Abstract
SOX (SRY type HMG box) proteins are transcription factors that are predominantly known for their roles during development. During melanocyte development from the neural crest, SOX10 regulates microphthalmia-associated transcription factor, which controls a set of genes critical for pigment cell development and pigmentation, including dopachrome tautomerase and tyrosinase. We report here that another SOX factor, SOX9, is expressed by melanocytes in neonatal and adult human skin and is up-regulated by UVB exposure. We demonstrate that this regulation is mediated by cAMP and protein kinase. We also show that agouti signal protein, a secreted factor known to decrease pigmentation, down-regulates SOX9 expression. In adult and neonatal melanocytes, SOX9 regulates microphthalmia-associated transcription factor, dopachrome tautomerase, and tyrosinase promoters, leading to an increase in the expression of these key melanogenic proteins and finally to a stimulation of pigmentation. SOX9 completes the complex and tightly regulated process leading to the production of melanin by acting at a very upstream level. This role of SOX9 in pigmentation emphasizes the poorly understood impact of SOX proteins in adult tissues.
Keywords: microphthalmia-associated transcription factor, tyrosinase, protein kinase A, melanocyte-stimulating hormone, agouti signal protein
Sox (SRY type HMG box) proteins are transcription factors that belong to the HMG box superfamily of DNA-binding proteins and play a key role during development. SOX9 belongs to the SOX-E subgroup, which includes SOX8, SOX9, and SOX10. The structures of these proteins show a high conservation and similar positions of their HMG boxes (1). SOX10 has been shown to play a key role in the regulation of melanocyte differentiation (2), and mutations in SOX10 lead to Waardenburg syndrome type 4, a genetic hypomelanosis with deafness and megacolon (3). During melanocyte development from the neural crest, SOX10 regulates the expression of microphthalmia-associated transcription factor (MITF), which in turn controls a set of genes critical for pigment cell development and pigmentation (4). Indeed, in conjunction with other transcription factors, MITF regulates dopachrome tautomerase (DCT), tyrosinase (the limiting enzyme for melanogenesis), and tyrosinase-related protein 1 (TYRP1). All of these proteins are essential for the full differentiation of melanocytes and are directly involved in melanin synthesis. SOX10 also acts as a critical transactivator of DCT, which MITF, on its own, is insufficient to stimulate (5–7).
SOX9 has a key role in sexual determination and chondrogenesis, and mutations in SOX9 can lead to campomelic dysplasia, a skeletal dysmorphology associated in most XY cases with sex reversal (8, 9). During embryonic development, the SOX9 gene becomes active in all prechondrocytic mesenchymal condensations, and its expression is maintained at high levels in fully differentiated chondrocytes. The direct target for SOX9 is a chondrocyte-specific enhancer in the gene for collagen type II (10, 11). With aging, the loss of expression of SOX9 in some disk cells may play a role in disk degeneration by resulting in decreased expression and production of collagen type II (12). There have been increased numbers of reports showing the active role of SOX9 in other tissues such as heart, kidney, or brain (13–15). Interestingly, ectopic SOX9 expression in the neural crest is sufficient to promote melanocytic differentiation (16), which suggests a role for SOX9 in melanocytic development. An active transcription of SOX9 protein in melanocytes within the skin also has been indirectly suggested by the presence of antibodies against SOX9 in the sera of vitiligo patients (17). Interestingly, the cAMP pathway, which plays a key role in melanocyte differentiation, also has been involved in the control of SOX9 function in chondrocytes (18, 19). These observations suggest that SOX9 might participate in the regulation of melanocyte differentiation by cAMP-elevating agents such as α-melanocyte-stimulating hormone (α-MSH) or UV radiation, that act, at least in part, through the cAMP pathway. In the present study, we show the presence of SOX9 at the RNA and protein levels in normal human melanocytes in vitro and in vivo. We show the up-regulation of SOX9 after UVB exposure and its increased nuclear localization. We demonstrate that the effects of UVB act at least through the cAMP pathway and increased levels of protein kinase A (PKA). We also show that agouti signal protein (ASP), a secreted factor known to decrease pigmentation and to antagonize the signaling pathway of α-MSH, down-regulates SOX9 expression. Moreover, we demonstrate the key role of SOX9 in regulating pigmentation. Indeed, we show that SOX9 regulates MITF and DCT promoters. Overexpression of SOX9 induces an increase of MITF, DCT, and tyrosinase proteins, which leads to an increased production of melanin within the cells.
Results
SOX9 Is Expressed in Melanocytes in Vivo.
