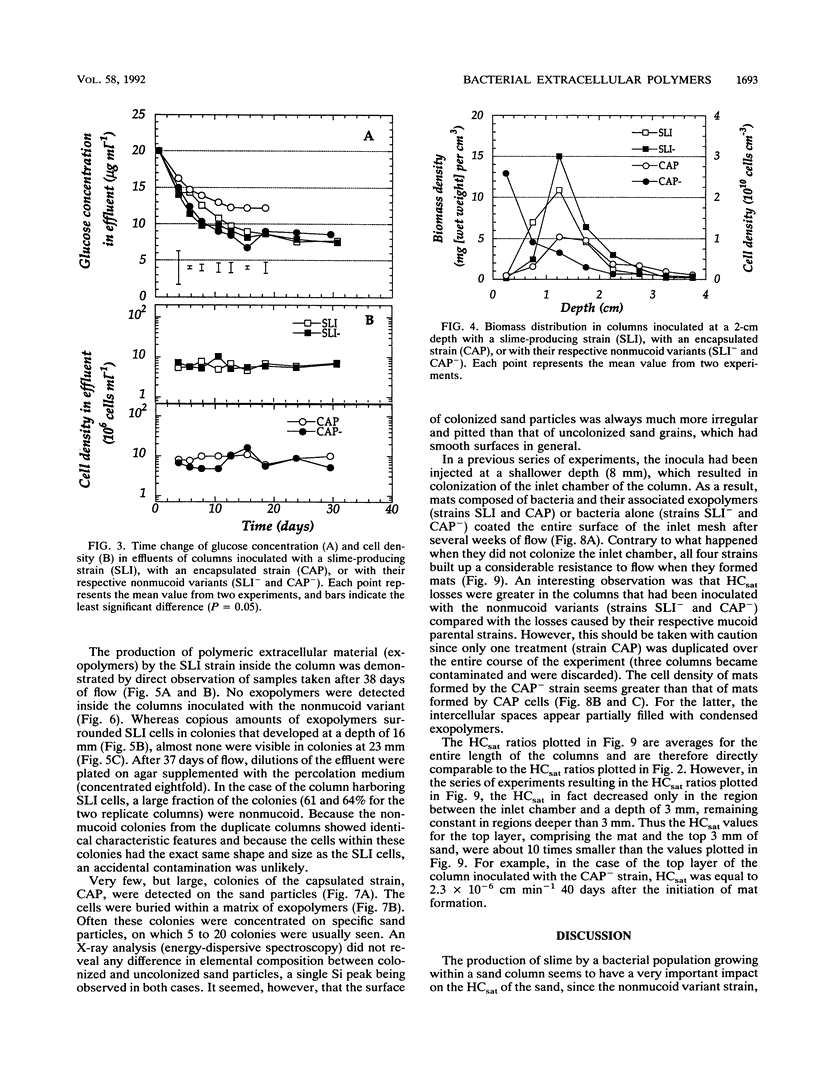
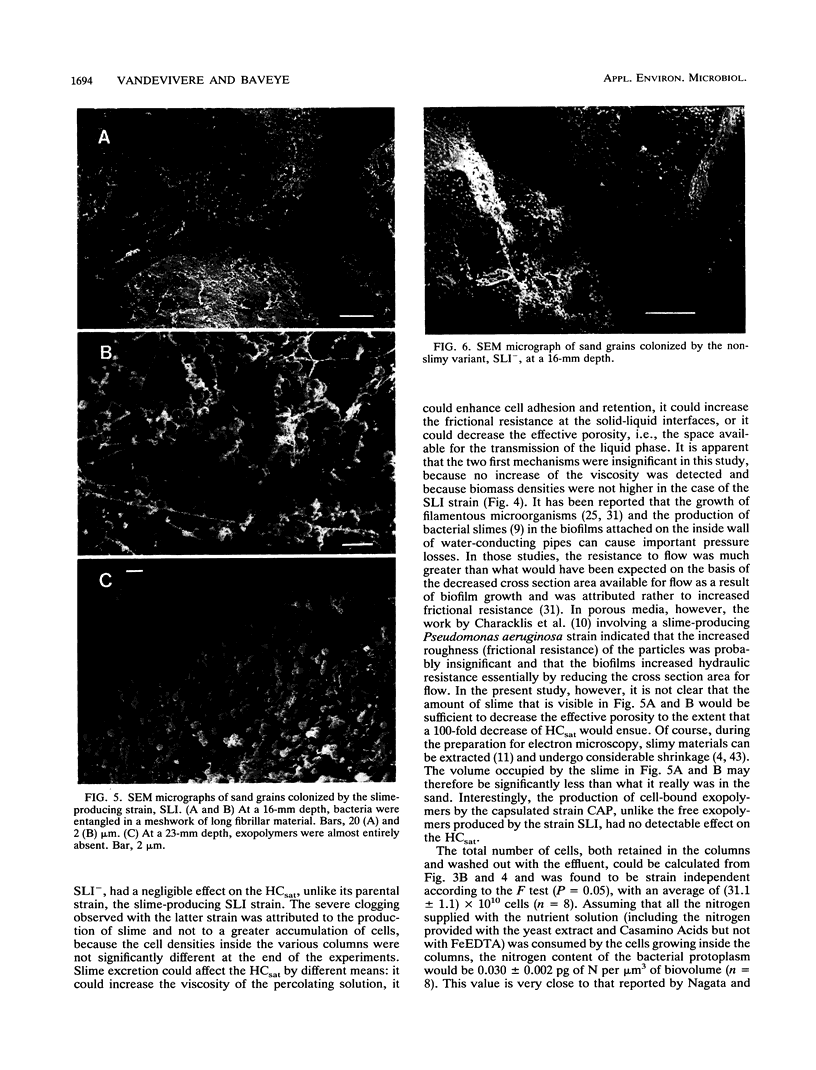
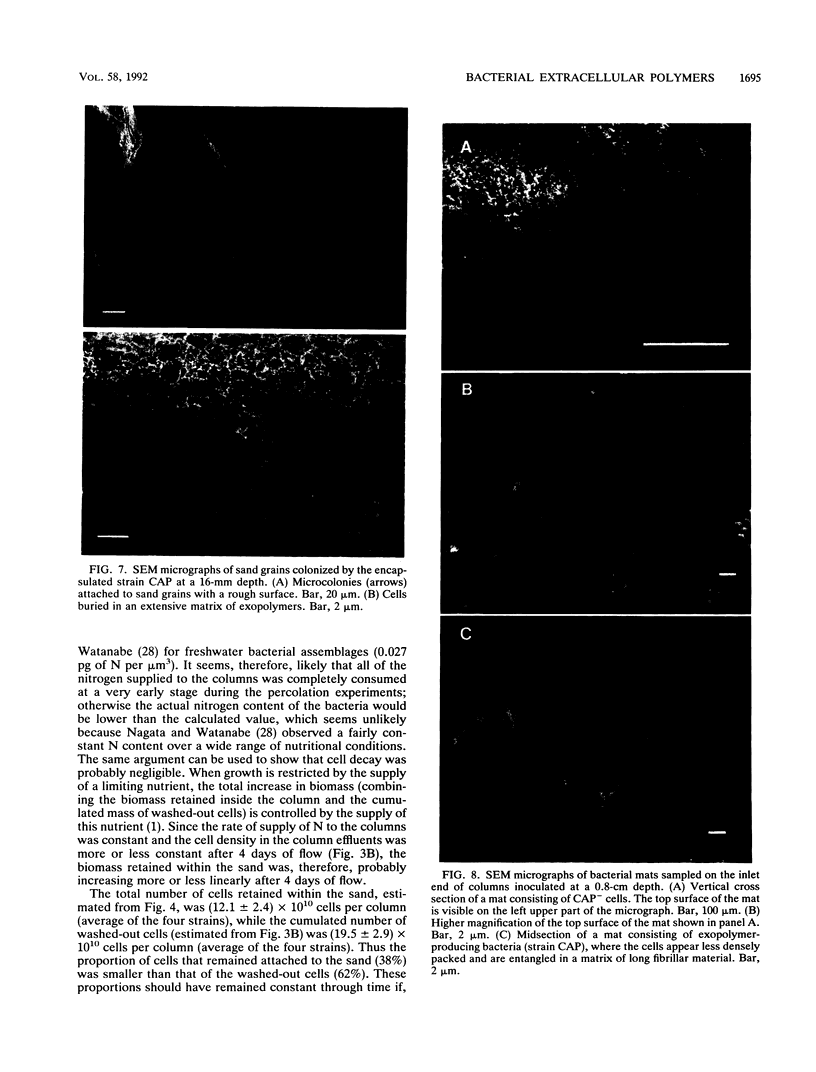
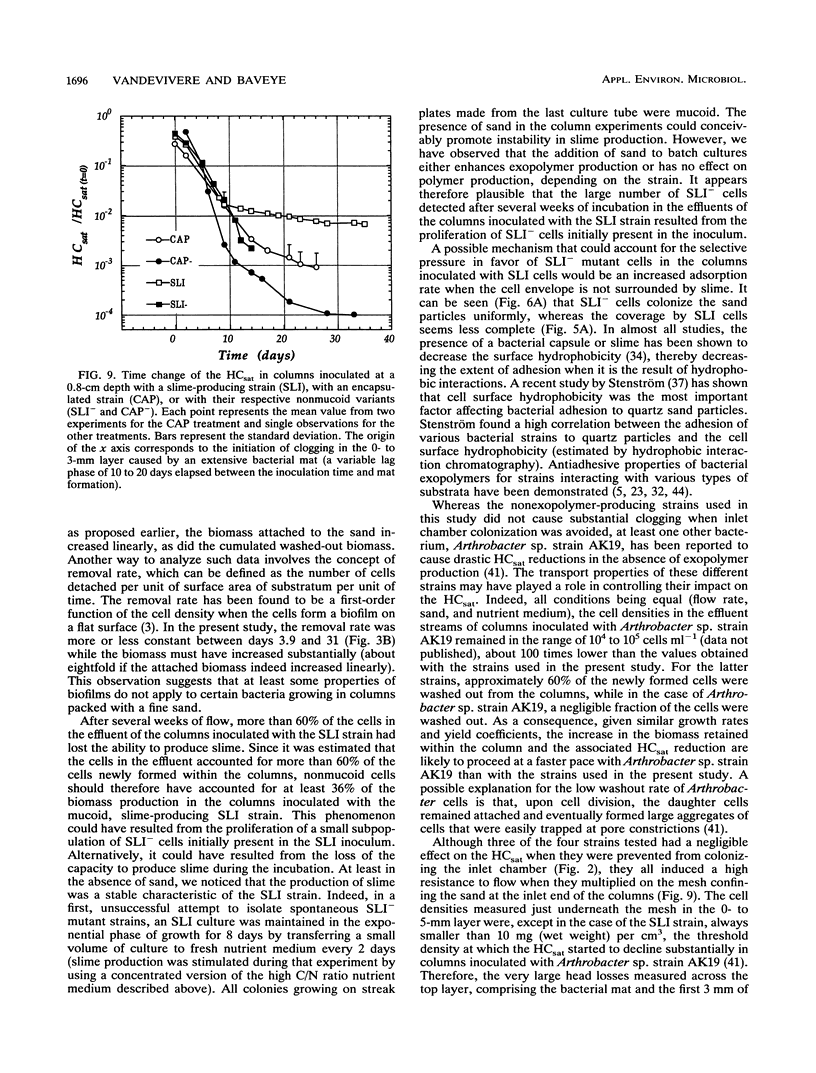
Abstract
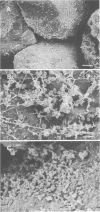
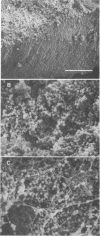
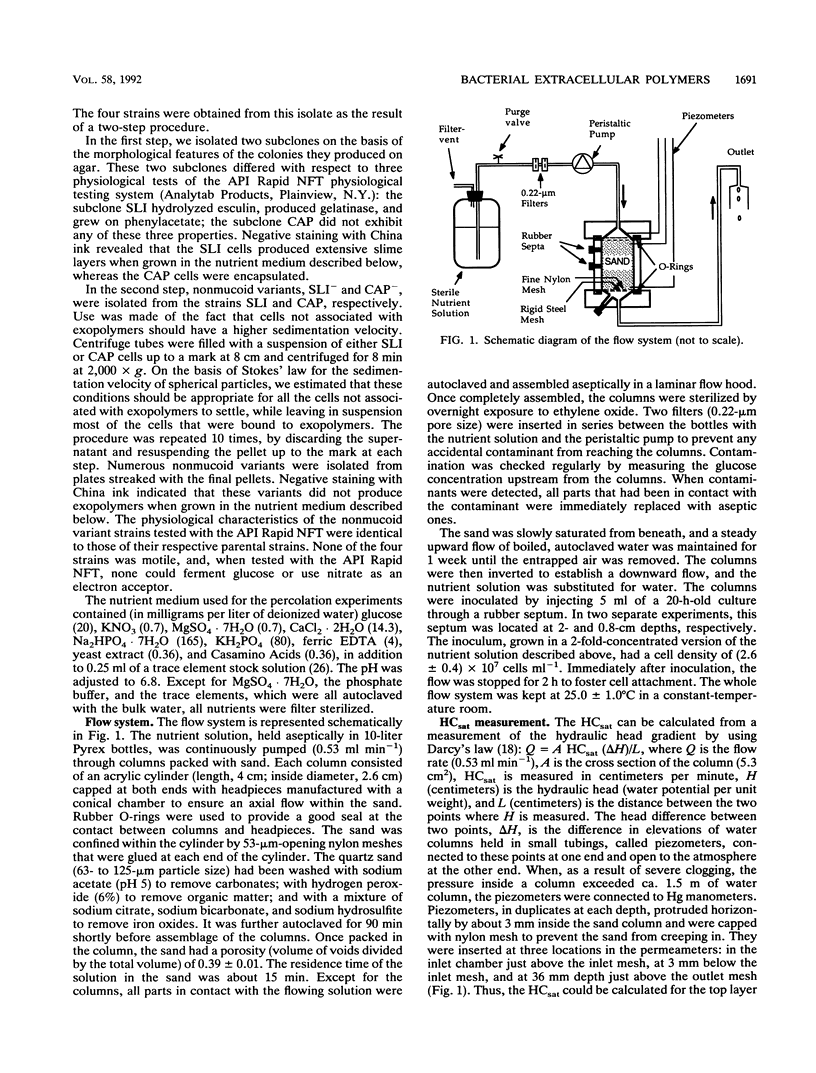
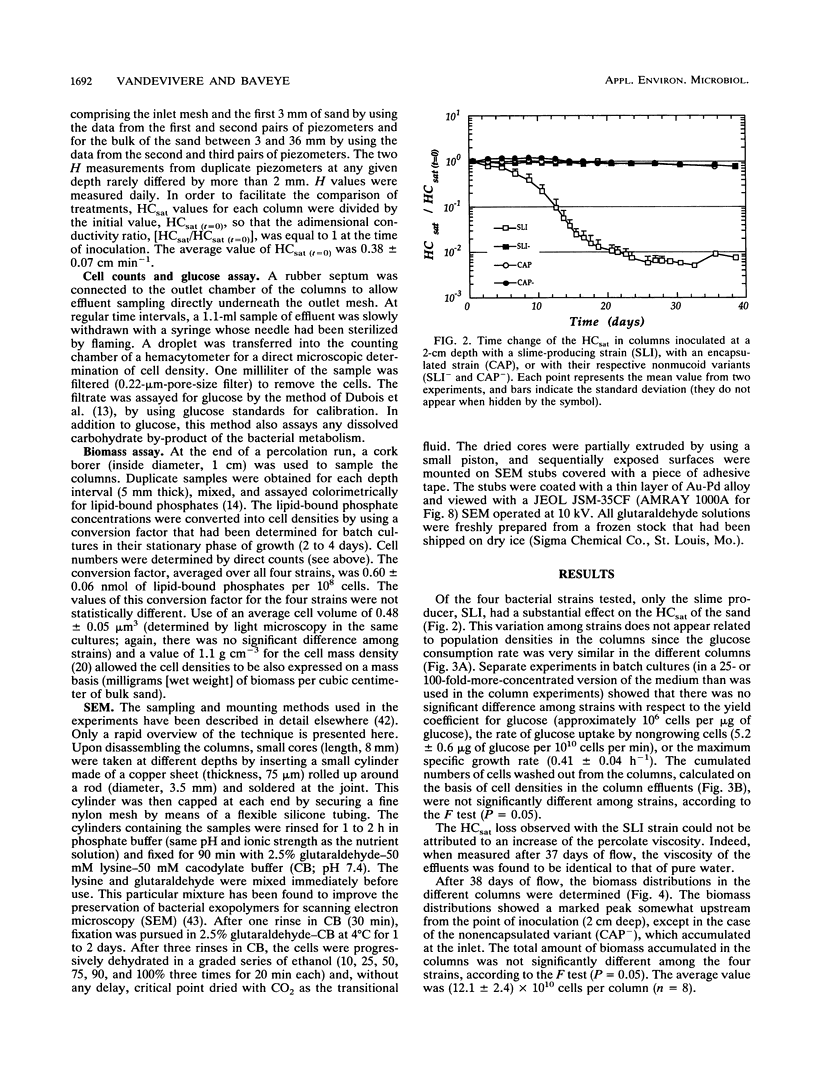
Columns were packed with clean quartz sand, sterilized, and inoculated with different strains of bacteria, which multiplied within the sand at the expense of a continuous supply of fresh nutrient medium. The saturated hydraulic conductivity (HCsat) of the sand was monitored over time. Among the four bacterial strains tested, one formed a capsule, one produced slime layers, and two did not produce any detectable exopolymers. The last two strains were nonmucoid variants of the first two. Only one strain, the slime producer, had a large