Abstract
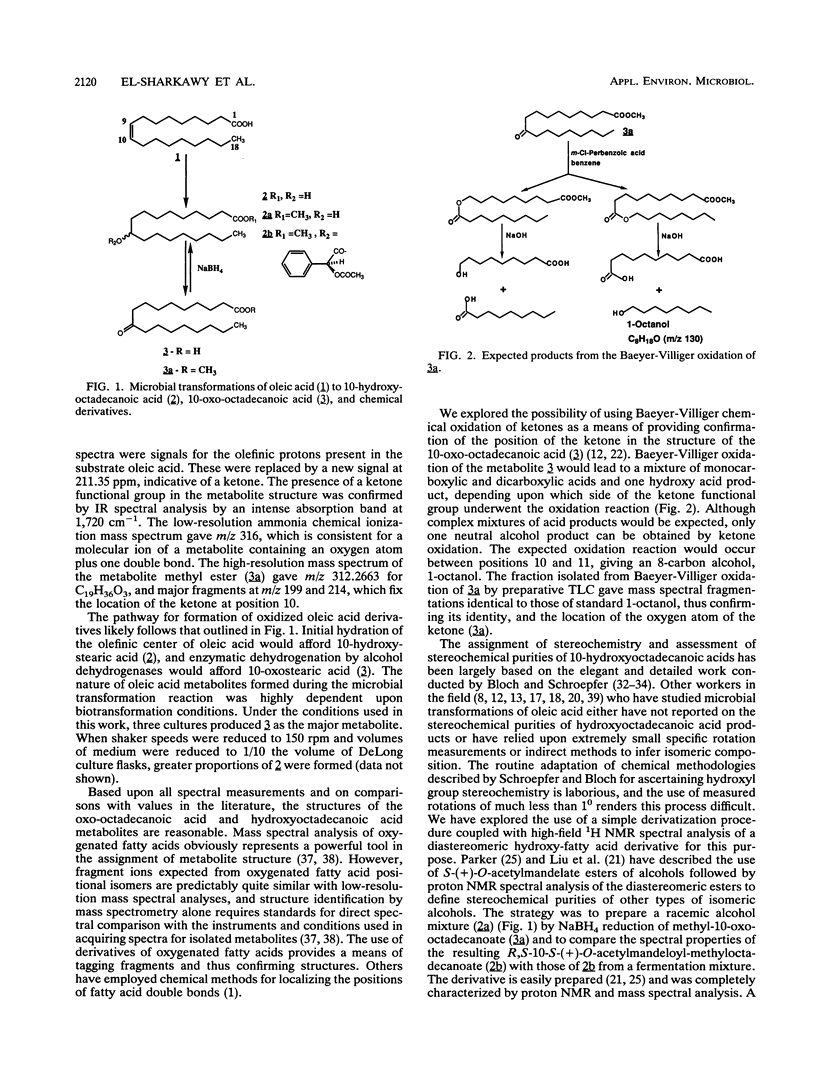
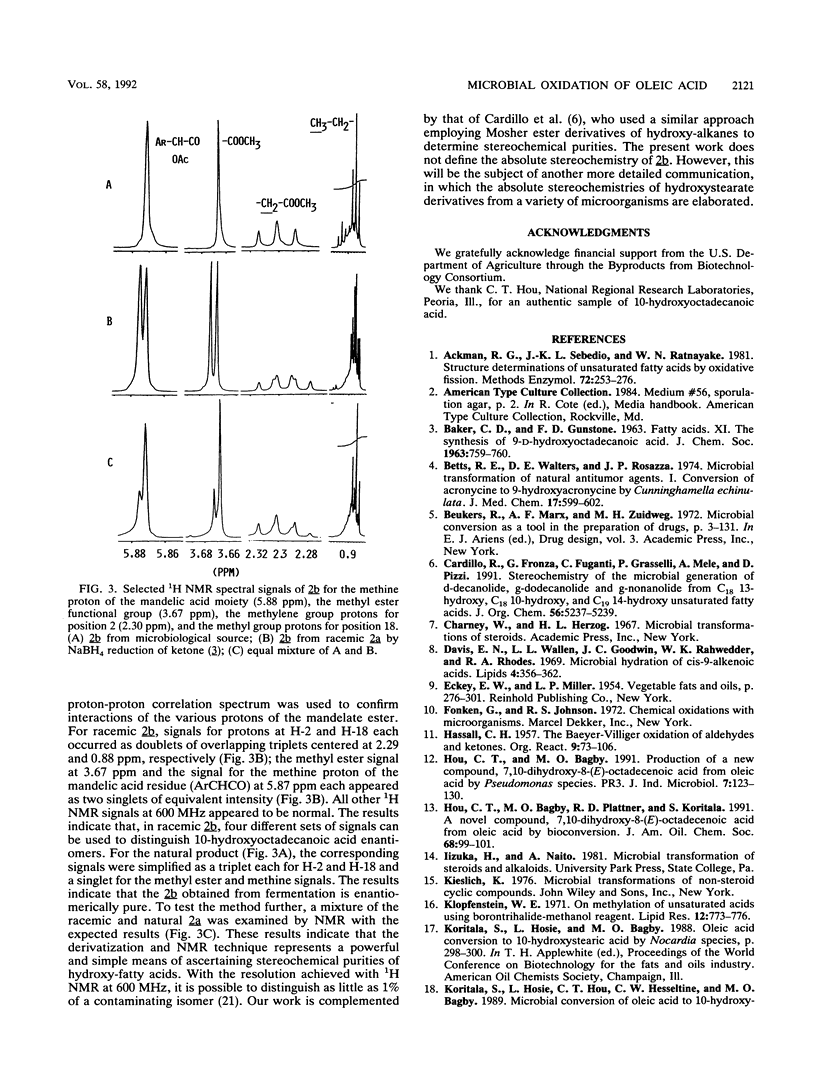
Resting cells of Saccharomyces cerevisiae (baker's yeast, type II; Sigma) were used to convert oleic acid into 10-hydroxyoctadecanoic acid with a 45% yield. Nocardia aurantia (ATCC 12674), Nocardia sp. (NRRL 5646), and Mycobacterium fortuitum (UI 53378) all converted oleic acid into 10-oxo-octadecanoic acid with 65, 55, and 80% yields, respectively. Structures of all metabolites were suggested by 1H and 13C nuclear magnetic resonance and by infrared and mass spectrometry. Structures of isomeric hydroxystearate and oxostearate derivatives and the stereochemical purity of hydroxystearates are difficult to prove unambiguously unless authentic standard compounds are available for spectral comparison. We describe the use of the chemical Baeyer-Villiger oxidation technique with 10-oxo-octadecanoic acid followed by mass spectral analysis of neutral extracts as a simple method to confirm the position of oxo-functional groups in the structures of fatty acid ketones. We further introduce a simple method based on 1H nuclear magnetic resonance analysis of diastereomeric S-(+)-O-acetylmandelate esters of hydroxystearates as a means of ascertaining stereochemical purities of hydroxy fatty acids.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackman R. G., Sebedio J. L., Ratnayake W. N. Structure determinations of unsaturated fatty acids by oxidative fission. Methods Enzymol. 1981;72:253–276. doi: 10.1016/s0076-6879(81)72014-7. [DOI] [PubMed] [Google Scholar]