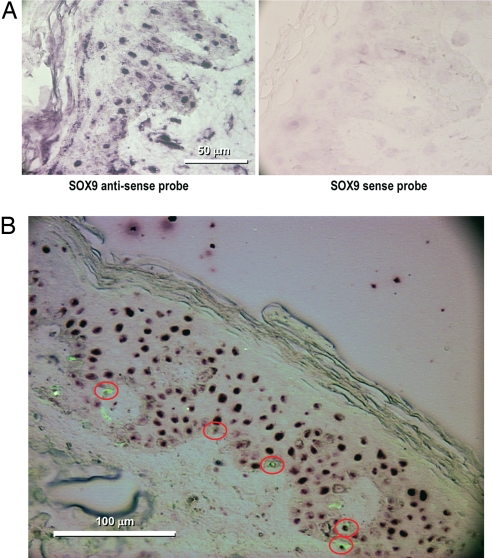
To determine whether SOX9 mRNA is expressed in human skin, we designed a specific probe directed against SOX9 and performed a tissue in situ hybridization (TISH) study. We were able to detect a strong positive staining in the epidermis with the antisense probe whereas staining with the sense probe was negative (Fig. 1A). To differentiate melanocytes from keratinocytes, we coupled the TISH protocol with standard immunohistochemistry performed with antibodies directed against MART1, a specific marker of melanocytes. Melanocytes identified by MART1 also were stained by the SOX9 TISH probe (Fig. 1B), demonstrating that melanocytes express SOX9 in human skin in vivo. The presence of SOX9-positive melanocytes also was detected at the protein level with immunohistochemistry by using antibodies directed against SOX9 and MART1 (data not shown).
Fig. 1.
TISH analysis of SOX9 expression in the skin in vivo. (A) Antisense probes directed against SOX9 are present in the skin with strong nuclear staining, whereas staining with the sense probe against SOX9 is negative and shows the presence of SOX9 RNA in the skin. (B) Coupling of TISH with antisense probes against SOX9 and immunohistochemistry with MART1 antibody as a melanocytic marker shows that SOX9 is expressed in keratinocytes and in melanocytes.
SOX9 Is Up-Regulated by UVB Exposure.
To investigate whether SOX9 is regulated during melanocyte differentiation, we exposed melanocytes to their most common external stimulus, UV radiation. We irradiated normal human epidermal melanocytes (NHM) obtained from adult lightly pigmented (a-LP) skin in culture with 21 mJ/cm2 UVB and compared them with nonirradiated controls. Using RT-PCR, we showed that the expression level of SOX9 was increased 1 h after UVB exposure compared with nonirradiated cells (Fig. 2A). At the protein level, an increase in SOX9 was observed starting 2 h after the UVB exposure, increased further until 8 h, and then decreased at 24 h (Fig. 2B). The stimulation of SOX9 expression was confirmed by immunoprecipitation of 35S-labeled SOX9. Finally, we compared the basal expression of SOX9 in neonatal lightly pigmented (n-LP) skin and in neonatal darkly pigmented (n-DP) skin NHM by using immunoblotting. The darker melanocytes expressed more SOX9 than did the lightly pigmented melanocytes (Fig. 2C).
Fig. 2.
SOX9 expression is up-regulated after UVB exposure. (A) Expression levels of SOX9 mRNA using RT-PCR in untreated NHM (basal) or 1 h after 21 mJ/cm2 UVB irradiation. β-actin expression served as a control. The histogram shows quantification of the data with the means ± SD of three independent experiments. Results are expressed as a ratio compared with the basal condition. (B) Protein expression levels of SOX9 using immunoblotting in a-LP NHM without treatment (basal) and after irradiation with 21 mJ/cm2 UVB at 0, 1, 2, 4, 6, 8, and 24 h. (C) Expression levels of SOX9 by immunoblotting in n-DP NHM without treatment (basal) and after irradiation with 21 mJ/cm2 UVB at 1, 2, and 4 h. Numbers above the gels indicate levels of intensity compared with GAPDH. (D) Comparison of the basal level expression of SOX9 in n-LP cells compared with n-DP cells using immunoblotting. The histogram shows quantification of the data with means ± SD in three independent experiments. Results are expressed as a ratio compared with the basal condition. SOX9 is expressed at higher levels in the more pigmented melanocytes.
The Action of UVB on SOX9 Is Mediated by cAMP and PKA.
The cAMP pathway plays a key role in regulating pigmentation and mediates most of the effects of UV on melanogenesis (20), so we next investigated the effect of cAMP on SOX9. After 35S metabolic labeling, NHM were exposed to 21 mJ/cm2 UVB or to forskolin, a cAMP-stimulating agent, and then SOX9 was immunoprecipitated. Treatment with either UVB or forskolin led to increased expression of SOX9 (Fig. 3A). PKA, the most important downstream target of cAMP, can be inhibited by H89. The expressions of SOX9 and SOX9 phosphorylated at S181 were both increased after UVB exposure, and in both cases, pretreatment with H89 prevented the increased expression of SOX9 by UVB (Fig. 3B).
Fig. 3.
The action of UVB on SOX9 is mediated through the cAMP pathway. (A) 35S labeling of a-LP NHM treated for 3 h with 20 μM forskolin or 21 mJ/cm2 UVB or untreated (Basal). Labeled proteins were immunoprecipitated with SOX9 or GAPDH antibodies. (B) UVB acts through the activation of PKA to up-regulate SOX9. 35S labeling of a-LP NHM without treatment (Basal) or with 21 mJ/cm2 UVB irradiation for 3 h with or without a PKA inhibitor (5 μM H89). Proteins were immunoprecipitated with antibodies against SOX9 and GAPDH.
