Abstract
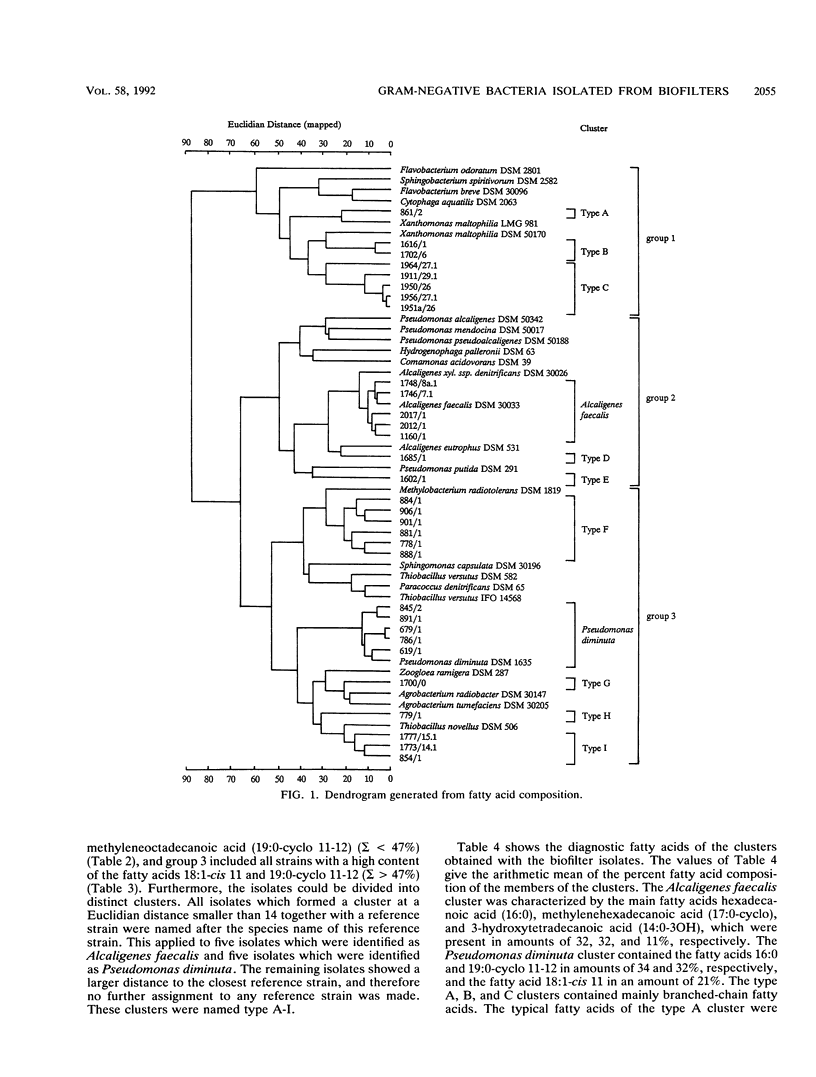
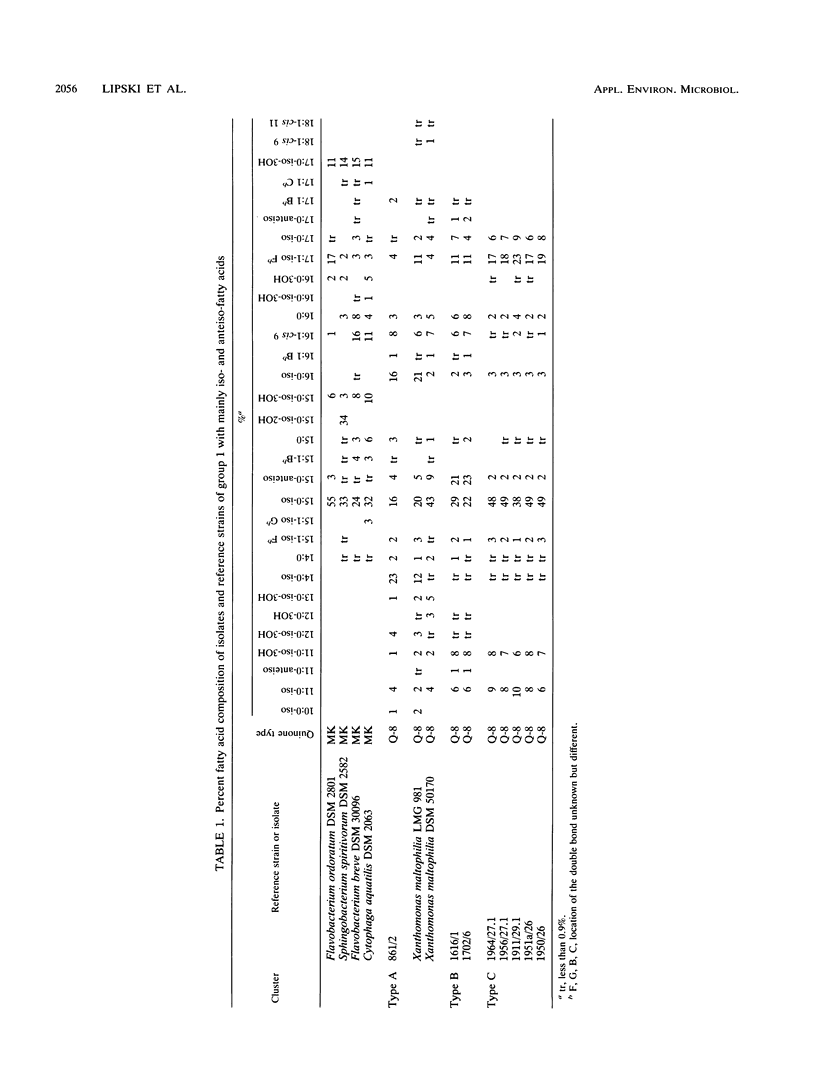
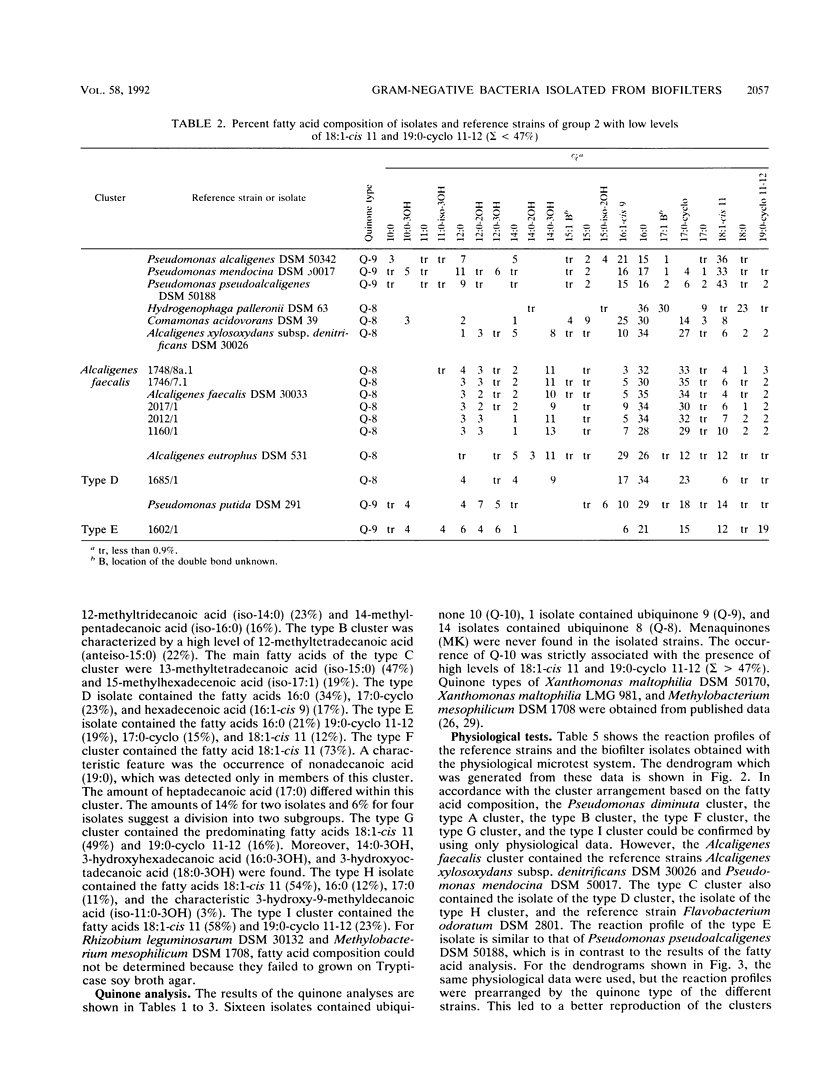
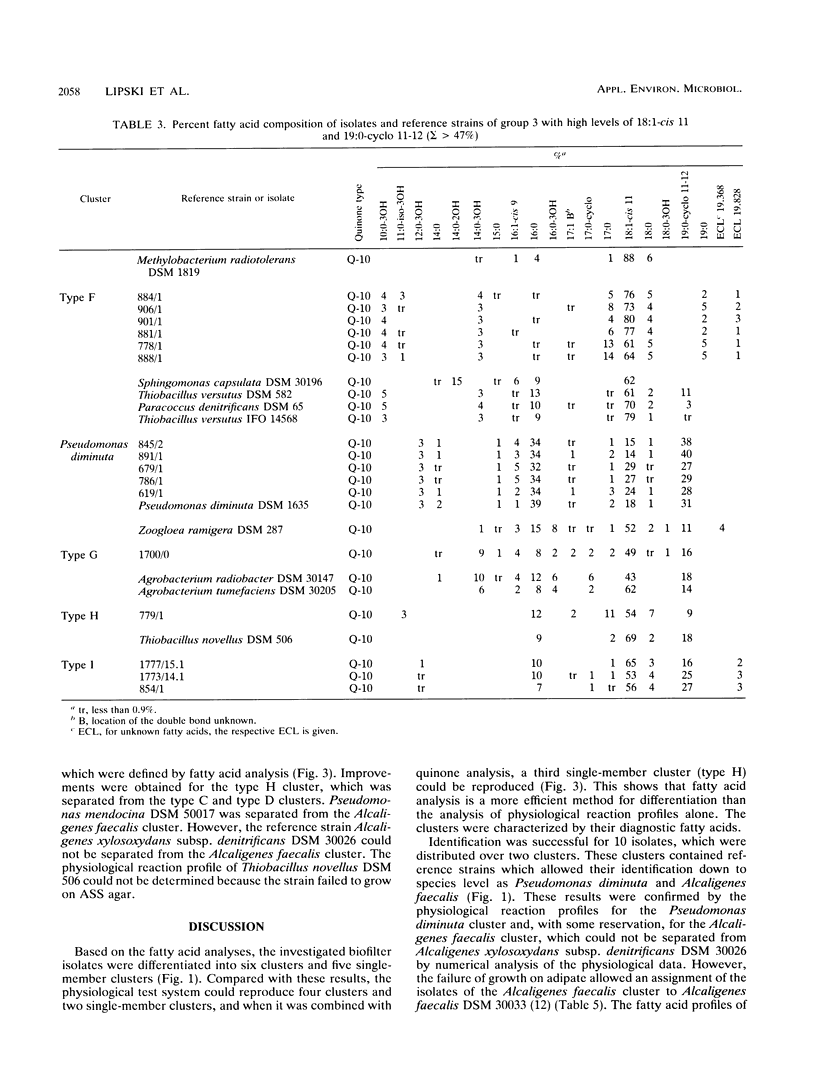
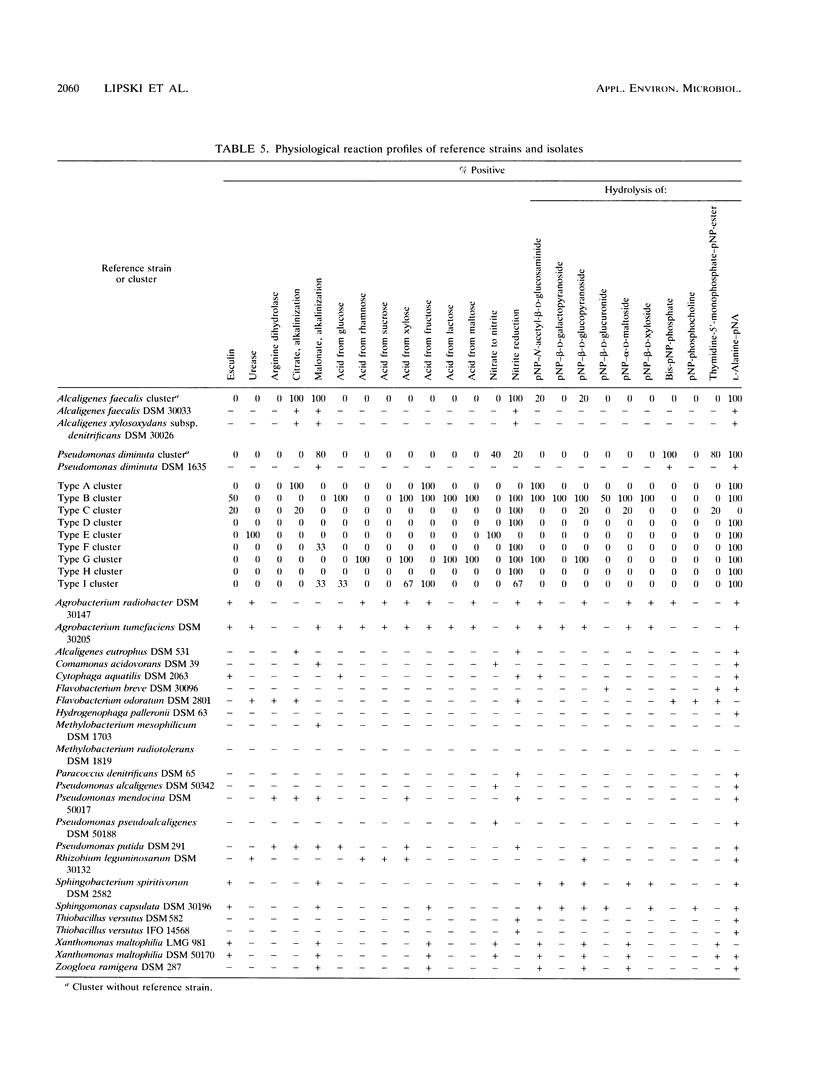
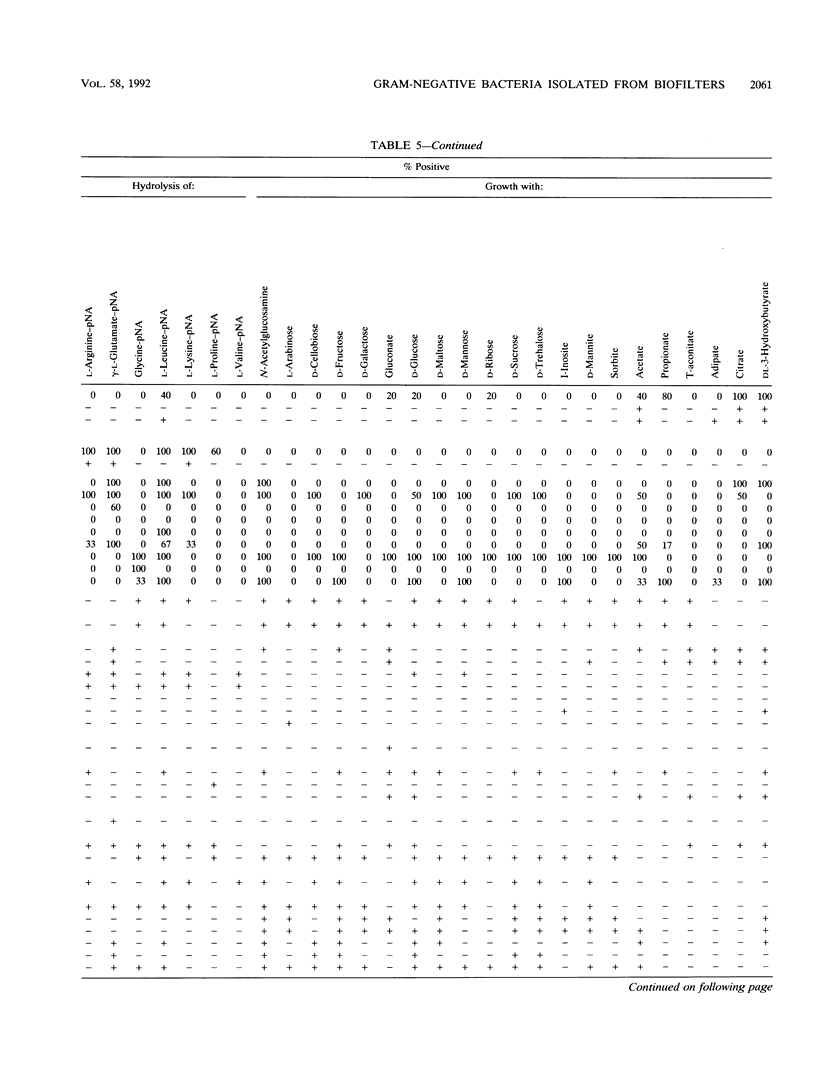
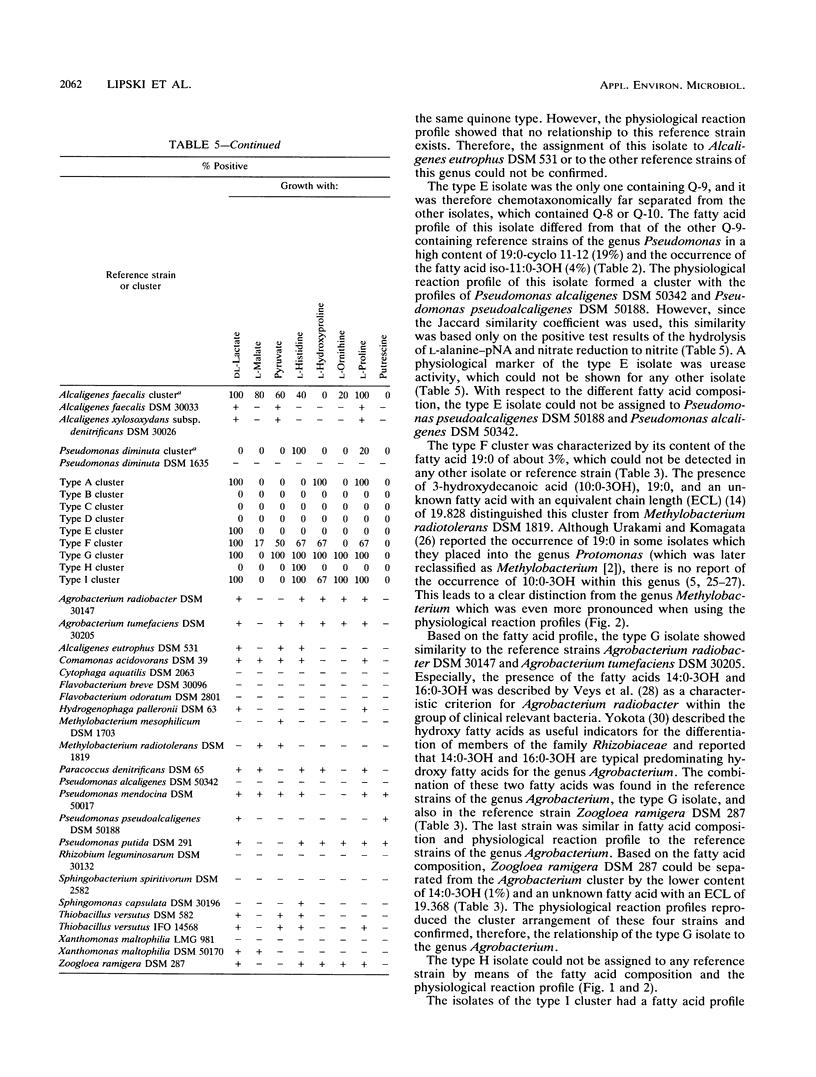
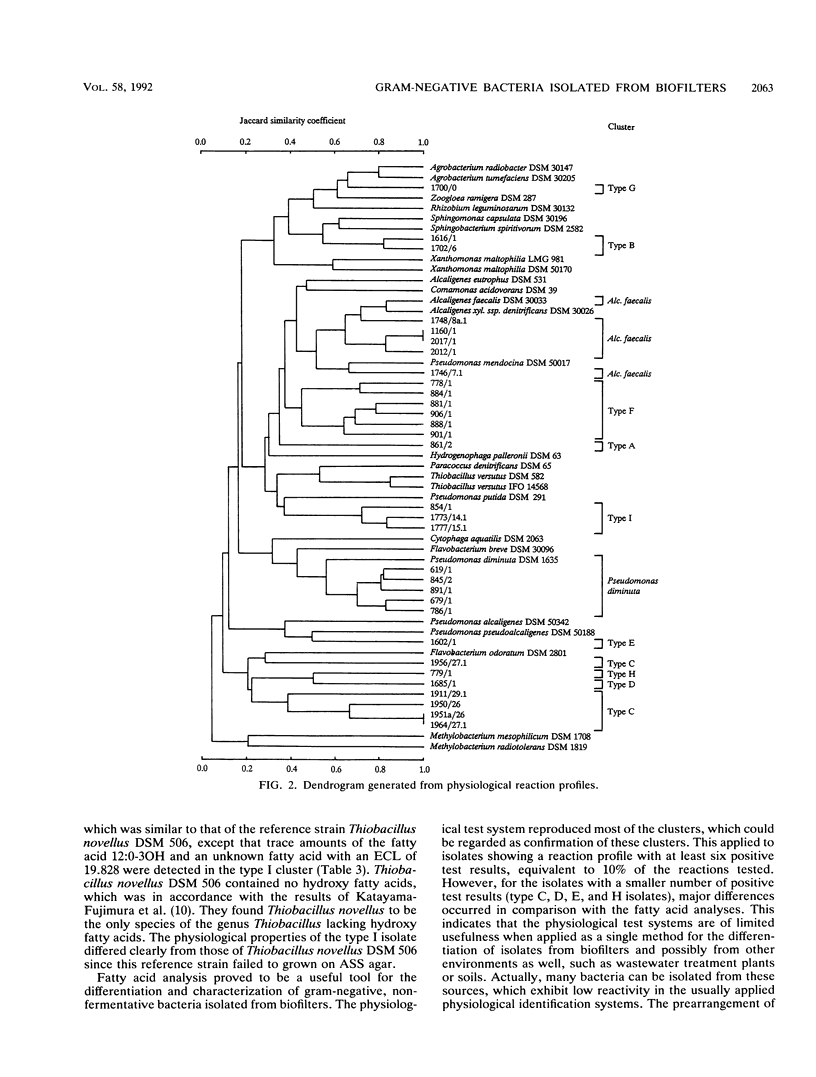
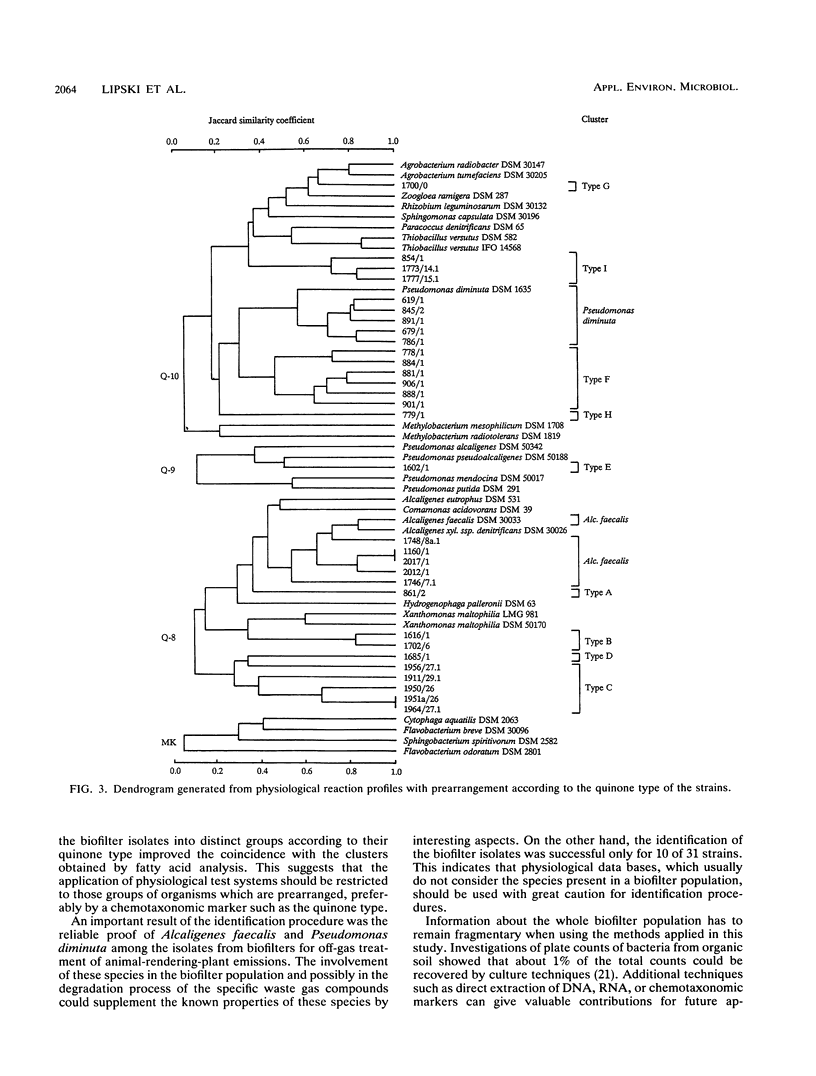