impact on the HCsat. The production of exopolymers had no effect on either cell multiplication within or movement through the sand columns. Therefore, the HCsat reduction observed with the slime producer was tentatively attributed to the obstruction of flow channels with slime. Compared with the results with Arthrobacter sp. strain AK19 used in a previous study, there was a 100-fold increase in detachment from the solid substratum and movement through the sand of the strains used in this study. All strains induced severe clogging when they colonized the inlet chamber of the columns. Under these conditions, the inlet end was covered by a confluent mat with an extremely low HCsat.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks M. K., Bryers J. D. Bacterial species dominance within a binary culture biofilm. Appl Environ Microbiol. 1991 Jul;57(7):1974–1979. doi: 10.1128/aem.57.7.1974-1979.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Carlemalm E., Kellenberger E. Capsule of Escherichia coli K29: ultrastructural preservation and immunoelectron microscopy. J Bacteriol. 1985 Jun;162(3):985–991. doi: 10.1128/jb.162.3.985-991.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle G. D. Fine structure and distribution of extracellular polymer surrounding selected aerobic bacteria. Can J Microbiol. 1975 Mar;21(3):395–408. doi: 10.1139/m75-055. [DOI] [PubMed] [Google Scholar]
- Findlay R. H., King G. M., Watling L. Efficacy of phospholipid analysis in determining microbial biomass in sediments. Appl Environ Microbiol. 1989 Nov;55(11):2888–2893. doi: 10.1128/aem.55.11.2888-2893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL R., NEVO Z. EFFECT OF BACTERIAL POLYSACCHARIDE ACCUMULATION ON INFILTRATION OF WATER THROUGH SAND. Appl Microbiol. 