The up-regulation of SOX9 after forskolin treatment also was observed in NHM by using immunocytochemistry. Four hours after forskolin treatment, the cells were fixed and stained with SOX9 antibodies. An increased expression of SOX9 was noted in NHM treated with forskolin (Fig. 4A). We also used a reconstructed skin model (MelanoDerm; MatTek, Ashland, MA) to assess the effect of UVB on SOX9 expression in the skin. After 4 days of growth, MelanoDerms were exposed or not to 21 mJ/cm2 UVB. The samples were fixed 8 h after the UV irradiation and were then double-stained with SOX9 and MART1 antibodies to identify melanocytes. The results confirmed the increased SOX9 staining in melanocytes with a predominant nuclear localization after UVB exposure (Fig. 4B).
Fig. 4.
UVB and cAMP up-regulate SOX9 and increase its nuclear concentration. (A) Immunocytochemical staining of a-LP NHM. Four hours after treatment without (Control) or with 20 μM forskolin (FSK), the cells were fixed and stained with SOX9 antibody (red). Counterstaining was performed with DAPI (blue) to visualize nuclei. An increased expression of SOX9 was noted in cells treated with forskolin. The transversal cuts shown beneath under the Control and FSK images confirmed the nuclear localization of SOX9. (B) Immunohistochemistry with MelanoDerm 8 h after 21 mJ/cm2 UVB irradiation or without UVB (Control). Samples were stained with antibodies to SOX9 (green) and MART1 (red; as a melanocytic marker). An increased staining of SOX9 was observed after UVB irradiation both in keratinocytes and in melanocytes, with a stronger increase in melanocytes and a clear nuclear localization.
SOX9 Is Down-Regulated by ASP.
ASP is an antagonist of the melanocortin 1 receptor, which plays a key role in regulating pigmentation (21). ASP inhibits melanin formation in mouse melanocytes and in melanoma cells as well as in NHM (22–24). We treated NHM obtained from neonatal moderately pigmented (n-MP) skin with ASP. RNAs then were extracted, and with RT-PCR, we showed that the expression level of SOX9 was decreased 4 h after ASP treatment compared with nontreated cells (Fig. 5A). Proteins were extracted from n-MP NHM treated for 48 h with ASP and were analyzed by immunoblotting. Concordantly with the RT-PCR results, SOX9 expression was decreased significantly in NHM after treatment with ASP (Fig. 5B).
Fig. 5.
SOX9 is down-regulated by ASP. (A) Expression levels of SOX9 gene using RT-PCR in n-MP NHM untreated (basal) or treated with ASP (10 nM) for 4 h. β-actin expression served as a control. The histogram shows quantification of the data with the means ± SD of three independent experiments. Results are expressed as a ratio compared with the basal condition. The expression of SOX9 is down-regulated in melanocytes treated by ASP. (B) Proteins from n-MP NHM treated 48 h with 10 nM ASP were extracted and analyzed by immunoblotting. The histogram shows quantification of the data with the means ± SD of three independent experiments. Results are expressed as a ratio compared with the basal condition. SOX9 protein expression was decreased significantly in NHM after treatment with ASP.
SOX9 Induces Transcription of MITF, DCT, and TYR.
As SOX9 was up-regulated after UVB exposure, we then investigated the effects of SOX9 on the activities of MITF, TYR, and DCT promoters in B16 melanoma cells. Overexpression of SOX9, or treatment with forskolin to a lesser extent, induced a strong activation of the MITF, DCT, and TYR promoters [supporting information (SI) Fig. 11]. Conversely, SOX9 silencing using siRNA decreased the MITF, DCT, and TYR promoter activities and impaired forskolin-induced stimulation (SI Fig. 11B). As a control, we showed that the stimulation of a cAMP response element (CRE)-containing promoter by forskolin was not affected by SOX9 silencing (SI Fig. 12). Finally, in HeLa cells, which, in contrast to B16 cells, do not express the protein MITF, overexpression of SOX9 stimulated MITF and DCT promoter activities but not the TYR promoter activity, and forskolin had no effect on the TYR and DCT promoter activities (data not shown). The sum of these data supports the direct activation of the MITF and DCT promoters by SOX9, whereas the TYR promoter seems not to be directly activated by SOX9.
To assess whether SOX9 directly regulates MITF expression, we performed ChIP assays on NHM before and 4 h after treatment with forskolin. The ChIP assays clearly showed that SOX9 directly occupies the MITF promoter and that the binding was significantly increased after treatment with forskolin (Fig. 6). To determine the relative roles of SOX9 and CRE-binding protein (CREB) on the activation of the MITF promoter, we studied the effects of SOX9 overexpression and of stimulation with forskolin on several truncated (in the 5′ flanking region) or mutated luciferase constructs of the MITF promoter (SI Fig. 13). The 217-bp construct, which has the CRE but lacks all putative SOX9 binding sites, showed no significant activation after SOX9 overexpression or forskolin treatment. As previously reported, mutation of the CRE prevents activation of the MITF promoter by forskolin (25). This mutation significantly decreased activation of the MITF promoter after SOX9 overexpression without completely preventing it. Finally, selectively mutating the SOX9 element (at residues −266 and −260) showed significant but incomplete decreases in cAMP or SOX9 responsiveness. This incomplete effect could be due to the action of several other putative SOX9 binding sites present on the MITF promoter.
