Abstract
Immunological memory is a key characteristic of specific immune responses. Persistence of increased levels of precursor T cells is antigen-independent and is often used as an indicator of T cell memory. This study documents that, depending on the chosen readout, cytotoxic T lymphocyte (CTL) memory against lymphocytic choriomeningitis virus (LCMV) appears long- or short-lived in the absence of persisting antigen. To study T cell memory in the absence of persisting antigen, either short-lived antigens were used for immunization or adoptive transfer methods were used to eliminate possibly persisting antigen. These experiments revealed that increased specific precursor frequencies and CTL-mediated protection against an i.v. infection with LCMV were long-lived. In contrast, CTL-mediated protection against a peripheral infection of the skin with LCMV, or of the ovary with recombinant vaccinia virus, was short-lived. These results show that maintenance of increased specific CTL precursor frequencies and central T cell memory in lymphoid tissue (where preexisting neutralizing antibodies usually provide protection anyway) is long-lived and antigen-independent. In contrast, in protection against peripheral viral infections, where the relative kinetics of virus growth and virus elimination by T cells are of key importance, T cell memory is short-lived in the absence of antigen. This indicates that peripheral T cell memory in antibody-inaccessible tissues is mediated by antigen-activated effector T cells and apparently not by specialized memory T cells.
The immune system remembers previous antigen experience. This immunological memory is a hallmark of the immune system and is most often mediated by antibodies, particularly against epidemiologically important childhood infections against which vaccines have been successfully developed. The question whether “memory” is a special quality of memory cells or whether it is maintained by antigen that activates specific B and T cells at low levels is being debated. The problem may be illustrated by immunity against pox viruses after immunization with vaccinia virus. Early studies reported by von Pirquet (1) had shown that in vaccinated children, protection against vaccinia lesions after revaccination disappeared after 4 weeks. Although protection against such peripheral challenge infection was rather short-lived, these children presumably were protected against general vaccinosis or encephalitis and smallpox. The latter diseases all depend on hematogenic spread of virus, which is essentially stopped by neutralizing antibodies after vaccination.
Antibody-dependent immunological memory requires increased levels of neutralizing antibodies. The protective antibodies are constantly produced by B cells stimulated by antigen–antibody complexes on follicular dendritic cells (FDCs) in germinal centers (2, 3); the stimulated B cells migrate to the bone marrow, where most of the antibody is produced (4–6). Without persisting antigen, protective B cell memory disappears despite presence of memory B cells as demonstrated by adoptive transfer experiments (7).
The key importance of memory B cells and elevated antibody titers is strongly substantiated by their role in protecting newborn offspring during the phase of physiological immunodeficiency. Due to major histocompatibility complex restriction and the ensuing potential danger of alloreactivity between mother and fetus, the transplacental exchange of T cells between the two is minimized; complex barriers exist, and T cell development is delayed in the fetus. This prevents transmission of T cell memory from mother to offspring, and protection of neonates is therefore solely mediated by antibody transferred via placenta and/or milk (8, 9). To maintain this elevated level of antibodies ready for transmission, B cells need to be activated by persisting or periodically encountered antigen (3).
This evidence suggests that antibodies play a key role in providing a survival advantage for the species by protecting against common infectious diseases during the period of physiological immunodeficiency early in life. In contrast, T cell memory may therefore reflect an ongoing low-level T cell response that probably is not critical for protection against cytopathic viruses. Rather, and this study provides supporting evidence, this low-level T cell response may keep persistent noncytopathic or poorly cytopathic viruses such as hepatitis B virus and HIV under control to prevent immunopathological disease in the individual (10).
How is this protective T cell memory response mediated by low-level T cell responses? Is it antigen-dependent? As pointed out above, increased levels of antibody necessary for protection have been shown to be antigen-dependent. Also, the 10-year and later 5- and finally 3-year repetitions of vaccination that finally led to the eradication of smallpox in the 1970s had suggested that protection against reinfection of the skin by cell-mediated immunity was rather short-lived and antigen-dependent. This view was in conflict with evidence from several recent studies that demonstrated long-lived increased cytotoxic T cell precursors and protection against LCMV replication in spleens after i.v. challenge infection, even under conditions that excluded antigen persistence (11, 12). Supporting evidence came from experiments with sendai virus (13). As with any experimental approach, it is important whether “what can be measured” is “what should be measured.” In the context of immunological memory, the answer may be as follows. Preexisting neutralizing antibody levels are key to protect against reinfections via blood and mucosal surfaces; they may immediately neutralize the virus and therefore act much more quickly than recruitable memory cytotoxic T lymphocytes (CTLs) present at elevated frequencies. The crucial role of memory CTLs should therefore be assessed at sites that are not readily reached by protective antibodies: the brain, the skin, or other solid peripheral organs infected by pathways other than via blood or mucosal surfaces.
