Abstract
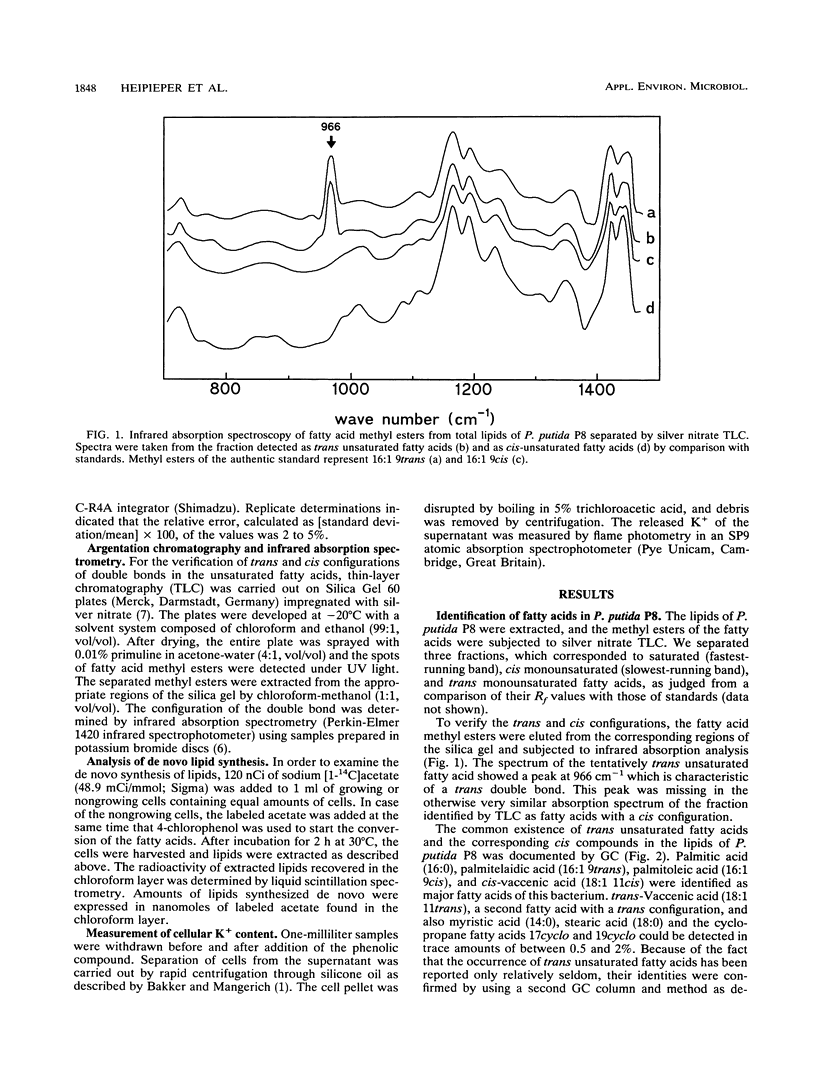
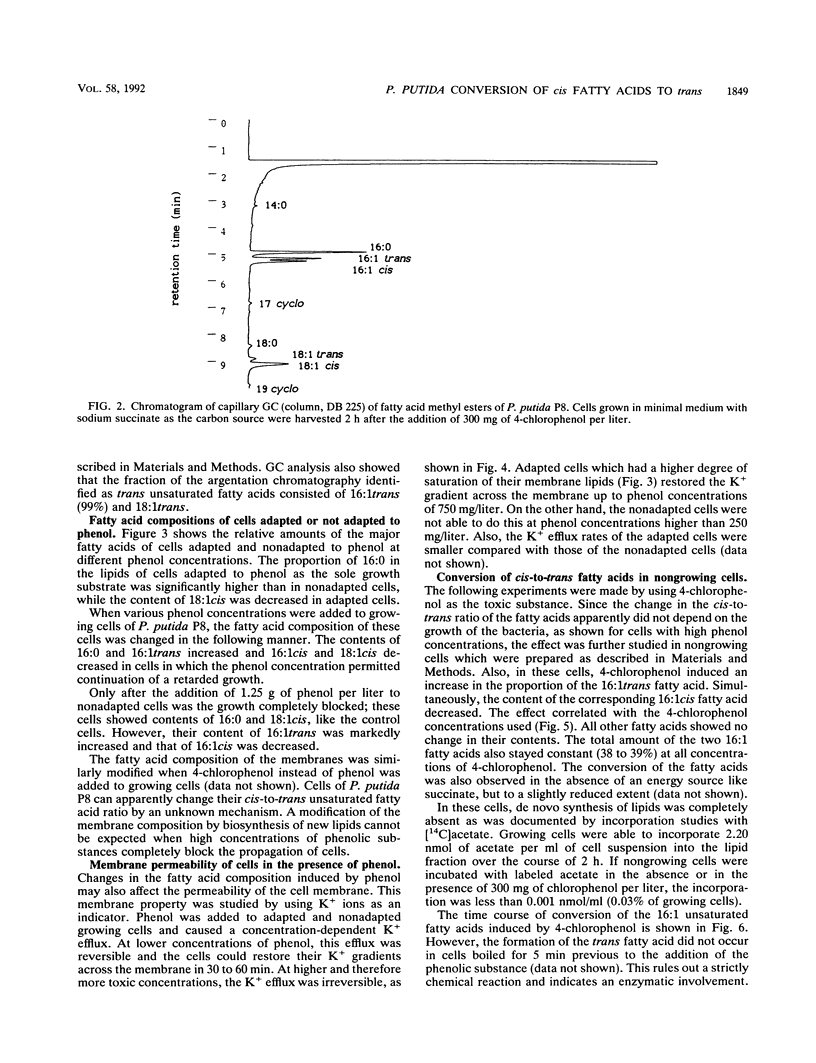
A trans unsaturated fatty acid was found as a major constituent in the lipids of Pseudomonas putida P8. The fatty acid was identified as 9-trans-hexadecenoic acid by gas chromatography, argentation thin-layer chromatography, and infrared absorption spectrometry. Growing cells of P. putida P8 reacted to the presence of sublethal concentrations of phenol in the medium with changes in the fatty acid composition of the lipids, thereby increasing the degree of saturation. At phenol concentrations which completely inhibited the growth of P. putida, the cells were still able to increase the content of the trans unsaturated fatty acid and simultaneously to decrease the proportion of the corresponding 9-cis-hexadecenoic acid. This conversion of fatty acids was also induced by 4-chlorophenol in nongrowing cells in which the de novo synthesis of lipids had stopped, as shown by incorporation experiments with labeled acetate. The isomerization of the double bond in the presence of chloramphenicol indicates a constitutively operating enzyme system. The cis-to-trans modification of the fatty acids studied here apparently is a new way of adapting the membrane fluidity to the presence of phenols, thereby compensating for the elevation of membrane permeability induced by these toxic substances.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Mangerich W. E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J Bacteriol. 1981 Sep;147(3):820–826. doi: 10.1128/jb.147.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfanz J., Rehm H. J. Biodegradation of 4-chlorophenol by adsorptive immobilized Alcaligenes sp. A 7-2 in soil. Appl Microbiol Biotechnol. 1991 Aug;35(5):662–668. doi: 10.1007/BF00169634. [DOI] [PubMed] [Google Scholar]
- CHAPMAN D. INFRARED SPECTROSCOPY OF LIPIDS. J Am Oil Chem Soc. 1965 May;42:353–371. doi: 10.1007/BF02635571. [DOI] [PubMed] [Google Scholar]
- Grogan D. W., Cronan J. E., Jr Characterization of Escherichia coli mutants completely defective in synthesis of cyclopropane fatty acids. J Bacteriol. 1986 Jun;166(3):872–877. doi: 10.1128/jb.166.3.872-877.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heipieper H. J., Keweloh H., Rehm H. J. Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl Environ Microbiol. 1991 Apr;57(4):1213–1217. doi: 10.1128/aem.57.4.1213-1217.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. A., Guckert J. B., White D. C., Deck F. Effect of nutrient deprivation on lipid, carbohydrate, DNA, RNA, and protein levels in Vibrio cholerae. Appl Environ Microbiol. 1986 Oct;52(4):788–793. doi: 10.1128/aem.52.4.788-793.