Abstract
In the cofermentation of glycerol with a sugar by Lactobacillus brevis and Lactobacillus buchneri, a 1,3-propanediol:NAD+ oxidoreductase provides an additional method of NADH disposal. The enzyme has been purified from both L. brevis B22 and L. buchneri B190 and found to have properties very similar to those reported for the enzyme from Klebsiella pneumoniae. The enzymes required Mn2+ and are probably octamers with a molecular mass of 350 kDa. Although not absolutely specific for 1,3-propanediol when tested as dehydrogenases, the enzymes have less than 10% activity with glycerol, ethanol, and 1,2-propanediol. These properties contrast sharply with those of a protein isolated from another Lactobacillus species (L. reuteri) that ferments glycerol with glucose and previously designated a 1,3-propanediol dehydrogenase.
Full text
PDF
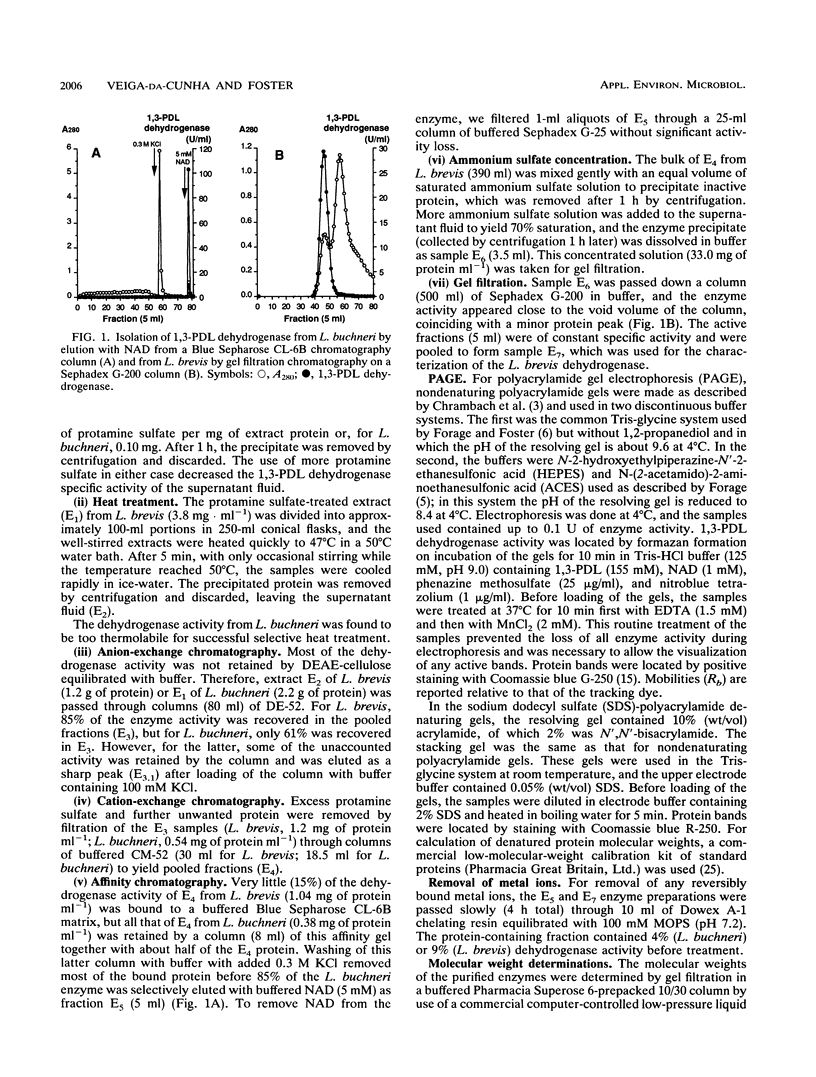
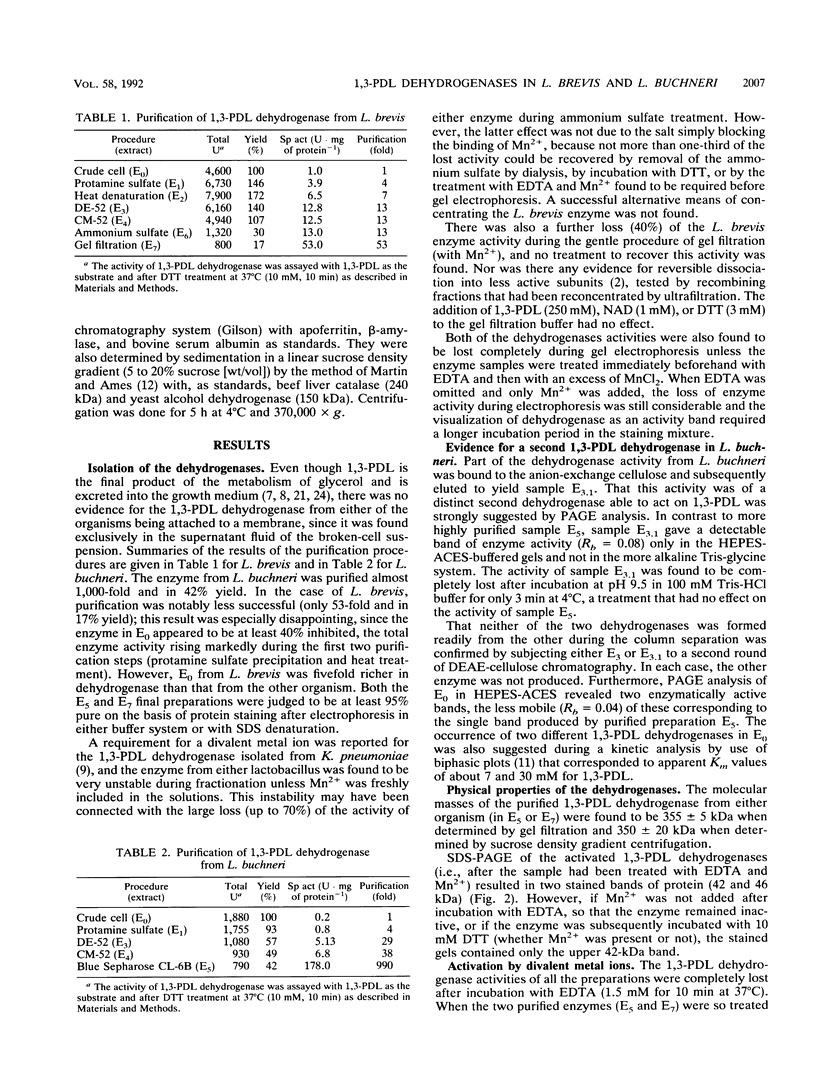


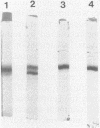
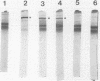
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELES R. H., LEE H. A., Jr An intramolecular oxidation-reduction requiring a cobamide coenzyme. J Biol Chem. 1961 Aug;236:2347–2350. [PubMed] [Google Scholar]
- Forage R. G., Foster M. A. Glycerol fermentation in Klebsiella pneumoniae: functions of the coenzyme B12-dependent glycerol and diol dehydratases. J Bacteriol. 1982 Feb;149(2):413–419. doi: 10.1128/jb.149.2.413-419.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forage R. G., Foster M. A. Resolution of the coenzyme B-12-dependent dehydratases of Klebsiella sp. and Citrobacter freundii. Biochim Biophys Acta. 1979 Aug 15;569(2):249–258. doi: 10.1016/0005-2744(79)90060-3. [DOI] [PubMed] [Google Scholar]
- Forage R. G., Lin E. C. DHA system mediating aerobic and anaerobic dissimilation of glycerol in Klebsiella pneumoniae NCIB 418. J Bacteriol. 1982 Aug;151(2):591–599. doi: 10.1128/jb.151.2.591-599.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Lin E. C. Klebsiella pneumoniae 1,3-propanediol:NAD+ oxidoreductase. J Bacteriol. 