In a first study, we had shown that continuous activation of T cells was important to protect mice against a peripheral challenge infection with vaccinia virus and that increased levels of CTLs alone were not protective against such an infection (14). The present study used lymphocytic choriomeningitis virus-glycoprotein (LCMV-GP)-transfected fibroblast cells to induce CTL memory. This immunization protocol avoids the induction of protective neutralizing antibodies, because the relevant epitopes are not present since the GP is not properly cleaved. The cells used for immunization could not persist because they were rejected within 10–14 days (15). In addition, cells were irradiated before injection, further ensuring that LCMV-GP did not persist on the cells used for immunization. LCMV-GP expressed by the transfected fibroblast is not taken up to be processed and presented on class I molecules on host antigen-presenting cells: intact cells are required for CTL induction, and no cross-priming is detectable (16). Therefore, memory could be induced under circumstances in which direct antigen-persistence could be excluded, and protective CTL memory could be tested in the absence of neutralizing antibodies. The experiments confirm that CTL memory assessed in vitro or as protection from systemic infections (central memory) was long-lived (11–13). In addition, they establish that protection against peripheral infection (peripheral memory) was short-lived in the absence of antigen. Collectively, these results document that, as for protection by elevated antibody levels, protection mediated by CTL memory against peripheral infections by noncytopathic viruses causing immunopathological disease requires persistence of antigens that keep few CTLs activated.
MATERIALS AND METHODS
Mice.
C57BL/6 (H-2b) mice were obtained from the breeding colony of the Institut für Zuchthygiene (Zürich). B cell-deficient mice have been described (17) and were generously provided by K. Rajewsky (University of Cologne, Cologne, Germany). Mice were kept in a conventional mouse house facility.
Detection of Virus-Specific Cytotoxic T Cells in Vitro.
Mice were immunized, and spleen cell suspensions were prepared at the indicated time points and restimulated for 5 days with peptide-pulsed [amino acids 33–41 derived from the LCMV-GP for LCMV (18) and amino acids 49–62 derived from the vesicular stomatitis virus-nucleoprotein (VSV-N) for VSV (19)] irradiated (25 Gy) spleen cells (106 cells per well) at a density of 4 × 106 spleen cells in 2 ml of Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% fetal calf serum. Restimulated spleen cells were resuspended in 0.5 ml of medium per culture well, and serial 3-fold dilutions of effectors were performed (referred to as dilution of standard culture) and tested in a conventional 51Cr release assay, using peptide-pulsed EL-4 cells as targets. Limiting dilutions were performed as described (20).
Determination of Virus Titers.
Titers of LCMV in spleen were determined as described (21). Footpads were homogenized with pestles and sand, and viral titers were determined as described (21). Titers of vaccinia virus in ovaries were determined as described (22).
RESULTS
Induction of Long-Lived Increased Cytotoxic T cell precursor (CTLp) Frequencies After Immunization with MC57 Cells Expressing LCMV-GP (GP2.9).
GP2.9 cells have been described previously to induce LCMV-GP-specific CTLs, but not neutralizing antibodies (16, 23). These GP2.9 cells have been shown to induce CTLs directly without the involvement of host antigen-presenting cells (16).
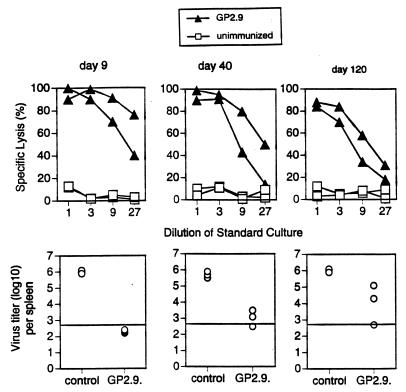
C57BL/6 mice were immunized with GP2.9 cells [3 × 106 irradiated (25 Gy) cells given i.p.], and spleen cells were restimulated with specific peptide after 9, 40, or 120 days and tested in a conventional 51Cr release assay (Fig. 1). No significant decrease in cell lysis was measurable up to 120 days after immunization. This demonstrated the presence of long-lived memory CTLp after immunization with GP2.9 cells and confirms previous findings by several groups (11–13). To assess protective LCMV-specific memory CTLs, mice were challenged i.v. with LCMV [strain WE, 200 plaque-forming units (pfu)], and virus titers were determined in the spleen 5 days later. Protection against LCMV replication in the spleen strictly correlated with the presence of CD8+ memory CTLs. Mice were well protected from LCMV replication in the spleen even 120 days after priming (Fig. 1). Thus, GP2.9 cells induced long-lived increased memory CTLp frequencies that protected against systemic infection via blood with the noncytopathic LCMV. These results are fully compatible with those of Lau et al. (11).
Figure 1.