- Betts R. E., Walters D. E., Rosazza J. P. Microbial transformations of antitumor compounds. 1. Conversion of acronycine to 9-hydroxyacronycine by Cunninghamella echinulata. J Med Chem. 1974 Jun;17(6):599–602. doi: 10.1021/jm00252a006. [DOI] [PubMed] [Google Scholar]
- Davis E. N., Wallen L. L., Goodwin J. C., Rohwedder W. K., Rhodes R. A. Microbial hydration of cis-9-alkenoic acids. Lipids. 1969 Sep;4(5):356–362. doi: 10.1007/BF02531006. [DOI] [PubMed] [Google Scholar]
- Klopfenstein W. E. On methylation of unsaturated acids using boron trihalide-methanol reagents. J Lipid Res. 1971 Nov;12(6):773–776. [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Torkelson A., Kisic A., Bednarczyk D. J., Schroepfer G. J., Jr Stereospecific hydration of the delta-9 double bond of oleic acid. J Biol Chem. 1970 Aug 10;245(15):3790–3797. [PubMed] [Google Scholar]
- SCHROEPFER G. J., Jr, BLOCH K. THE STEREOSPECIFIC CONVERSION OF STEARIC ACID TO OLEIC ACID. J Biol Chem. 1965 Jan;240:54–63. [PubMed] [Google Scholar]
- SCHROEPFER G. J., Jr ENZYMATIC STEREOSPECIFICITY IN THE CONVERSION OF OLEIC ACID TO 10-HYDROXYSTERARIC ACID. J Am Chem Soc. 1965 Mar 20;87:1411–1412. doi: 10.1021/ja01084a067. [DOI] [PubMed] [Google Scholar]
- Schroepfer G. J., Jr Stereospecific conversion of oleic acid to 10-hydroxystearic acid. J Biol Chem. 1966 Nov 25;241(22):5441–5447. [PubMed] [Google Scholar]
- WALLEN L. L., BENEDICT R. G., JACKSON R. W. The microbiological production of 10-hydroxystearic acid from oleic acid. Arch Biochem Biophys. 1962 Nov;99:249–253. doi: 10.1016/0003-9861(62)90006-1. [DOI] [PubMed] [Google Scholar]


