Abstract
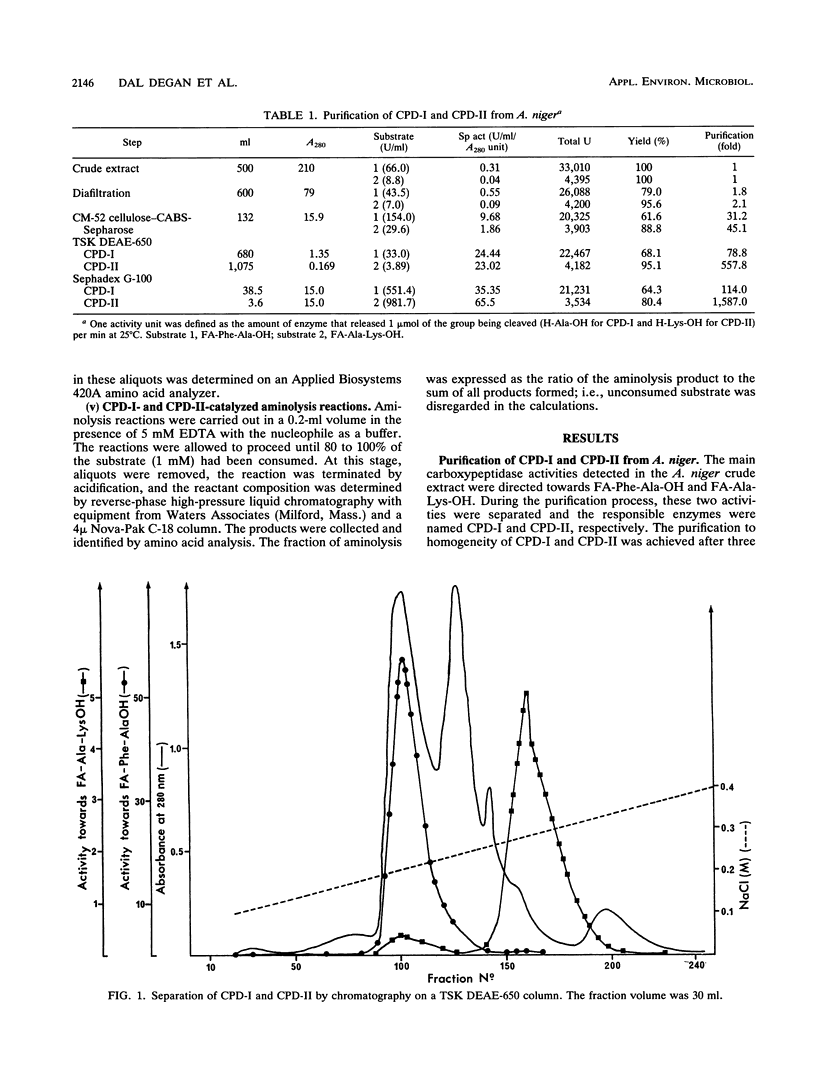
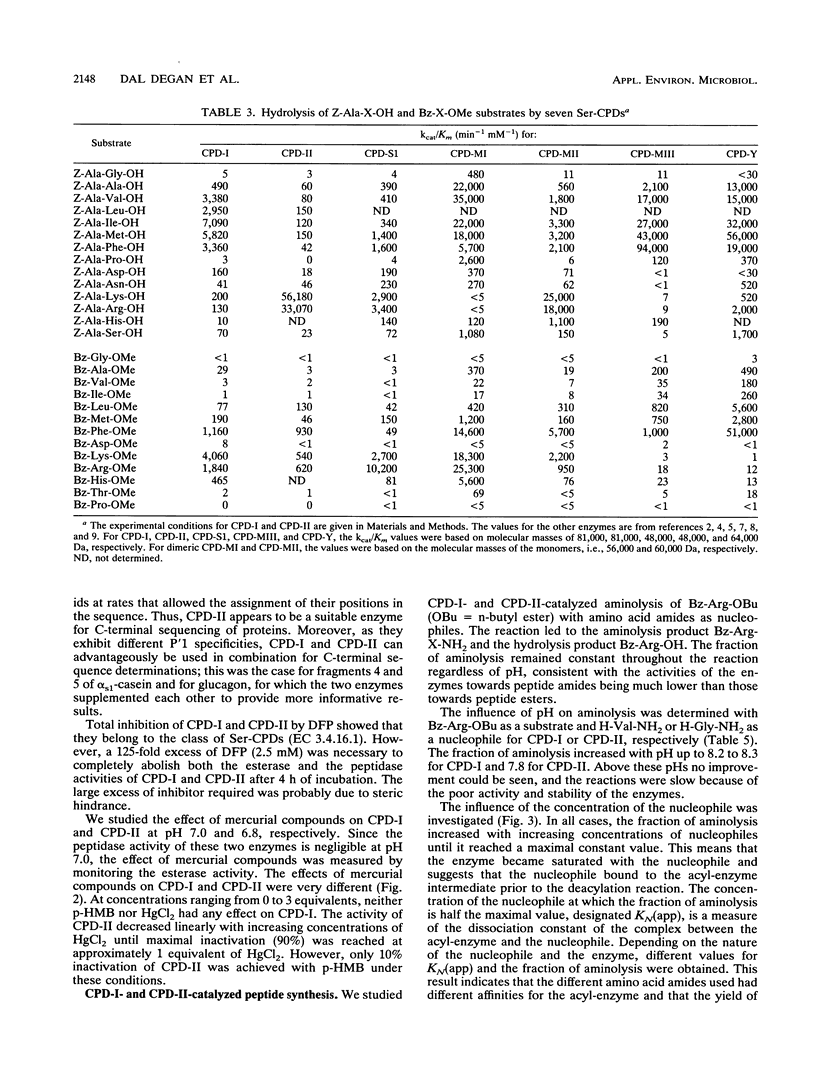
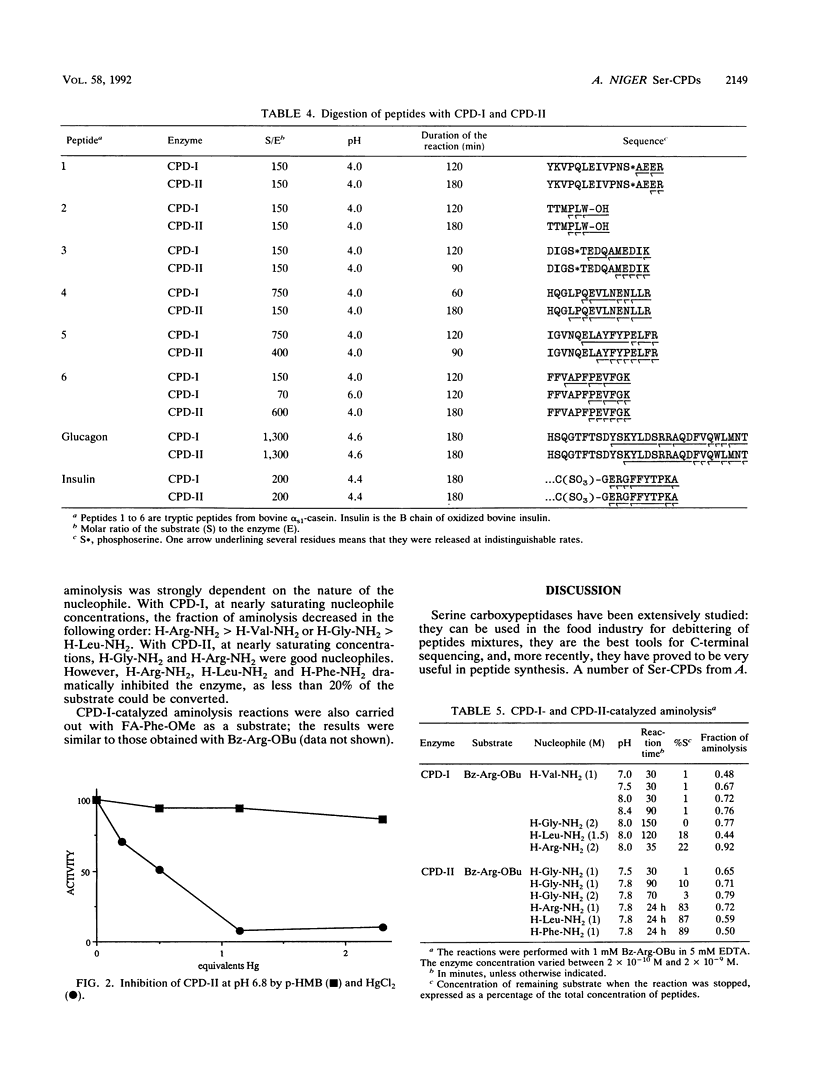
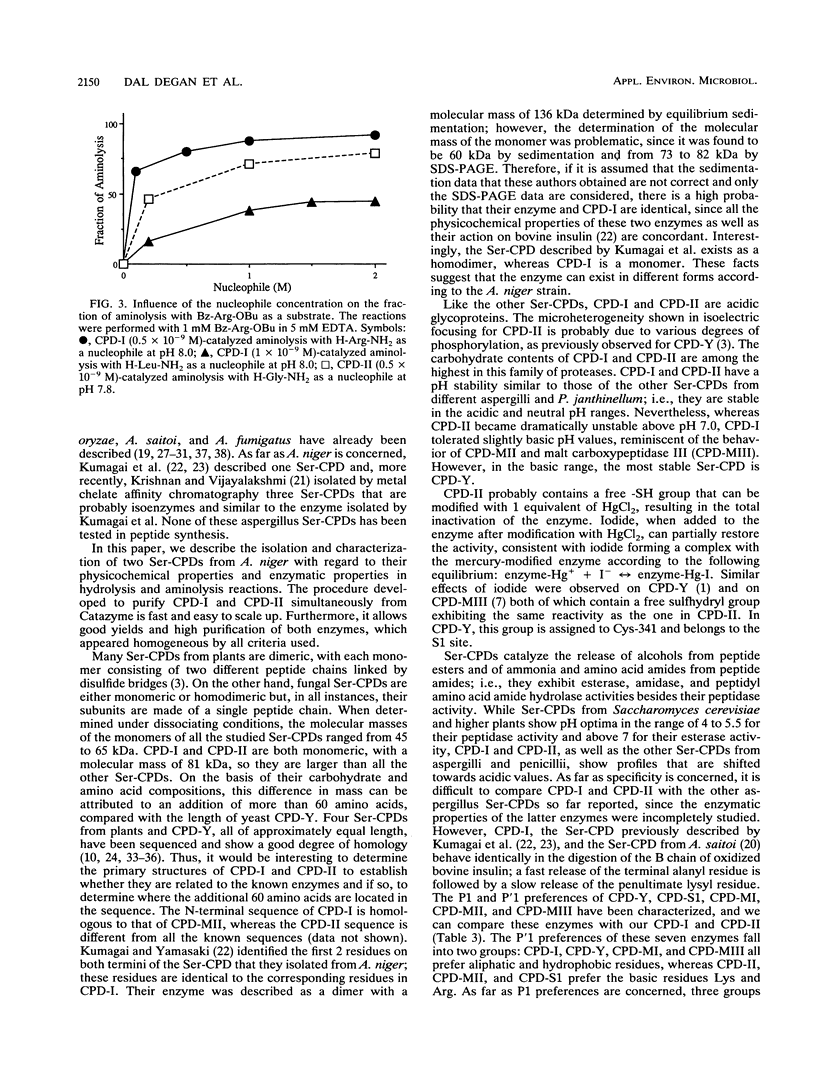
A procedure was developed to prepare in large amounts two carboxypeptidases, CPD-I and CPD-II, from Aspergillus niger. They were each shown to be serine proteases and single-chain monomers with molecular masses of ca. 81 kDa and containing 22% carbohydrates. Amino acid analysis, carbohydrate determination, and N-terminal sequencing (20 to 25 residues) were performed on each enzyme. CPD-I showed sequence homologies with malt carboxypeptidase II, while the N terminus of CPD-II was different from that of any known serine carboxypeptidase. Like carboxypeptidase Y from Saccharomyces cerevisiae and carboxypeptidase III from malt, CPD-II contained a free sulfhydryl group that could play a role in catalysis. Both A. niger enzymes had pH optima of about 4 and were unstable above pH 7. Their