Fig. 6.
SOX9 directly regulates the MITF promoter. ChIP of NHM was performed with SOX9 or IgG antibodies with or without 4-h treatment with 20 μM forskolin. The DNA recovered was subjected to amplification by PCR (30 cycles) using primers for human MITF promoter and human GAPDH promoter.
To confirm the role of SOX9 on MITF expression, we used immunocytochemistry to examine the effects of silencing SOX9. NHM were transfected with SOX9 siRNA, and, after 24 h, the cells were fixed and stained with SOX9 and MITF antibodies. Cells silenced for SOX9 showed a marked decrease in the expression of MITF (Fig. 7).
Fig. 7.
SOX9 regulates MITF expression. n-MP NHM were silenced for SOX9 using siRNA. After 24 h, they were fixed, and an immunocytochemistry was performed using SOX9 (red) and MITF (green) antibodies. DAPI (blue) served as a counterstaining. Cells silenced for SOX9 (white arrow) showed no or slight expression of MITF.
Effects of SOX9 Are Independent of SOX10 Activation.
SOX10 has been shown to interact with the MITF and DCT promoters, at least during embryogenesis (7). Thus, some effects of SOX9 could be mediated by SOX10. Therefore, we investigated the putative action of SOX9 on SOX10 expression. After silencing SOX9 in NHM, we extracted proteins 48 h later and analyzed them by Western blotting. The decrease of SOX9 did not affect the level of SOX10 protein (Fig. 8Left). We then overexpressed SOX9 in NHM and extracted proteins 24 h later and then analyzed them by Western blotting. Again, the increase of SOX9 showed no effect on SOX10 protein levels (Fig. 8 Right). These results show that, in NHM, the effects of SOX9 on DCT and MITF targets are not mediated or facilitated through SOX10 regulation.
Fig. 8.
SOX9 does not regulate SOX10. SOX9 was silenced in n-DP NHM by using siRNA for 48 h, after which proteins were extracted and analyzed by immunoblotting. (Left) The silencing of SOX9 does not affect the expression of SOX10. SOX9 was overexpressed for 24 h in n-DP NHM, and again, after extraction, proteins were analyzed by immunoblotting. (Right) The expression of SOX10 was not affected when SOX9 was overexpressed.
SOX9 Increases Expression of Melanogenic Proteins and Enhances Pigmentation.
To investigate the action of SOX9 on proteins involved in pigmentation, we overexpressed or silenced SOX9 in n-LP NHM, whereas control n-LP NHM were transfected with siRNA scrambles or overexpression mocks. Proteins then were extracted and analyzed with Western blot. The expression of DCT and tyrosinase proteins was increased when SOX9 was overexpressed. Concomitantly, when siRNA was used to silence SOX9, NHM expressed less DCT and tyrosinase proteins compared with the controls (Fig. 9A). As a final target, we analyzed the amount of melanin produced in n-MP NHM after silencing or overexpressing SOX9 for 24 and 48 h. We found that the amount of melanin was increased by the overexpression of SOX9 and was decreased by its silencing (Fig. 9B).
Fig. 9.
SOX9 regulates proteins involved in melanogenesis and increases pigmentation of melanocytes. (A) SOX9 was silenced in n-LP NHM by using siRNA for 24 h; then, proteins were extracted and analyzed by immunoblotting. (Left) Concomitantly with the silencing of SOX9, the expression of DCT and tyrosinase proteins was decreased. SOX9 was overexpressed for 24 h in n-LP NHM. Again, after extraction, proteins were analyzed by immunoblotting. (Right) A parallel increase of DCT and tyrosinase proteins was observed when SOX9 was overexpressed. (B) n-MP NHM were silenced or overexpressed for SOX9 for 24 and 48 h. After protein extraction, the amount of melanin for each condition was calculated and adjusted against the total protein concentration. The histogram shows quantification of the data with the means ± SD of three independent experiments. Results are expressed as a ratio compared with the basal condition. Except after 24 h of SOX9 silencing, there was a significant decrease of melanin after 48 h of SOX9 silencing and a significant increase after overexpression of SOX9.
