel, B. (2005). Auxin: Regulation, action, and interaction. Ann. Bot. (Lond.) 95 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Q., Frugis, G., Colgan, D., and Chua, N.H. (2000). Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development. Genes Dev. 14 3024–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Q., Guo, H.S., Dallman, G., Fang, S., Weissman, A.M., and Chua, N.H. (2002). SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature 419 167–170. [DOI] [PubMed] [Google Scholar]
- Zhao, Y.D., Christensen, S.K., Fankhauser, C., Cashman, J.R., Cohen, J.D., Weigel, D., and Chory, J. (2001). A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291 306–309. [DOI] [PubMed] [Google Scholar]
- Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L., and Gruissem, W. (2004). GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.