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976 Feb;125(2):670–678. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Changes in lipid composition of Escherichia coli resulting from growth with organic solvents and with food additives. Appl Environ Microbiol. 1977 May;33(5):1233–1236. doi: 10.1128/aem.33.5.1233-1236.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keweloh H., Diefenbach R., Rehm H. J. Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch Microbiol. 1991;157(1):49–53. doi: 10.1007/BF00245334. [DOI] [PubMed] [Google Scholar]
- Keweloh H., Weyrauch G., Rehm H. J. Phenol-induced membrane changes in free and immobilized Escherichia coli. Appl Microbiol Biotechnol. 1990 Apr;33(1):66–71. doi: 10.1007/BF00170572. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Kametani F., Tsuchiya K., Sakurai H. ESR analysis with long-chain alkyl spin labels in bovine blood platelets. Relationship between the increase in membrane fluidity by alcohols and phenolic compounds and their inhibitory effects on aggregation. Biochim Biophys Acta. 1990 Aug 24;1027(2):123–129. doi: 10.1016/0005-2736(90)90075-y. [DOI] [PubMed] [Google Scholar]
- Li D. Y., Eberspächer J., Wagner B., Kuntzer J., Lingens F. Degradation of 2,4,6-trichlorophenol by Azotobacter sp. strain GP1. Appl Environ Microbiol. 1991 Jul;57(7):1920–1928. doi: 10.1128/aem.57.7.1920-1928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Makula R. A. Phospholipid composition of methane-utilizing bacteria. J Bacteriol. 1978 Jun;134(3):771–777. doi: 10.1128/jb.134.3.771-777.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama H., Okajima N., Sasaki S., Higashi S., Murata N. The cis/trans isomerization of the double bond of a fatty acid as a strategy for adaptation to changes in ambient temperature in the psychrophilic bacterium, Vibrio sp. strain ABE-1. Biochim Biophys Acta. 1991 Jun 19;1084(1):13–20. doi: 10.1016/0005-2760(91)90049-n. [DOI] [PubMed] [Google Scholar]
- Okuyama H., Sasaki S., Higashi S., Murata N. A trans-unsaturated fatty acid in a psychrophilic bacterium, Vibrio sp. strain ABE-1. J Bacteriol. 1990 Jun;172(6):3515–3518. doi: 10.1128/jb.172.6.3515-3518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz B. F., Rehm H. J. Terminale und subterminale Oxidation von n-Alkanen durch Schimmelpilze. Arch Mikrobiol. 1973;92(2):153–170. [PubMed] [Google Scholar]
- Shimp R. J., Pfaender F. K. Effect of adaptation to phenol on biodegradation of monosubstituted phenols by aquatic microbial communities. Appl Environ Microbiol. 1987 Jul;53(7):1496–1499. doi: 10.1128/aem.53.7.1496-1499.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