Discussion
Acquired pigmentation, so called “tanning,” is a complex process that leads to an increase in the production of melanin within specialized organelles called melanosomes. These melanosomes are produced within melanocytes and then are transported to the extremities of their dendrites and are finally transferred to surrounding keratinocytes. This physiological phenomenon is induced by UV exposure and helps protect the skin from the carcinogenic action of UV irradiation. The activation of melanocortin I receptor receptors at the surface of melanocytes by α-MSH secreted by surrounding keratinocytes leads to the activation of the cAMP pathway, which plays a key role not only in the process of melanogenesis but also in the transport of melanosomes (20, 26). The downstream activation of CRE, then MITF, and finally the DCT and tyrosinase promoters is considered the main pathway leading to the production of melanin (27, 28). However, although MITF is required, it does not seem to be sufficient to induce the expression of melanogenic enzymes (29). Concomitantly, it has been demonstrated that SOX10 is able to bind the MITF promoter and acts with CREB to activate its transcription (25). The importance of SOX10 in pigmentation already was known because mutations in that gene lead to a genetic hypomelanosis disorder, the Waardenburg syndrome (3). Recently, it has been shown that as melanoblasts differentiate to melanocytes, the expression of SOX10 decreases, but, at the same time, the expression of SOX9 increases (30). Of interest, the authors of ref. 30 report that SOX9 was expressed at higher levels in pigmented melanoma cell lines compared with nonpigmented ones.
In this study, we demonstrate that SOX9 is expressed by NHM in culture as well in adult human skin. We also show that the expression of SOX9 is up-regulated in melanocytes after UVB irradiation. As SOX9 has no UV-responsive element in its promoter, the action of UV should act through an intermediate to activate SOX9. It has been recently demonstrated that the SOX9 promoter is regulated directly by CREB and sp1 in chondrocytes (31). The increase of CREB through the cAMP pathway could explain, at least in part, the effect of UV on SOX9 levels in melanocytes. However, it is highly probable that the regulation of SOX9 is more complex and that other factors might act concomitantly with CREB. Indeed, a putative binding site for LEF1 also is present in the SOX9 promoter. LEF1 binding sites also are present on the MITF promoter (32). Indeed, the Wnt/β-catenin pathway could be involved in SOX9 regulation. This pathway already is known to be involved in pigmentation through MITF activation (32). Moreover, Wnt/β-catenin also appears to be a key pathway for melanoma (33) and could link SOX9 not only to differentiation but also to proliferation. We showed that, as for most of the key players in melanogenesis, the up-regulation of SOX9 is mediated by the cAMP pathway. The transcription factor Usf-1 also has been shown to mediate the UV response in melanocytes by binding the conserved E-Box elements in the tyrosinase promoter (34). We show in this study the activation and role of SOX9, another transcription factor, in response to UV stimulation. Indeed, response to UV radiation appears to be tightly regulated and involves several actors, including USF-1 and SOX9, which regulate differentiation in melanocytic cells.
The regulation observed in melanocytes of adult skin suggests an active role of SOX9 after embryogenesis. However, although mutations of SOX10 induce strong pigmentary defects, such as those observed in Waardenburg syndrome, mutations of SOX9 lead to phenotypes in which pigmentation defects are slight (pigmented ears and light coat pigmentation) (35). To the best of our knowledge, pigmentary disorders have not been reported in patients suffering from campomelic dysplasia. This could be due to the severity of the other abnormalities that lead in most cases to death in early childhood, which occupies the attention of the clinicians. A possible compensation by SOX10 probably explains why SOX9 was never previously investigated for its role(s) in the pigmentary process. Interestingly, inappropriate expression of SOX9 during embryogenesis in mice leads to microphthalmia and pigmentary defects (36). Thus, it cannot be ruled out that some Waardenburg-like syndrome features in humans could be due to not-yet-recognized mutations in the SOX9 gene. We have shown in this study that the regulation of SOX9 does not affect the expression of SOX10 in neonatal and adult NHM and that the effects of SOX9 on differentiation are not mediated through an increase of SOX10. Although SOX10 plays a key role in regulating the development of melanocytes, its expression decreases as they differentiate, whereas SOX9 expression increases (30). Thus, in contrast to SOX10, SOX9 could have a minor role in early stages of melanocyte development but could then play an increasing role in regulating differentiation. The hypothesis already has been suggested that, once MITF expression is established, there may not be a continued requirement for the same factors later in development and that the role of SOX10 might well be undertaken by other members of the SOX family later in development (37). Indeed, we show that SOX9 directly binds and regulates the MITF promoter, increases the expression of DCT and tyrosinase, and, as a result, increases the pigmentation of melanocytic cells. This finding was corroborated by the results obtained when SOX9 was silenced, showing very slight or no activation of MITF, DCT, or TYR promoters after forskolin stimulation. These results emphasize the key roles of both MITF and SOX9 in the melanogenic process in neonatal and adult melanocytes. Moreover, the relative importance of SOX9/SOX10 after embryogenesis and their potential synergistic action required further investigations. The schematic role of SOX9 in UV-induced pigmentation is summarized in Fig. 10.
Fig. 10.
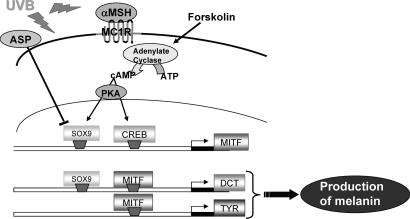
Schematic representation of the role of SOX9 in regulating pigmentation. UVB radiation, at least through the activation of cAMP via PKA, increases SOX9 and CREB expression. Those two transcription factors regulate the MITF promoter. SOX9 and MITF then act together to regulate the DCT promoter, whereas MITF also will act on the TYR promoter, finally leading to increased production of melanin within melanosomes.