Induction of long-lived increased CTLp frequencies with GP2.9 cells. Mice were immunized i.p. with irradiated (2500 cGy) GP2.9 cells (3 × 106 cells) (solid triangles) or left untreated (open squares). At the indicated time points, spleen cells were restimulated for 5 days in vitro and tested in a conventional 51Cr release assay on EL-4 cells pulsed with the relevant peptide (Upper). Alternatively, mice were challenged i.v. with LCMV (strain WE, 200 pfu), and virus titers in spleens were determined 5 days later (Lower). The horizontal line indicates the detection limit. The number of points below the line indicates the number of mice with no detectable virus. One representative experiment of two is shown.
Are Viral Proteins Persisting on FDCs Necessary for the Maintenance of CTL Memory?
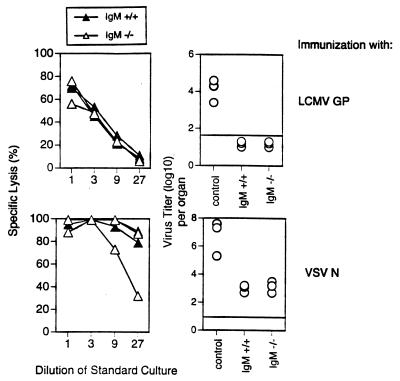
Although GP2.9 cells are not measurably processed by host antigen-presenting cells and no GP2.9-derived peptides are thus expected to persist on antigen-presenting cells, two other possibilities, regarding how GP2.9-derived antigens could persist in the host, had to be considered. (i) GP2.9 cells could persist in the host as intact cells, or (ii) proteins can persist as immune complexes on FDCs for a long time in the host and restimulate specific B cells (2, 5, 24, 25). Since proteins including the GP of LCMV have been shown to induce CTLs in their native form (26–28), it was conceivable that such proteins persisting as immune complexes on FDCs may be processed and restimulate memory CTLs. The first possibility seems unlikely, because irradiated (25 Gy) cells were used and because GP2.9 cells are rejected by the host unless very high doses (>5 × 106 cells) of proliferating cells are injected (ref. 29 and unpublished observations). Earlier studies had excluded that in LCMV-infected mice, antibodies retaining antigen on FDCs were crucial to maintain CTL memory (30, 31). To evaluate a possible influence of protein-immune complexes persisting on FDCs under conditions in which persistence of live virus itself is excluded, IgM-deficient mice (17) were used to assess CTL memory using recombinant viral proteins for immunization. We have shown previously that i.v. injection of 10 μg of recombinant LCMV-GP or the N of VSV induces a strong CD8+ CTL response that was as long-lived as after viral infection (26). IgM-deficient and control mice were therefore immunized i.v. with LCMV-GP protein or VSV-N protein (10 μg). Eight to 12 weeks later, spleen cells were restimulated in vitro with specific peptide and tested in a 51Cr release assay in vitro. Spleen cells from IgM −/− and control mice similarly lysed target cells (Fig. 2). Note, however, that in some cases, restimulation of CTLs from IgM −/− mice was rather difficult for technical reasons, because these mice exhibited very small spleens, containing only about 107 lymphocytes. As additional readouts for memory, two antiviral protection assays were performed. LCMV-GP protein-primed mice (8 weeks earlier) were challenged i.v. with LCMV (200 pfu), and 5 days later LCMV titers were determined in the spleen. Both IgM-deficient and control mice were protected against LCMV replication. VSV-primed mice are protected from replication of a recombinant vaccinia virus expressing the VSV-N for several months after immunization (14). This protection against recombinant vaccinia virus expressing VSV N replication has been shown to correlate strictly with the presence of CD8+ memory CTLs in H-2b mice. Neither CD4+ T cells nor antibodies play a measurable role (22, 26). VSV-N protein-primed mice (8 weeks earlier) were challenged i.p. with a recombinant vaccinia virus expressing VSV N (5 × 106 pfu). Five days later, ovaries were removed and virus titers were determined. IgM-deficient mice were protected as efficiently as control mice. Thus, viral proteins persisting on FDCs are not necessary for the maintenance of elevated CTLp or for protective CTL-mediated immunity. These results confirm three recent reports (30–32) and extend them to experimental conditions in which antigen-persistence in any form of live virus is excluded.
Figure 2.
Antigen persisting as immune complexes on FDCs are not necessary to maintain CTL memory. Mice deficient for IgM expression (−/−, solid triangles) that cannot produce antibody and control mice (+/+, open triangles) were immunized with 10 μg of recombinant LCMV-GP protein (Upper) or 10 μg of recombinant VSV-N protein (Lower). Eight to 12 weeks later, spleen cells were restimulated in vitro with the relevant peptides and tested 5 days later in a conventional 51Cr release assay on peptide-pulsed EL-4 cells. Alternatively, LCMV-GP-primed mice were challenged with LCMV (200 pfu), and virus titers were determined in the spleen 5 days later. VSV-N primed mice were challenged i.p. with recombinant vaccinia virus expressing VSV-N (5 × 106 pfu), and virus titers were determined 5 days later in ovaries. The horizontal line indicates the detection limit. One representative experiment of three is shown.