specificities for substrate positions P1 and P'1 were characterized by use of, as substrates, a series of N-blocked amino acid esters and dipeptides. Both enzymes were specific for Arg, Lys, and Phe in P1. CPD-I preferred hydrophobic residues in P'1, while CPD-II was highly specific for Arg and Lys in this position. Each displayed an original specificity when P1 and P'1 were considered together. The specificities were also studied by analyzing the time course of the release of amino acids from eight different peptides of various lengths. CPD-I and CPD-II appeared to be quite suitable for C-terminal sequence studies as well as for the synthesis of peptide bonds. The latter was studied with two peptide esters as aminolysis substrates and a series of amino acid amides as nucleophiles.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breddam K. Carboxypeptidase S-1 from Penicillium janthinellum: enzymatic properties in hydrolysis and aminolysis reactions. Carlsberg Res Commun. 1988;53(5):309–320. doi: 10.1007/BF02904436. [DOI] [PubMed] [Google Scholar]
- Breddam K., Widmer F., Meldal M. Amidation of growth hormone releasing factor (1-29) by serine carboxypeptidase catalysed transpeptidation. Int J Pept Protein Res. 1991 Feb;37(2):153–160. doi: 10.1111/j.1399-3011.1991.tb00096.x. [DOI] [PubMed] [Google Scholar]
- Carles C., Gueguen P., Ribadeau-Dumas B. C-terminal labelling of beta-casein. FEBS Lett. 1987 Feb 9;212(1):163–167. doi: 10.1016/0014-5793(87)81578-8. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichishima E., Arai T. Specificity and mode of action of acid carboxypeptidase from Aspergillus saitoi. Biochim Biophys Acta. 1973 Feb 15;293(2):444–450. doi: 10.1016/0005-2744(73)90351-3. [DOI] [PubMed] [Google Scholar]
- Ichishima E. Purification and characterization of a new type of acid carboxypeptidase from Aspergillus. Biochim Biophys Acta. 1972 Jan 20;258(1):274–288. doi: 10.1016/0005-2744(72)90985-0. [DOI] [PubMed] [Google Scholar]
- Krishnan S., Vijayalakshmi M. A. Purification and some properties of three serine carboxypeptidases from Aspergillus niger. J Chromatogr. 1986 Dec 5;370(2):315–326. doi: 10.1016/s0021-9673(00)94702-2. [DOI] [PubMed] [Google Scholar]
- Kumagai I., Yamasaki M. Enzymatic properties of an acid carboxypeptidase from Aspergillus niger var. macrosporus. Biochim Biophys Acta. 1981 Jun 15;659(2):344–350. doi: 10.1016/0005-2744(81)90060-7. [DOI] [PubMed] [Google Scholar]
- Kumagai I., Yamasaki M., Ui N. Isolation, purification and some chemical properties of an acid carboxypeptidase from Aspergillus niger var. Macrosporus. Biochim Biophys Acta. 1981 Jun 15;659(2):334–343. doi: 10.1016/0005-2744(81)90059-0. [DOI] [PubMed] [Google Scholar]
- Sørensen S. B., Svendsen I., Breddam K. Primary structure of carboxypeptidase III from malted barley. Carlsberg Res Commun. 1989;54(5):193–202. doi: 10.1007/BF02904473. [DOI] [PubMed] [Google Scholar]


