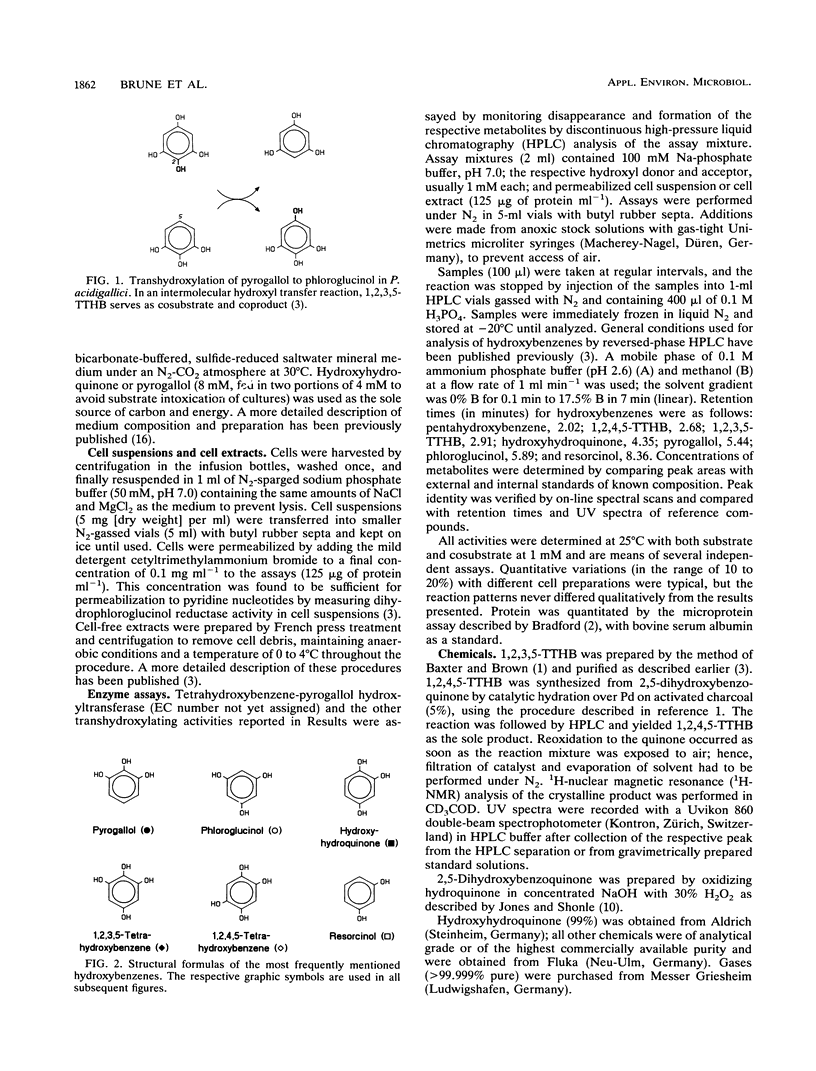
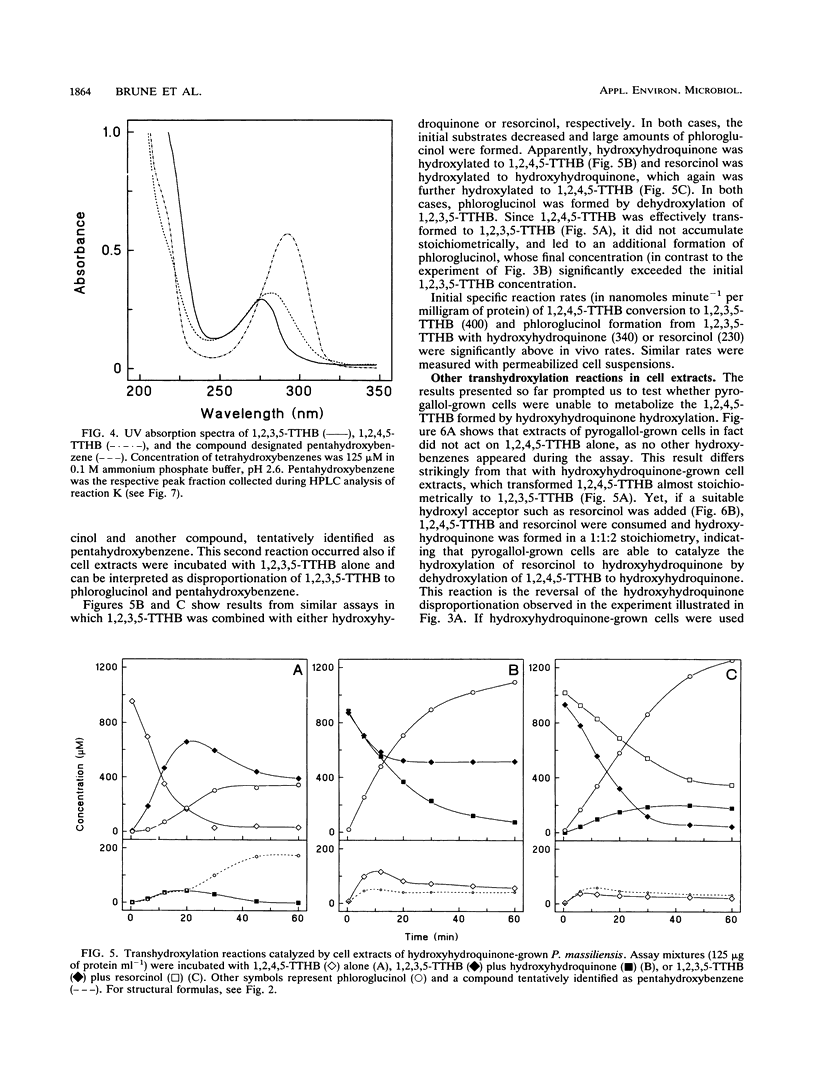
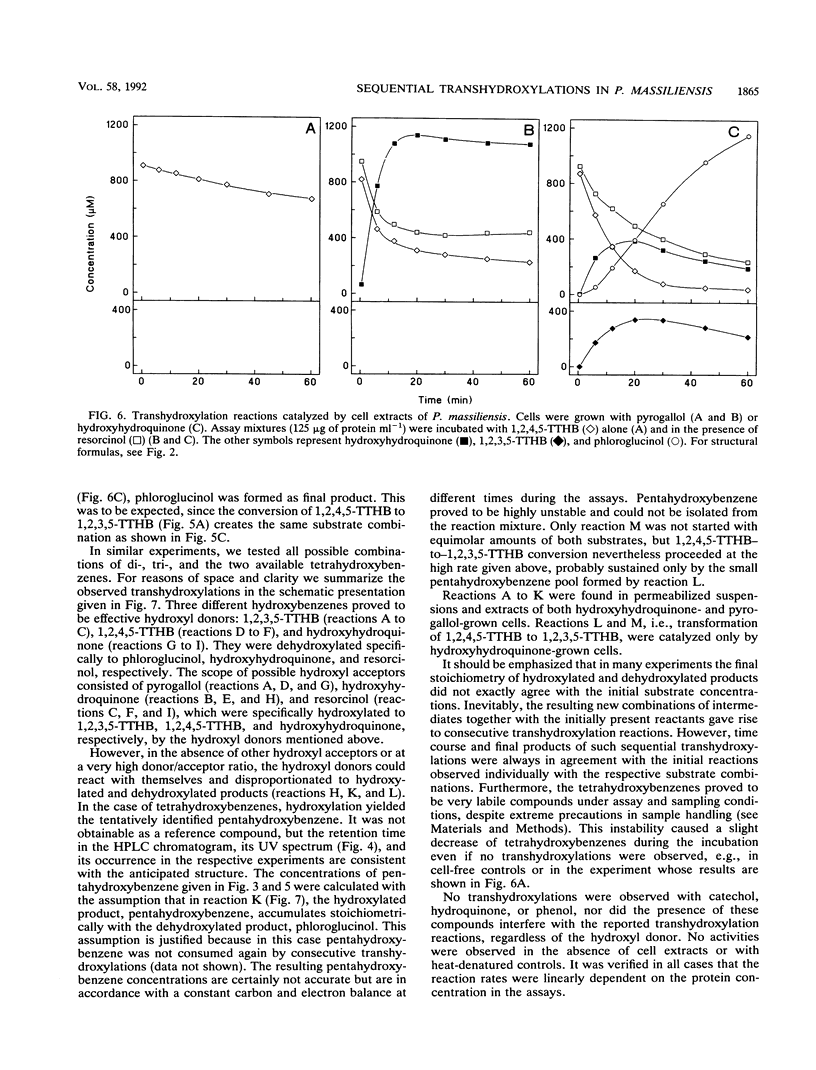
Abstract
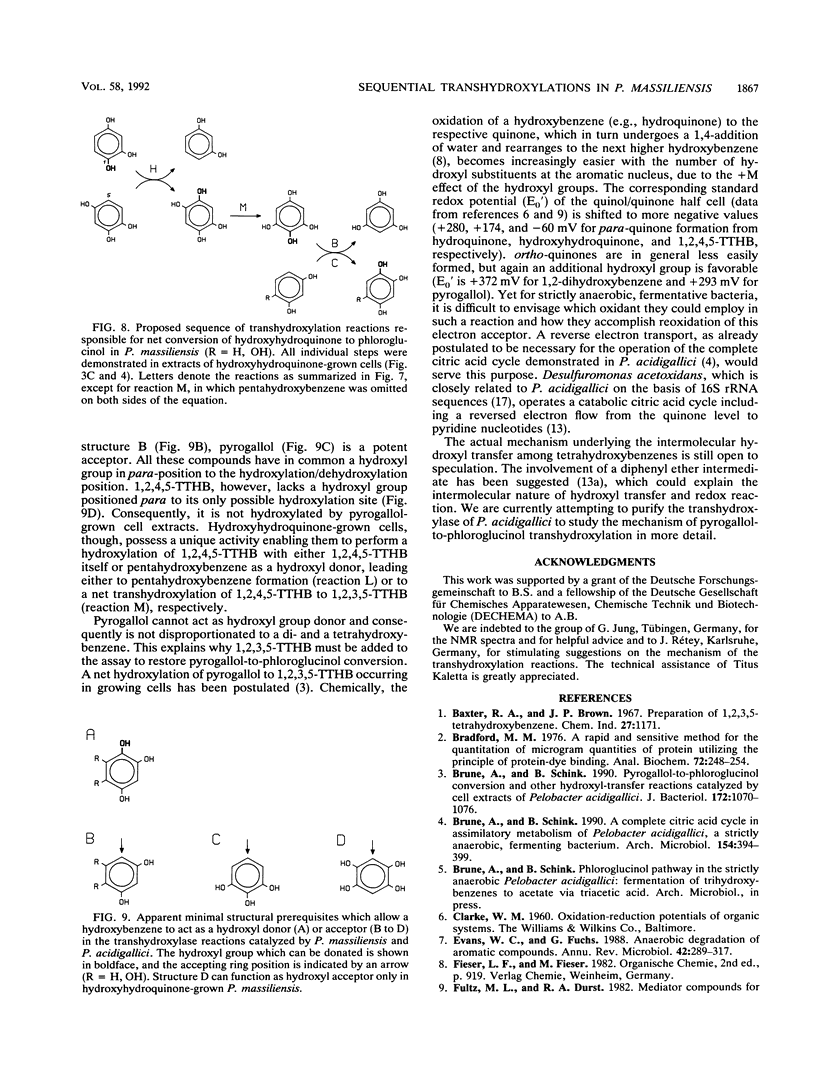
The recently isolated fermenting bacterium Pelobacter massiliensis is the only strict anaerobe known to grow on hydroxyhydroquinone (1,2,4-trihydroxybenzene) as the sole source of carbon and energy, converting it to stoichiometric amounts of acetate. In this paper, we report on the enzymatic reactions involved in the conversion of hydroxyhydroquinone and pyrogallol (1,2,3-trihydroxybenzene) to phloroglucinol (1,3,5-trihydroxybenzene). Cell extracts of P. massiliensis transhydroxylate pyrogallol to phloroglucinol after addition of 1,2,3,5-tetrahydroxybenzene (1,2,3,5-TTHB) as cosubstrate in a reaction identical to that found earlier with Pelobacter acidigallici (A. Brune and B. Schink, J. Bacteriol. 172:1070-1076, 1990). Hydroxyhydroquinone conversion to phloroglucinol is initiated in cell extracts without an external addition of cosubstrates. It involves a minimum of