UVB radiation, through at least the activation of the cAMP pathway by α-MSH, increases SOX9 expression in melanocytes. However, we also found a decrease in SOX9 expression after treatment with ASP. Interestingly, ASP is known to decrease pigmentation in mouse and human melanocytes (22–25). Transfection of the ASP gene in the skin and hair follicles of rats decreases tyrosinase expression and pigmentation (38). However, little is known about the mechanism of action of ASP on melanocytes. It has been demonstrated that MITF is inhibited by ASP (39). The basic helix–loop–helix transcription factor (ITF2) has been shown to be involved in the action of ASP on pigmentation (40). Indeed, ITF2 is up-regulated by ASP and is down-regulated by α-MSH. The overexpression of ITF2 leads to the decreased expression of MITF, DCT, and tyrosinase, whereas its silencing induces opposite effects (41). Here, we show that SOX9 is up-regulated by α-MSH but is down-regulated by ASP in human melanocytes. According to our results on the effects of SOX9 on MITF, DCT, and tyrosinase expression, the down-regulation of SOX9 after ASP treatment could explain, at least in part, the mechanism used by ASP to decrease pigmentation.
Recently, SOX9 has been shown to be expressed in keratinocytes and in the sebaceous glands of adult skin (42). These data are concordant with the results obtained in our study that demonstrate the expression of SOX9 at protein and RNA levels in keratinocytes. The previously unrecognized role of SOX9 in pigmentation in neonatal and in adult melanocytes emphasizes the poor understanding of the roles of SOX proteins in adult tissues. In melanocytic cells, SOX9 completes the complex and tightly regulated process leading to the production of melanin by acting at a very upstream level.
Materials and Methods
For full details regarding reagents, cell lines, culture conditions, plasmid construction, siRNA, transfection, immunoblotting, immunohistochemistry, and immunocytochemical staining, see SI Materials and Methods.
Reconstructed Skin.
The epidermal equivalent MelanoDerm was obtained from MatTek (Ashland, MA), and normal human keratinocytes and melanocytes were obtained from Asian neonatal foreskin tissues. MelanoDerms were grown at the air/liquid interface of the maintenance medium MEL–NHM-113 (MatTek), and culture medium was renewed every 2 days.
RT-PCR.
Total RNA was reverse-transcribed by using SuperScript III (Invitrogen). PCRs consisted of 25 cycles for β-actin and 35 cycles for SOX9 using TaqDNA polymerase (Invitrogen, Carlsbad, CA). PCR products for β-actin and SOX9 were 838 and 288 bp, respectively, and were electrophoresed in parallel with DNA molecular mass markers (Invitrogen). Full details can be found in SI Materials and Methods.
TISH.
Oligonucleotide probes specific for human SOX9 were designed. Target sites were selected based on the analysis of sequence matches and mismatches BLAST (GenBank). Probes showed no evidence of cross-reaction with sequences of other genes, including other SOX family genes. Full details can be found in SI Materials and Methods.
Metabolic Labeling.
Radioactive metabolic labeling and immunoprecipitation was performed as described previously (43). Where indicated, cells were irradiated with 21 mJ/cm2 UVB, and/or chemical agents were added from the “pulse” period to the “chase” period. Cell extracts were incubated with normal rabbit serum (Vector, Burlingame, CA) and then were incubated with protein G beads (GE Healthcare, Buckinghamshire, U.K.). The supernatants were incubated with SOX9 (Abcam, Cambridge, MA) or GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. The immune complexes were separated by incubation with beads and were washed further with immunoprecipitate lysis buffer. The pellets were eluted, electrophoresed, and visualized by autoradiography.
Luciferase Assay.
B16 melanoma cells or HeLa cells were seeded in 24-well dishes and were transfected with 0.3 μg of the luciferase reporter plasmid and 0.05 μg of pCMVβ-Gal (Promega, Madison, WI) to control the variability of transfection efficiency. In some experiments, SOX9 was overexpressed by using 0.1 μg of the pCDNA3 encoding SOX9 or was silenced with 100 nM SOX9 siRNA. Empty pCDNA3 or siRNA scrambles were used as controls depending on the experiments. All transfections were made with Lipofectamine (Invitrogen) according to the manufacturer's instructions. Thirty-six hours later, cells were washed with a saline phosphate buffer and lysed with reporter lysis buffer (Promega). Soluble extracts were harvested and assayed for luciferase and β-gal activities. All transfections were repeated at least three times with different plasmid preparations and gave similar results. The pMITF wild-type and mutant pDCT, pTYR, and pCRE constructs used have been described previously (4, 27, 44).
ChIP.