Life-Span of Antivirally Protective CTL Memory in the Absence of Persisting Antigen: Assessment by Protection Against i.v. Challenge Infection with LCMV.
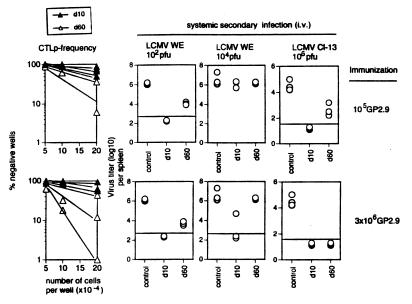
To measure the life-span of CTL memory in assays revealing antiviral protection, C57BL/6 mice were immunized with irradiated GP2.9 cells (3 × 106 cells or 105 cells), and 10 or 60 days later, specific CTLp frequencies were assessed in some mice by limiting dilution, and other mice were challenged i.v. with LCMV. Limiting-dilution analysis revealed that CTLp frequencies had not dropped over the 2-month period (Fig. 3). Mice challenged with a low dose of LCMV WE (200 pfu) were largely protected from LCMV replication in the spleen when titers were assessed 5 days after challenge (Figs. 1 and 3). Similarly, mice that were challenged with a very high dose of LCMV Cl-13 (106 pfu), a virus strain that easily establishes persistent infections, were protected if virus titers were assessed 10 days after challenge (Fig. 3), fully supporting findings by Lau et al. (11). It is noteworthy that, if mice were challenged with an intermediate dose of LCMV WE (104 pfu) and virus titers were assessed already 3 days after challenge infection, only mice primed with a high dose of GP2.9 cells 10 days, but not 60 days, earlier were protected (Fig. 3). Mice primed with the limiting dose of 105 GP2.9 cells were not fully protected also at the earlier time point.
Figure 3.
Antigen dependence of protective CTL memory varies with the readout: i.v. challenge infection with different virus doses. Mice were immunized with irradiated (25 Gy) GP2.9 cells (3 × 106 or 105 cells) and frequencies of LCMV-GP-specific CTLp were determined 10 (solid triangles) or 60 days (open triangles) later. Alternatively, mice were challenged i.v. with 200 pfu of LCMV WE, and virus titers were assessed 5 days later, or they were challenged with 104 pfu of LCMV WE and virus titers were assessed 3 days later. Additional groups of mice were challenged with 106 pfu of LCMV Cl-13, and virus titers were assessed in the blood 10 days later. The horizontal line indicates the detection limit. One representative experiment of two is shown.
Life-Span of Antivirally Protective CTL Memory in the Absence of Persisting Antigen: Assessment by Protection Against Peripheral Challenge Infections with LCMV or Vaccinia Virus.
Within the same experimental setup as described in Fig. 3, mice primed with GP2.9 cells were challenged peripherally, either with LCMV or a recombinant vaccinia virus expressing LCMV GP (vacc-GP). LCMV WE (200 pfu) was injected into the footpads, and virus titers in footpads were determined 7 days later. Protection against virus growth was rather limited, and no clear difference could be seen between the early and late time points after priming (Table 1). If virus titers were determined 5 days after challenge, no protection at all could be seen (not shown). Alternatively, mice were challenged intracranially (i.c.) with 300 pfu of LCMV, and survival was monitored. Only mice primed with a high cell dose were used in the experiment, because it had been shown previously that vaccination with low cell doses did not confer protection against i.c. challenge (23). Mice challenged i.c. on day 10 after immunization were all protected, whereas mice challenged on day 60 after immunization were not protected (Table 1). Similarly, mice challenged i.p. with vacc-GP exhibited reduced viral titers in ovaries if challenged early, but not late, after immunization.
Table 1.
Peripheral CTL-mediated antiviral protection is short-lived in the absence of antigen
| Challenge infection | Immunization* | Virus titer/organ (log10 ± SEM) in time of challenge infection after immunization
|
No. of surviving mice
|
||
|---|---|---|---|---|---|
| Day 10 | Day 60 | Day 10 | Day 60 | ||
| LCMV i.f. | 3 × 106 GP2.9 | 2.0 ± 0.1 | 2.1 ± 0.3 | ||
| 1 × 105 GP2.9 | 2.1 ± 0.2 | 2.5 ± 0.1 | |||
| 3 × 106 MC57 | 3.8 ± 0.2 | 3.7 ± 0.1 | |||
| vacc-GP i.p. | 3 × 106 GP2.9 | 1.1 ± 0.1 | 5.9 ± 0.3 | ||
| 1 × 105 GP2.9 | 4.0 ± 0.2 | 5.2 ± 0.1 | |||
| 3 × 106 MC57 | 6.1 ± 0.1 | 6.0 ± 0.1 | |||
| LCMV i.c. | 3 × 106 GP2.9 | 6/6 | 2/6 | ||
| 3 × 106 MC57 | 0/6 | 0/6 | |||
Mice of the same groups as in Fig. 3 were challenged into the footpad (i.f.) with LCMV WE (30 pfu), and 7 days later virus titers were determined in the footpad (LCMV WE i.f.). Alternatively, mice were challenged i.p. with recombinant vaccinia virus expressing LCMV-GP, and virus titers were assessed 5 days later in ovaries (vacc-GP i.p.). Alternatively, mice primed with a high cell dose (6 per group) were challenged i.c. with LCMV WE (30 pfu), and survival was monitored (LCMV WE i.c.).