1964 May;12:219–223. doi: 10.1128/am.12.3.219-223.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy W. F., Bryers J. D., Robbins J., Costerton J. W. Observations of fouling biofilm formation. Can J Microbiol. 1981 Sep;27(9):910–917. doi: 10.1139/m81-143. [DOI] [PubMed] [Google Scholar]
- McLee A. G., Kormendy A. C., Wayman M. Isolation and characterization of n-butane-utilizing microorganisms. Can J Microbiol. 1972 Aug;18(8):1191–1195. doi: 10.1139/m72-186. [DOI] [PubMed] [Google Scholar]
- Nagata T., Watanabe Y. Carbon- and Nitrogen-to-Volume Ratios of Bacterioplankton Grown under Different Nutritional Conditions. Appl Environ Microbiol. 1990 May;56(5):1303–1309. doi: 10.1128/aem.56.5.1303-1309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow S., Egorin M. J., Hahn D., Markus S., Leroy A., Chang P., Klein M., Bachur N. R., Wiernik P. H. Cis-Dichlorodiammine platinum and adriamycin therapy for advanced gynecological and genitourinary neoplasms. Cancer. 1980 Oct 15;46(8):1715–1721. doi: 10.1002/1097-0142(19801015)46:8<1715::aid-cncr2820460802>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Shaw J. C., Bramhill B., Wardlaw N. C., Costerton J. W. Bacterial fouling in a model core system. Appl Environ Microbiol. 1985 Mar;49(3):693–701. doi: 10.1128/aem.49.3.693-701.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenström T. A. Bacterial hydrophobicity, an overall parameter for the measurement of adhesion potential to soil particles. Appl Environ Microbiol. 1989 Jan;55(1):142–147. doi: 10.1128/aem.55.1.142-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C. Bacterial extracellular polysaccharides. Can J Microbiol. 1988 Apr;34(4):415–420. doi: 10.1139/m88-073. [DOI] [PubMed] [Google Scholar]