three consecutive transhydroxylation reactions characterized by the transient accumulation of two different TTHB isomers. Chemical synthesis of the TTHB intermediates allowed the resolution of the distinct transhydroxylation steps in this sequence. In an initial transhydroxylation, the hydroxyl group in the 1-position of a molecule of hydroxyhydroquinone is transferred to the 5-position of another molecule of hydroxyhydroquinone to give 1,2,4,5-TTHB and resorcinol (1,3-dihydroxybenzene) as products. Following this disproportionation of hydroxyhydroquinone, the 1,2,4,5-isomer is converted to 1,2,3,5-TTHB, an enzymatic activity present only in hydroxyhydroquinone-grown cells. Finally, phloroglucinol is formed from 1,2,3,5-TTHB by transfer of the 2-hydroxyl group to either hydroxyhydroquinone or resorcinol. The resulting coproducts are again cosubstrates in earlier reactions of this sequence. From the spectrum of hydroxybenzenes transhydroxylated by the cell extracts, the minimum structural prerequisites that render a hydroxybenzene a hydroxyl donor or acceptor are deduced.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brune A., Schink B. Pyrogallol-to-phloroglucinol conversion and other hydroxyl-transfer reactions catalyzed by cell extracts of Pelobacter acidigallici. J Bacteriol. 1990 Feb;172(2):1070–1076. doi: 10.1128/jb.172.2.1070-1076.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Fuchs G. Anaerobic degradation of aromatic compounds. Annu Rev Microbiol. 1988;42:289–317. doi: 10.1146/annurev.mi.42.100188.001445. [DOI] [PubMed] [Google Scholar]
- Krumholz L. R., Bryant M. P. Characterization of the pyrogallol-phloroglucinol isomerase of Eubacterium oxidoreducens. J Bacteriol. 1988 Jun;170(6):2472–2479. doi: 10.1128/jb.170.6.2472-2479.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz L. R., Crawford R. L., Hemling M. E., Bryant M. P. Metabolism of gallate and phloroglucinol in Eubacterium oxidoreducens via 3-hydroxy-5-oxohexanoate. J Bacteriol. 1987 May;169(5):1886–1890. doi: 10.1128/jb.169.5.1886-1890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