Life-Span of CTL Memory After Adoptive Transfer.
The classical way to eliminate persisting antigen is the adoptive transfer experiment (33). Therefore, we compared our results obtained with GP2.9 cell-primed hosts with the protective capacity of CTLp transferred into antigen-free recipients. Mice were immunized with LCMV, and 100 days later spleen cells were adoptively transferred into normal recipients (3 × 107 cells per recipient). To avoid unspecific activation of transferred cells, recipient mice were not irradiated (see below). One or 45 days later, CTLp were assessed by limiting dilution, and antiviral protective memory was assessed by challenging recipient mice with either LCMV i.v. (200 pfu) or vacc-GP i.p. (5 × 106 pfu) (Table 2). Similar to the results with GP2.9 cells, CTLp frequencies did not change significantly after transfer, and protection against i.v. challenge with LCMV was long-lived. In contrast, protection against peripheral infection with vacc-GP was short-lived. A long life-span of transferred CTLp was not due to accidentally cotransferred antigen, because the same results were obtained with magnetic bead-purified CD8+ T cells (not shown), as had been shown previously by others (11, 13).
Table 2.
Transfer of primed T cells: Kinetics of CTLp and of protection depended on time after transfer, route of infection, and irradiation of recipient
| Treatment of recipient* | Time after transfer† | CTLp | Virus titer (log10 )
|
Survival LCMV i.c.¶ | |
|---|---|---|---|---|---|
| LCMV i.v.‡ | vacc-GP i.p.§ | ||||
| – | Control | <1:106 | 5.8 ± 0.1 | 5.9 ± 0.2 | 0/3 |
| – | d + 1 | 1:4 × 104 | <2.3 | 1.1 ± 1.0 | 0/3 |
| – | d + 45 | 1:3 × 104 | <2.3 | 5.8 ± 0.3 | 0/3 |
| Irradiated | d + 45 | 1:3 × 104 | <2.3 | <1 | 3/3 |
Mice were irradiated with 4.5 Gy.
Spleen cells (3 × 107) from LCMV-immune mice (day 100) were injected i.v.; control mice received spleen cells from unimmunized mice.
Mice were challenged i.v. with 200 pfu LCMV WE.
Mice were challenged i.p. with 5 × 106 pfu vacc-GP.
Mice were challenged i.c. with 30 pfu LCMV WE.
An Analysis of the Influence of Irradiating the Recipient Mice on T-Cell Memory.
It has been reported that transfer of T cells into irradiated recipients leads to nonspecific activation of the transferred spleen cells (14, 34, 35). To reveal whether this may influence the life-span of protective CTL memory upon transfer, the adoptive transfer experiments were repeated using irradiated recipients (4.5 Gy). Forty-five days after transfer, CTLp frequencies were assessed (Table 2). No significant difference between irradiated and nonirradiated mice could be seen. Also, both groups were equally well-protected against an i.v. challenge infection with LCMV when virus titers were assessed 5 days after the challenge in the spleen. However, if mice were challenged i.c. at different time points after transfer, a gradual increase in level of protection could be observed in the group of irradiated mice; 25 days after transfer, one mouse of three was protected (not shown), whereas 45 days after transfer, three of three mice in the irradiated group were protected, and none of the nonirradiated recipient mice were protected (Table 2). Finally, irradiated, but not normal, recipient mice were fully protected from replication of vacc-GP in ovaries after peripheral infections. These results indicate that, although the CTLp frequencies are comparable in irradiated and nonirradiated mice 45 days after adoptive transfer, memory CTLs are significantly more efficient in protecting against peripheral infections in irradiated mice than in nonirradiated recipients. This data could mean that CTLs transferred into an irradiated host gain or retain an increased state of activation. Analysis of CD44, CD69, or MEL-14 expression, however, did not reveal significant differences (not shown).
DISCUSSION
Whether CTL Memory Is Antigen Dependent or Not Has Been Widely Debated.