1987 May;169(5):2050–2054. doi: 10.1128/jb.169.5.2050-2054.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McGregor W. G., Phillips J., Suelter C. H. Purification and kinetic characterization of a monovalent cation-activated glycerol dehydrogenase from Aerobacter aerogenes. J Biol Chem. 1974 May 25;249(10):3132–3139. [PubMed] [Google Scholar]
- Pawelkiewicz J., Zagalak B. Enzymic conversion of glycerol into beta-hydroxy-propionaldehyde in a cell-free extract from Aerobacter aerogenes. Acta Biochim Pol. 1965;12(3):207–218. [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- SMILEY K. L., SOBOLOV M. A cobamide-requiring glycerol dehydrase from an acrolein-forming Lactobacillus. Arch Biochem Biophys. 1962 Jun;97:538–543. doi: 10.1016/0003-9861(62)90118-2. [DOI] [PubMed] [Google Scholar]
- SOBOLOV M., SMILEY K. L. Metabolism of glycerol by an acrolein-forming lactobacillus. J Bacteriol. 1960 Feb;79:261–266. doi: 10.1128/jb.79.2.261-266.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico T. L., Axelsson L. T., Novotny J., Fiuzat M., Dobrogosz W. J. Utilization of Glycerol as a Hydrogen Acceptor by Lactobacillus reuteri: Purification of 1,3-Propanediol:NAD Oxidoreductase. Appl Environ Microbiol. 1990 Apr;56(4):943–948. doi: 10.1128/aem.56.4.943-948.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico T. L., Dobrogosz W. J. Purification and Characterization of Glycerol Dehydratase from Lactobacillus reuteri. Appl Environ Microbiol. 1990 Apr;56(4):1195–1197. doi: 10.1128/aem.56.4.1195-1197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraya T., Fukui S. Immunochemical evidence for the difference between coenzyme-B12-dependent diol dehydratase and glycerol dehydratase. Eur J Biochem. 1977 Jun 1;76(1):285–289. doi: 10.1111/j.1432-1033.1977.tb11594.x. [DOI] [PubMed] [Google Scholar]
- Veiga da Cunha M., Foster M. A. Sugar-glycerol cofermentations in lactobacilli: the fate of lactate. J Bacteriol. 1992 Feb;174(3):1013–1019. doi: 10.1128/jb.174.3.1013-1019.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]