Abstract
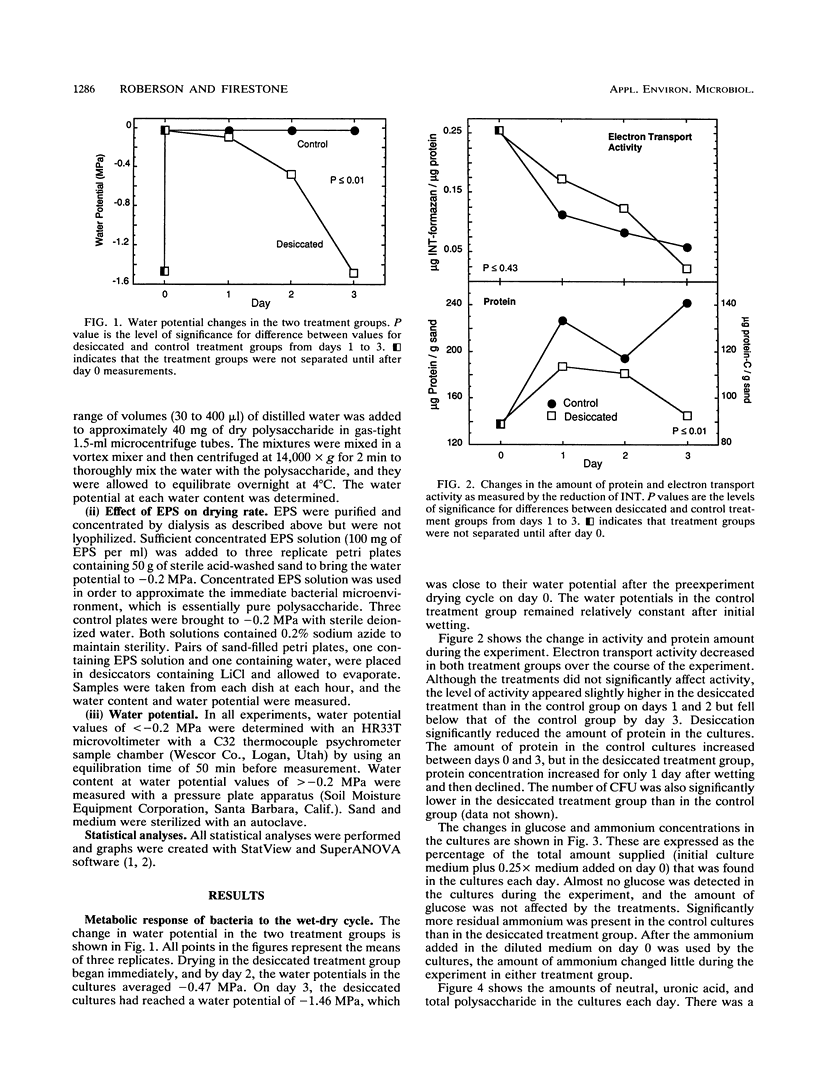
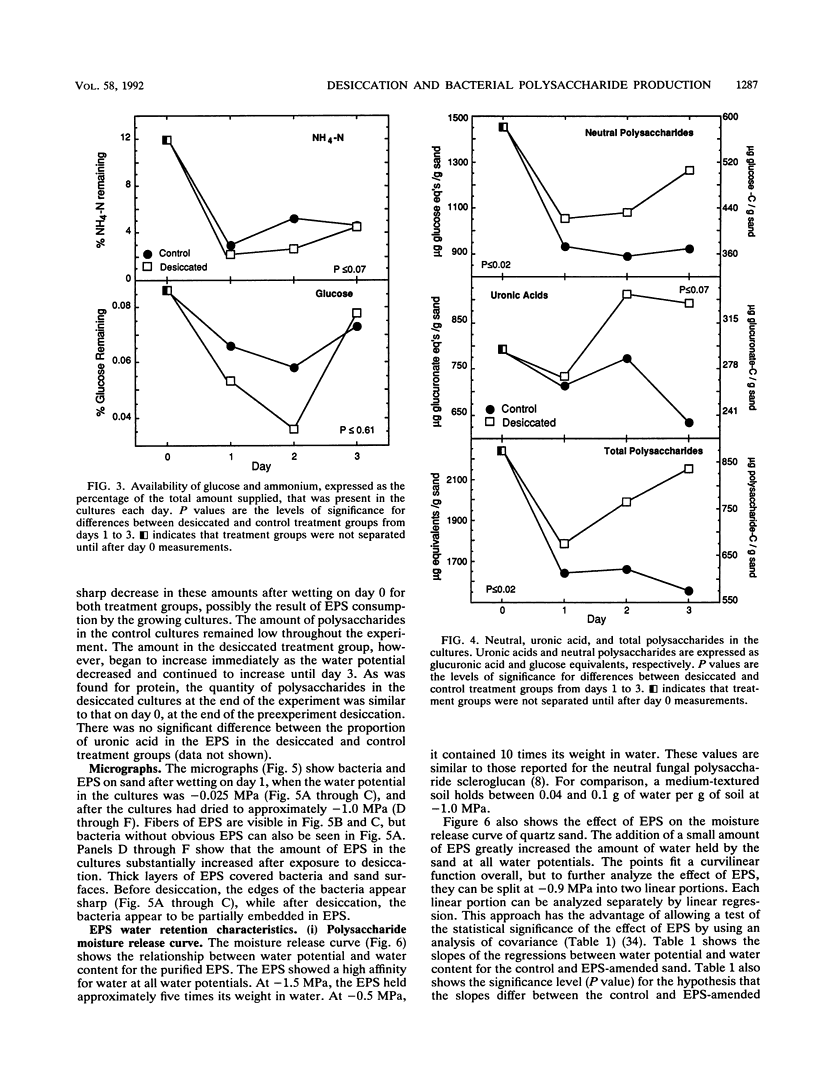
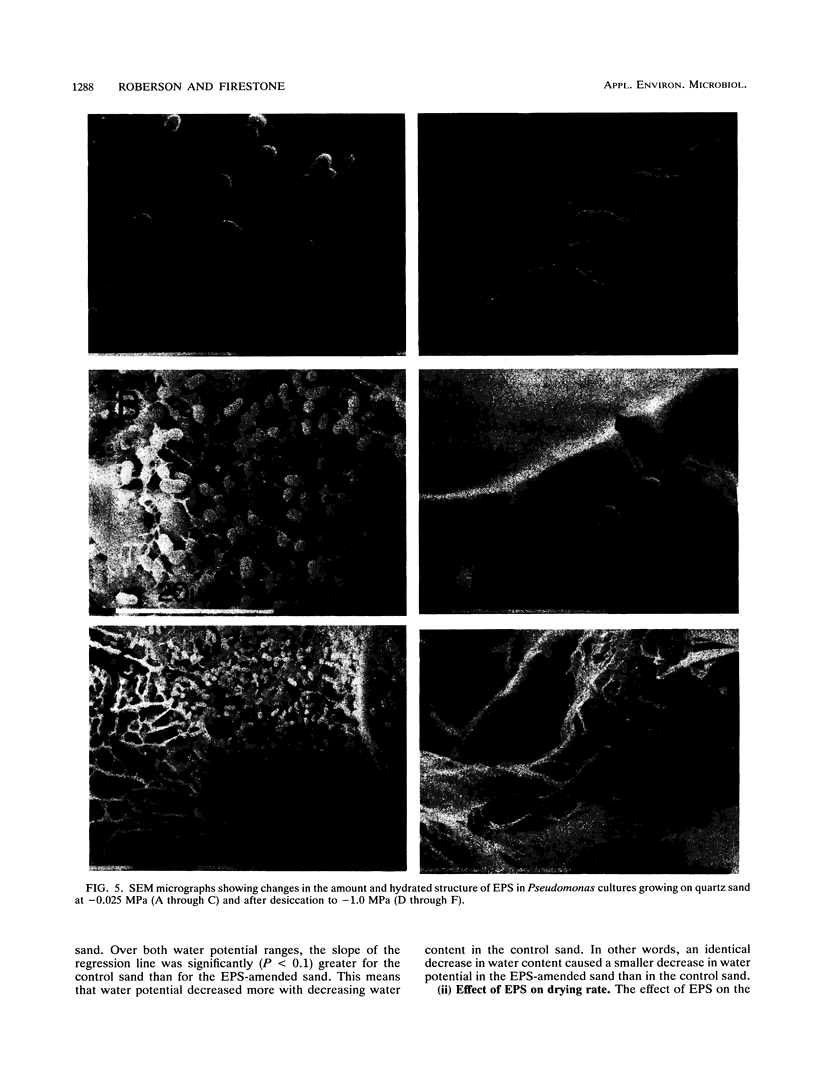
The relationship between desiccation and the production of extracellular polysaccharides (EPS) by soil bacteria was investigated by using a Pseudomonas species isolated from soil. Cultures subjected to desiccation while growing in a sand matrix contained more EPS and less protein than those growing at high water potential, suggesting that resources were allocated to EPS production in response to desiccation. Desiccation did not have a significant effect on activity as measured by reduction of iodonitrotetrazolium. Purified EPS produced by the Pseudomonas culture contained several times its weight in water at low water potential. Sand amended with EPS held significantly more water and dried significantly more slowly than unamended sand, implying that an EPS matrix may buffer bacterial colonies from some effects of desiccation. We conclude that bacteria may use EPS production to alter their microenvironment to enhance survival of desiccation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Carpenter J. F., Aurell Wistrom C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987 Feb 15;242(1):1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. C. Polysaccharides in soil fabrics. Science. 1981 Nov 6;214(4521):665–667. doi: 10.1126/science.214.4521.665. [DOI] [PubMed] [Google Scholar]
- Hershkovitz N., Oren A., Cohen Y. Accumulation of trehalose and sucrose in cyanobacteria exposed to matric water stress. Appl Environ Microbiol. 1991 Mar;57(3):645–648. doi: 10.1128/aem.57.3.645-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killham K., Firestone M. K. Salt stress control of intracellular solutes in streptomycetes indigenous to saline soils. Appl Environ Microbiol. 1984 Feb;47(2):301–306. doi: 10.1128/aem.47.2.301-306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measures J. C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature. 1975 Oct 2;257(5525):398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- Osa-Afiana L. O., Alexander M. Differences among cowpea rhizobia in tolerance to high temperature and desiccation in soil. Appl Environ Microbiol. 1982 Feb;43(2):435–439. doi: 10.1128/aem.43.2.435-439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON J. F. The extracellualr polysaccharides of bacteria. Bacteriol Rev. 1958 Mar;22(1):46–73. doi: 10.1128/br.22.1.46-73.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe H. H. Matric potential of several plant tissues and biocolloids. Plant Physiol. 1966 Nov;41(9):1439–1442. doi: 10.1104/pp.41.9.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]