Evidence for both short- and long-lived CTL memory in the absence of persisting antigen has been presented. Although in a first report (14) we used mainly vaccinia recombinants or major histocompatibility complex-binding peptides to assess memory effector T cell function, this report concentrated on protection against LCMV challenge infection in mice immunized with LCMV-GP associated with transfected cells. The present results indicate that some of the controversies may be explained by the different readouts used to measure memory, i.e., assessment of increased CTLp numbers and antiviral protection in central lymphoid organs (spleen) vs. protective memory assessed in peripheral solid tissue.
Long-Lived Central vs. Short-Lived Peripheral CTL Memory.
To measure CTL memory in the absence of persisting antigen, two different experimental approaches can be used. Either the antigen used for immunization is short-lived, or, alternatively, the antigen is removed experimentally after priming. The first approach has rarely been used so far. Vaccination with short-lived peptides has documented a very short-lived protective CTL memory (14). Experimental removal of persisting antigen is usually done by adoptive transfer experiments. Both approaches have disadvantages. In particular, it is very difficult, if not impossible, to completely exclude any residual persisting antigen after immunization with a (presumably) short-lived antigen. However, the same is true, although to a considerably lesser extent, for adoptive transfer experiments, because cotransfer of antigen cannot really be excluded (in particular if CD8+ T cells themselves are postulated to be a potential source of persisting antigen). The adoptive transfer setup has a second drawback, namely, that partially unpredictable changes are introduced to the system by the transfer of purified cell populations, taken out of their natural anatomical environment and put into new recipients. In this study, we used both approaches and obtained comparable results. Although, as explained in the introduction, GP2.9 cells cannot persist functionally, they nevertheless induce long-lived increased memory CTLp frequencies. Also, protection against an i.v. challenge infection with a low dose of LCMV could be observed 3 months after priming. These results confirm previous data demonstrating that central CTL memory is long-lived in the absence of persisting antigen (11–13). Similarly, and confirming the results of Ahmed and collaborators (11), protection against an i.v. challenge infection with a high dose of LCMV was long-lived if protection was assessed 10 days after challenge. On the other hand, protection against a challenge infection with a high dose of LCMV was short-lived if assessed 3 days after challenge. The rather long-lived protective capacity against systemic infection assessed in the spleen reflects efficient activation of CTLs within 1–3 days after infection; this reaction is not sufficient to clear the virus by day 3, but will be efficient enough to eliminate a noncytopathic virus within 5–10 days. In contrast to most of these systemic infections via blood or the spleen, protection against peripheral, subcutaneous, or intracerebral infection with LCMV and protection against recombinant vaccinia virus replication in ovaries as well was generally short-lived under the various conditions tested. Comparable results were obtained when persisting antigen was removed by adoptive transfer experiments.
Comparison with Previous Findings.
The findings presented here are in agreement with several reports documenting both short- and long-lived CTL memory. Studies revealing long-lived CTL memory in the absence of persisting antigen either measured increased frequencies of CTLp in vitro to assess memory or showed protection against i.v. challenge infection. These findings are fully confirmed here. On the other hand, short-lived protective T cell memory was found if protection against infections of ovaries or the skin with vaccinia virus (1, 14, 36) or influenza virus (37) was assessed. Also, when functional T-helper cell memory accelerating Ig isotype switch from IgM to IgG was assessed after immunization with VSV, T cell memory was found to be short-lived, since it lasted for only 3 weeks (38). Similar findings are documented here after peripheral challenge infection with LCMV or recombinant vaccinia virus. The findings here with adoptive transfer studies in C57BL/6 mice contradict earlier reports documenting short-lived increased memory CTLp in the absence of persisting antigen in adoptive transfer experiments by our own group using BALB/c mice and LCMV (20), and by others using the H-Y antigen (39). In these earlier studies, antigen doses used for priming and numbers of transferred T cells may have been limiting by chance; under such circumstances, protection against the used dose for i.v. challenge with LCMV when assessed on day 4 also may have waned within 2 weeks. Such a possibility is also supported by the absence of protection 3 days after i.v. challenge infection with 104 pfu of LCMV. Alternatively, differences in endogenous viruses [as, e.g., mammary tumor viruses (40, 41)] and/or transplantation antigens between mice from different colonies may have been responsible for a rejection of donor CTLs, thereby contributing to the observed more rapid waning of T cell memory in our earlier study (20).
Influence of Irradiating Recipient Mice.
In a normal host, B- and T cell numbers are tightly regulated; it can be expected, therefore, that transfer of few T cells into a recipient largely devoid of lymphocytes due to irradiation or other general immunosuppression interferes with the life-span of the transferred T cells, because competition with endogenous T cells is poor. In addition, these conditions may cause nonspecific activation (35). Such an activation of lymphocytes may not only interfere with their life-span, but also alter their activation state. In the experiments reported here, memory CTLp as determined in vitro were the same in irradiated and nonirradiated recipients. This was also true for absolute numbers of CTLp, because overall spleen cell numbers were comparable. Nevertheless, irradiated recipients were more efficiently protected against peripheral infections than nonirradiated recipients. These data are compatible with earlier results, indicating that transferred T cells were activated in the irradiated recipient mice (14). Although it remains unclear how T cells are kept activated, it may explain why LCMV-specific memory T cells are largely pgp-1high upon transfer into irradiated recipients in the absence of antigen (11).