ChIP assays were performed as previously described (46) with 4 μg of SOX9 antibody (Chemicon, Billerica, MA) or 4 μg of nonspecific IgG (Invitrogen). The DNA recovered was subjected to amplification by PCR before analysis with agarose gel electrophoresis. The primers used for the PCRs were the human MITF promoter region (5′-GATGATGTCTCCTCCAAAGG-3′ and 5′-AGCCCTACGAGTTTGGTCTT-3′) and the GAPDH promoter (5′-CGGTGCGTGCCCAGTTG-3′ and 5′-GCGACGCAAAAGAAGATG-3′).
Melanin Content Assay.
Melanin content was determined as described previously (45). Melanin contents are expressed as nanograms of melanin divided by the total protein in micograms. Values are reported compared with values obtained in controls and are reported as ratios. Each experiment was repeated at least three times.
Statistical Analysis.
Data are presented as means ± SD. Student's t test was used to analyze differences. Values of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Karine Bille for her technical support. This research was supported by the Intramural Research Program of the National Cancer Institute.
Abbreviations
- a-LP
adult lightly pigmented
- α-MSH
α-melanocyte-stimulating hormone
- ASP
agouti signal protein
- CRE
cAMP response element
- CREB
CRE-binding protein
- DCT
dopachrome tautomerase
- MITF
microphthalmia-associated transcription factor
- n-LP
neonatal lightly pigmented
- n-DP
neonatal darkly pigmented
- NHM
normal human epidermal melanocytes
- n-MP
neonatal moderately pigmented
- TISH
tissue in situ hybridization
- TYRP1
tyrosinase-related protein 1
- PKA
protein kinase A.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705117104/DC1.
References
- 1.Bowles J, Schepers G, Koopman P. Dev Biol. 2000;227:239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- 2.Mollaaghababa R, Pavan WJ. Oncogene. 2003;22:3024–3034. doi: 10.1038/sj.onc.1206442. [DOI] [PubMed] [Google Scholar]
- 3.Pingault V, Bondurand N, Kuhlbrodt K, Goerich DE, Prehu MO, Puliti A, Herbarth B, Hermans-Borgmeyer I, Legius E, Matthijs G, et al. Nat Genet. 1998;18:171–173. doi: 10.1038/ng0298-171. [DOI] [PubMed] [Google Scholar]
- 4.Verastegui C, Bille K, Ortonne JP, Ballotti R. J Biol Chem. 2000;275:30757–30760. doi: 10.1074/jbc.C000445200. [DOI] [PubMed] [Google Scholar]
- 5.Potterf SB, Mollaaghababa R, Hou L, Southard-Smith EM, Hornyak TJ, Arnheiter H, Pavan WJ. Dev Biol. 2001;237:245–257. doi: 10.1006/dbio.2001.0372. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig A, Rehberg S, Wegner M. FEBS Lett. 2004;556:236–244. doi: 10.1016/s0014-5793(03)01446-7. [DOI] [PubMed] [Google Scholar]
- 7.Jiao Z, Mollaaghababa R, Pavan WJ, Antonellis A, Green ED, Hornyak TJ. Pigment Cell Res. 2004;17:352–362. doi: 10.1111/j.1600-0749.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- 8.Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, et al. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 9.Kwok C, Weller PA, Guioli S, Foster JW, Mansour S, Zuffardi O, Punnett HH, Dominguez-Steglich MA, Brook JD, Young ID, et al. Am J Hum Genet. 1995;57:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 10.Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS. Nat Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 11.Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber HE, Norton HJ, Ingram JA, Hanley EN., Jr Spine. 2005;30:625–630. doi: 10.1097/01.brs.0000155420.01444.c6. [DOI] [PubMed] [Google Scholar]
- 13.Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B. Proc Natl Acad Sci USA. 2004;101:6502–6507. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pepicelli CV, Kispert A, Rowitch DH, McMahon AP. Dev Biol. 1997;192:193–198. doi: 10.1006/dbio.1997.8745. [DOI] [PubMed] [Google Scholar]
- 15.Pompolo S, Harley VR. Brain Res. 2001;906:143–148. doi: 10.1016/s0006-8993(01)02574-4. [DOI] [PubMed] [Google Scholar]
- 16.Cheung M, Briscoe J. Development (Cambridge, UK) 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 17.Hedstrand H, Ekwall O, Olsson MJ, Landgren E, Kemp EH, Weetman AP, Perheentupa J, Husebye E, Gustafsson J, Betterle C, et al. J Biol Chem. 2001;276:35390–35395. doi: 10.1074/jbc.M102391200. [DOI] [PubMed] [Google Scholar]