Gram-negative, nonfermentative bacteria isolated from biofilters for off-gas treatment of animal-rendering-plant emissions were differentiated by whole-cell fatty acid analysis, quinone analysis, and numerical taxonomy based on their physiological reaction profiles. The last system consisted of 60 physiological tests and was arranged as a microtest system on microtitration plates. Based on fatty acid analyses, 31 isolates were separated into six clusters and five single-member clusters. The isolates of two clusters were identified as Alcaligenes faecalis and Pseudomonas diminuta. The remaining nine clusters were characterized by their fatty acid profiles, quinone systems, and physiological reaction profiles. Clusters resulting from fatty acid analyses were compared with those resulting from physiological reaction profiles. Six clusters could be confirmed this way. The efficiency of the physiological test system was increased by the prearrangement of the isolates according to their quinone type.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dees S. B., Moss C. W. Cellular fatty acids of Alcaligenes and Pseudomonas species isolated from clinical specimens. J Clin Microbiol. 1975 May;1(5):414–419. doi: 10.1128/jcm.1.5.414-419.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991 Jun;55(2):288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämpfer P., Bette W., Dott W. Phenotypic differentiation of members of the family Vibrionaceae using miniaturized biochemical tests. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Oct;266(3-4):425–437. doi: 10.1016/s0176-6724(87)80223-7. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Dees S. B. Cellular fatty acids and metabolic products of Pseudomonas species obtained from clinical specimens. J Clin Microbiol. 1976 Dec;4(6):492–502. doi: 10.1128/jcm.4.6.492-502.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Wallace P. L., Hollis D. G., Weaver R. E. Cultural and chemical characterization of CDC groups EO-2, M-5, and M-6, Moraxella (Moraxella) species, Oligella urethralis, Acinetobacter species, and Psychrobacter immobilis. J Clin Microbiol. 1988 Mar;26(3):484–492. doi: 10.1128/jcm.26.3.484-492.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veys A., Callewaert W., Waelkens E., Van den Abbeele K. Application of gas-liquid chromatography to the routine identification of nonfermenting gram-negative bacteria in clinical specimens. J Clin Microbiol. 1989 Jul;27(7):1538–1542. doi: 10.1128/jcm.27.7.1538-1542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