Conclusion.
This report documents for LCMV infection in C57BL/6 mice that increased memory CTLp frequencies are independent of persisting antigen, whereas protection against peripheral challenge infection (as a model situation for induction of persistent, secluded infection by noncytopathic virus in the host) requires the presence of persisting antigen. Therefore, the data presented here support the concept that persisting antigen keeps memory CTLs activated (8, 42); when persisting antigen wanes, CTLp lose activation and therefore cannot emigrate into solid tissue immediately after peripheral infection. The finding that some virus-specific memory CTLp lose their activation markers long after immunization is compatible with this notion (43). The duration of the “activated” or protective CTL memory, therefore, is dependent on the antigen used for immunization; this has also been found for VSV (44): VSV is cytopathic and cannot usually persistently infect mice; protective CTL memory is therefore comparably short-lived (in contrast to increased CTLp frequencies, which are long-lived). In contrast, LCMV is noncytopathic and often persists in infected mice at very low levels. Protective CTL memory therefore is more long-lived(14). An extreme case is presented in this report using GP2.9 cells; they cannot persist and therefore lead to short-lived protective CTL memory against peripheral infections. These findings are reminiscent of those by North (45) and Jungi (46), who suggested that protective T cell-mediated memory was rather short-lived against Listeria infection.
From a theoretical point of view, CTL memory has an antigen-independent aspect, fighting central infections, and an antigen-dependent aspect, responsible for protection against peripheral infections. From the practical point of view, however, the central lymphoid organs are protected by antibodies, so that T cell memory is more important for peripheral infections; such T cell memory is antigen-dependent. Because such peripheral challenge infections are rare except for transmission via animal bites through skin, the most important task of CTL memory is probably to prevent viruses that established low-level persistent infections in kidney, testes, salivary glands, and neurons from widely spreading in the host; in this way, chronic immunopathological disease is prevented. This is no different from the ongoing T cell control of granulomatous lesions due to infection by facultative intracellular bacteria, a concept originally called infection or concomitant immunity (47, 48). Thus, protective T cell memory is not really due to “memory T cells,” but rather to low levels of continuously induced and activated effector T cells.
Acknowledgments
We thank Dr. K. Rajewsky (Cologne) for generously providing B cell-deficient mice, A. Althage for excellent technical assistance, and D. Speiser, A. Oxenius, and T. Fehr for helpful discussions. This work was supported by the Swiss National Science Foundation (31-32179.91), the Human Frontier Science Program, and the Kanton Zürich.
Footnotes
Abbreviations: FDC, follicular dendritic cell; CTL, cytotoxic T lymphocyte; LCMV, lymphocytic choriomeningitis virus; GP, glycoprotein; VSV, vesicular stomatitis virus; N, nucleoprotein; CTLp, cytotoxic T cell precursors; GP2.9, MC57 cell transfected to express LCMV GP; vacc-GP, recombinant vaccinia virus expressing LCMV-GP; i.c., intracranially.
References
- 1.von Pirquet C. Von Klinischen Studien über Vakzination und Vakzinale Allergie. Leibzig: F. Denticke; 1907. [Google Scholar]
- 2.Tew J G, Phipps R P, Mandel T E. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann M F, Odermatt B, Hengartner H, Zinkernagel R M. J Exp Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benner R, Hijmans W, Haaijman J J. Clin Exp Immunol. 1981;46:1–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann M F, Kündig T M, Odermatt B, Hengartner H, Zinkernagel R M. J Immunol. 1994;153:3386–3397. [PubMed] [Google Scholar]
- 6.Slifka M K, Matloubian M, Ahmed R. J Virol. 1995;69:1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinhoff U, Müller U, Schertler A, Hengartner H, Aguet M, Zinkernagel R M. J Virol. 1995;64:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinkernagel R M, Bachmann M F, Kündig T M, Oehen S, Hengartner H. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 9.Zinkernagel R M. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 10.Chazoullieres O, Mamish D, Kim M, Carey K, Ferrell L, Roberts J P, Ascher N L, Wright T L. Lancet. 1994;343:142–146. doi: 10.1016/s0140-6736(94)90934-2. [DOI] [PubMed] [Google Scholar]
- 11.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Nature (London) 1994;369:648–652. [Google Scholar]
- 12.Müllbacher A. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou S, Hyland L, Ryan K W, Portner A, Doherty P C. Nature (London) 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 14.Kündig T M, Bachmann M F, Oehen S, Hoffmann U W, Simard J J L, Castelmur I, Pircher H, Ohashi P S, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiPaolo, C. (1990) Diplomarbeit, ETH, Zürich.