- 18.Huang W, Zhou X, Lefebvre V, de Crombrugghe B. Mol Cell Biol. 2000;20:4149–4158. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Mejean C, Berta P, Poulat F, Boizet-Bonhoure B. EMBO J. 2005;24:1798–1809. doi: 10.1038/sj.emboj.7600660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busca R, Ballotti R. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 21.Rouzaud F, Hearing VJ. Peptides. 2005;26:1858–1870. doi: 10.1016/j.peptides.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 22.Hunt G, Thody AJ. J Endocrinol. 1995;147:R1–R4. doi: 10.1677/joe.0.147r001. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki I, Tada A, Ollmann MM, Barsh GS, Im S, Lamoreux ML, Hearing VJ, Nordlund JJ, Abdel-Malek ZA. J Invest Dermatol. 1997;108:838–842. doi: 10.1111/1523-1747.ep12292572. [DOI] [PubMed] [Google Scholar]
- 24.Sviderskaya EV, Hill SP, Balachandar D, Barsh GS, Bennett DC. Dev Dyn. 2001;221:373–379. doi: 10.1002/dvdy.1153. [DOI] [PubMed] [Google Scholar]
- 25.Huber WE, Price ER, Widlund HR, Du J, Davis IJ, Wegner M, Fisher DE. J Biol Chem. 2003;278:45224–45230. doi: 10.1074/jbc.M309036200. [DOI] [PubMed] [Google Scholar]
- 26.Passeron T, Bahadoran P, Bertolotto C, Chiaverini C, Busca R, Valony G, Bille K, Ortonne JP, Ballotti R. FASEB J. 2004;18:989–991. doi: 10.1096/fj.03-1240fje. [DOI] [PubMed] [Google Scholar]
- 27.Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, Ballotti R. J Cell Biol. 1998;142:827–835. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertolotto C, Bille K, Ortonne JP, Ballotti R. J Cell Biol. 1996;134:747–755. doi: 10.1083/jcb.134.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaggioli C, Busca R, Abbe P, Ortonne JP, Ballotti R. Pigment Cell Res. 2003;16:374–382. doi: 10.1034/j.1600-0749.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 30.Cook AL, Smith AG, Smit DJ, Leonard JH, Sturm RA. Exp Cell Res. 2005;308:222–235. doi: 10.1016/j.yexcr.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Piera-Velazquez S, Hawkins DF, Whitecavage MK, Colter DC, Stokes DG, Jimenez SA. Exp Cell Res. 2007;313:1069–1079. doi: 10.1016/j.yexcr.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy C, Khaled M, Fisher DE. Trends Mol Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Larue L, Delmas V. Front Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- 34.Galibert MD, Carreira S, Goding CR. EMBO J. 2001;20:5022–5031. doi: 10.1093/emboj/20.17.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, Behringer RR, Overbeek PA. Nat Genet. 2000;26:490–494. doi: 10.1038/82652. [DOI] [PubMed] [Google Scholar]
- 36.Qin Y, Kong LK, Poirier C, Truong C, Overbeek PA, Bishop CE. Hum Mol Genet. 2004;13:1213–1218. doi: 10.1093/hmg/ddh141. [DOI] [PubMed] [Google Scholar]
- 37.Lee M, Goodall J, Verastegui C, Ballotti R, Goding CR. J Biol Chem. 2000;275:37978–37983. doi: 10.1074/jbc.M003816200. [DOI] [PubMed] [Google Scholar]
- 38.Yang CH, Shen SC, Lee JC, Wu PC, Hsueh SF, Lu CY, Meng CT, Hong HS, Yang LC. Gene Ther. 2004;11:1033–1039. doi: 10.1038/sj.gt.3302264. [DOI] [PubMed] [Google Scholar]
- 39.Aberdam E, Bertolotto C, Sviderskaya EV, de Thillot V, Hemesath TJ, Fisher DE, Bennett DC, Ortonne JP, Ballotti R. J Biol Chem. 1998;273:19560–19565. doi: 10.1074/jbc.273.31.19560. [DOI] [PubMed] [Google Scholar]
- 40.Furumura M, Sakai C, Potterf SB, Vieira WD, Barsh GS, Hearing VJ. Proc Natl Acad Sci USA. 1998;95:7374–7378. doi: 10.1073/pnas.95.13.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furumura M, Potterf SB, Toyofuku K, Matsunaga J, Muller J, Hearing VJ. J Biol Chem. 2001;276:28147–28154. doi: 10.1074/jbc.M101626200. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Yang CC, Liao CY, Hung CL, Tsai SJ, Chen KF, Sheu HM, Zouboulis CC. J Eur Acad Dermatol Venereol. 2006;20:846–852. doi: 10.1111/j.1468-3083.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 43.Yasumoto K, Watabe H, Valencia JC, Kushimoto T, Kobayashi T, Appella E, Hearing VJ. J Biol Chem. 2004;279:28330–28338. doi: 10.1074/jbc.M401269200. [DOI] [PubMed] [Google Scholar]
- 44.Larribere L, Hilmi C, Khaled M, Gaggioli C, Bille K, Auberger P, Ortonne JP, Ballotti R, Bertolotto C. Genes Dev. 2005;19:1980–1985. doi: 10.1101/gad.335905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virador VM, Kobayashi N, Matsunaga J, Hearing VJ. Anal Biochem. 1999;270:207–219. doi: 10.1006/abio.1999.4090. [DOI] [PubMed] [Google Scholar]
- 46.Carreira S, Goodall J, Aksan I, La Roca SA, Galibert MD, Denat L, Larue L, Goding CR. Nature. 2005;433:764–769. doi: 10.1038/nature03269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.