- 16.Kündig T M, Bachmann M F, DiPaolo C, Simard J, Battegay M, Lother H, Gessner A, Kühlcke K, Ohashi P S, Hengartner H, Zinkernagel R M. Science. 1995;268:1343–1347. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura D, Rajewsky K. Nature (London) 1992;356:154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- 18.Pircher H P, Moskophidis D, Rohrer U, Bürki K, Hengartner H, Zinkernagel R M. Nature (London) 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 19.van Bleek G M, Nathenson S G. Nature (London) 1990;348:213–215. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- 20.Oehen S, Waldner H P, Kündig T H, Hengartner H, Zinkernagel R M. J Exp Med. 1992;176:1273–1281. doi: 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battegay M, Cooper S, Althage A, Baenziger J, Hengartner H, Zinkernagel R M. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 22.Kündig T, Castelmur I, Bachmann M F, Abraham D, Binder D, Hengartner H, Zinkernagel R M. J Virol. 1993;67:3680–3683. doi: 10.1128/jvi.67.6.3680-3683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castelmur I, DiPaolo C, Bachmann M F, Hengartner H, Zinkernagel R M, Kündig T M. Cell Immunol. 1993;151:460–466. doi: 10.1006/cimm.1993.1254. [DOI] [PubMed] [Google Scholar]
- 24.Nossal G, Ada G L. In: Antigens, Lymphold Cells and the Immune Response. Dixon S J, Kunkel H G, editors. New York: Academic; 1971. [Google Scholar]
- 25.Bachmann M F, Kündig T M, Hengartner H, Zinkernagel R M. Eur J Immunol. 1994;24:2567–2570. doi: 10.1002/eji.1830241046. [DOI] [PubMed] [Google Scholar]
- 26.Bachmann M F, Kündig T M, Freer G, Li Y, Bishop D H, Hengartner H, Zinkernagel R M. Eur J Immunol. 1994;24:2228–2236. doi: 10.1002/eji.1830240944. [DOI] [PubMed] [Google Scholar]
- 27.Rock K L, Gamble S, Rothstein L. Science. 1990;249:918–921. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- 28.Staerz U D, Karasuyama H, Garner A M. Nature (London) 1987;329:449–451. doi: 10.1038/329449a0. [DOI] [PubMed] [Google Scholar]
- 29.Kohler M, Rüttner B, Cooper S, Hengartner H, Zinkernagel R M. Cancer Immunol Immunother. 1990;32:117–124. doi: 10.1007/BF01754208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano M S, Ahmed R. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bründler M-A, Aichele P, Bachmann M F, Kitamura D, Rajewsky K, Zinkernagel R M. Eur J Immunol. 1996;26:2257–2262. doi: 10.1002/eji.1830260943. [DOI] [PubMed] [Google Scholar]
- 32.Di Rosa F, Matzinger P. J Exp Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celada F. Prog Allergy. 1971;15:223–267. [PubMed] [Google Scholar]
- 34.Tough D F, Sprent J. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprent J, Tough D F. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 36.Fenner F. Intervirology. 1979;11:137–157. doi: 10.1159/000149027. [DOI] [PubMed] [Google Scholar]
- 37.Liang S, Mozdzanowska K, Palladino G, Gerhard W. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- 38.Roost H P, Charan S, Zinkernagel R M. Eur J Immunol. 1990;20:2547–2554. doi: 10.1002/eji.1830201204. [DOI] [PubMed] [Google Scholar]
- 39.Gray D, Matzinger P. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elder J H, Jensen F C, Bryant M L, Lerner R A. Nature (London) 1977;267:23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- 41.Acha O H, Held W, Waanders G A, Shakhov A N, Scarpellino L, Lees R K, MacDonald H R. Immunol Rev. 1993;131:5–26. doi: 10.1111/j.1600-065x.1993.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 42.Kündig T M, Bachmann M F, Ohashi P S, Pircher H, Hengartner H, Zinkernagel R M. Immunol Rev. 1996;150:63–90. doi: 10.1111/j.1600-065x.1996.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 43.Tripp R A, Hou S, Doherty P C. J Immunol. 1995;154:5870–5875. [PubMed] [Google Scholar]
- 44.Kündig T H, Althage A, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1992;89:7757–7761. doi: 10.1073/pnas.89.16.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.North R J. Cell Immunol. 1973;7:166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- 46.Jungi T W. J Reticuloendothel Soc. 1980;28:405–417. [PubMed] [Google Scholar]
- 47.Hotchin J. Cold Spring Harbor Symp Quant Biol. 1962;27:479–499. doi: 10.1101/sqb.1962.027.001.046. [DOI] [PubMed] [Google Scholar]
- 48.Mackaness G B. J Exp Med. 1969;129:973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]