Abstract
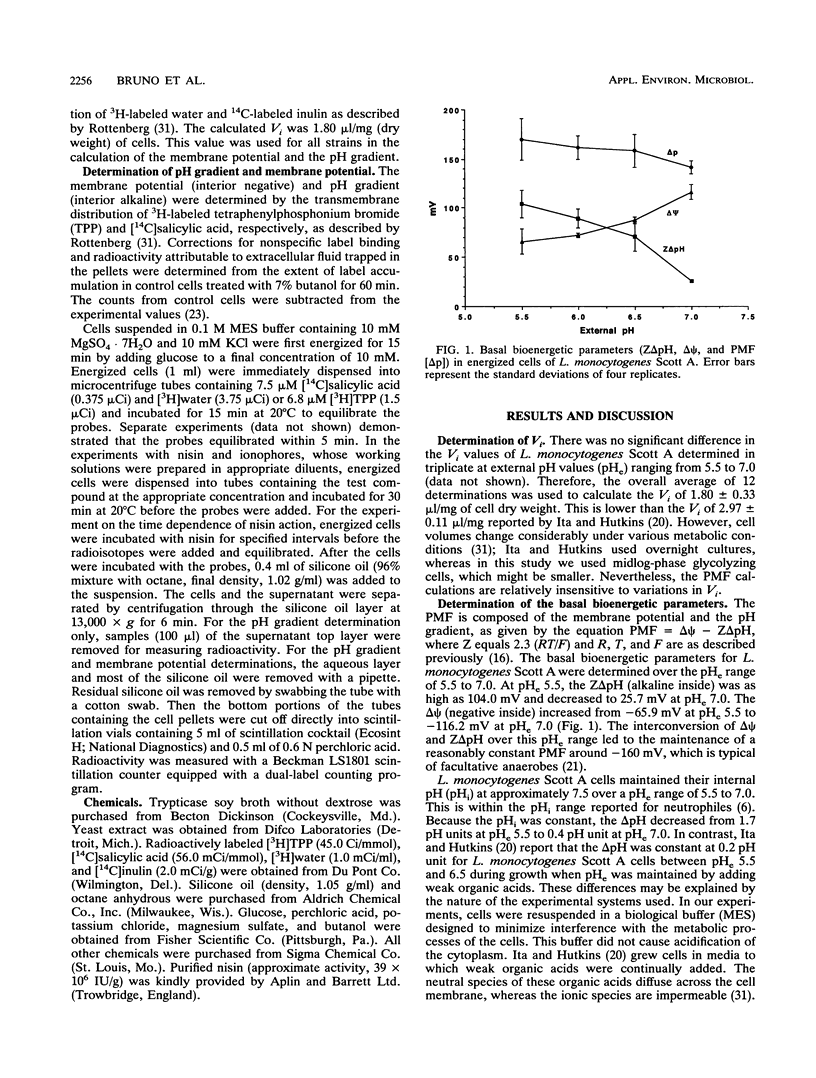
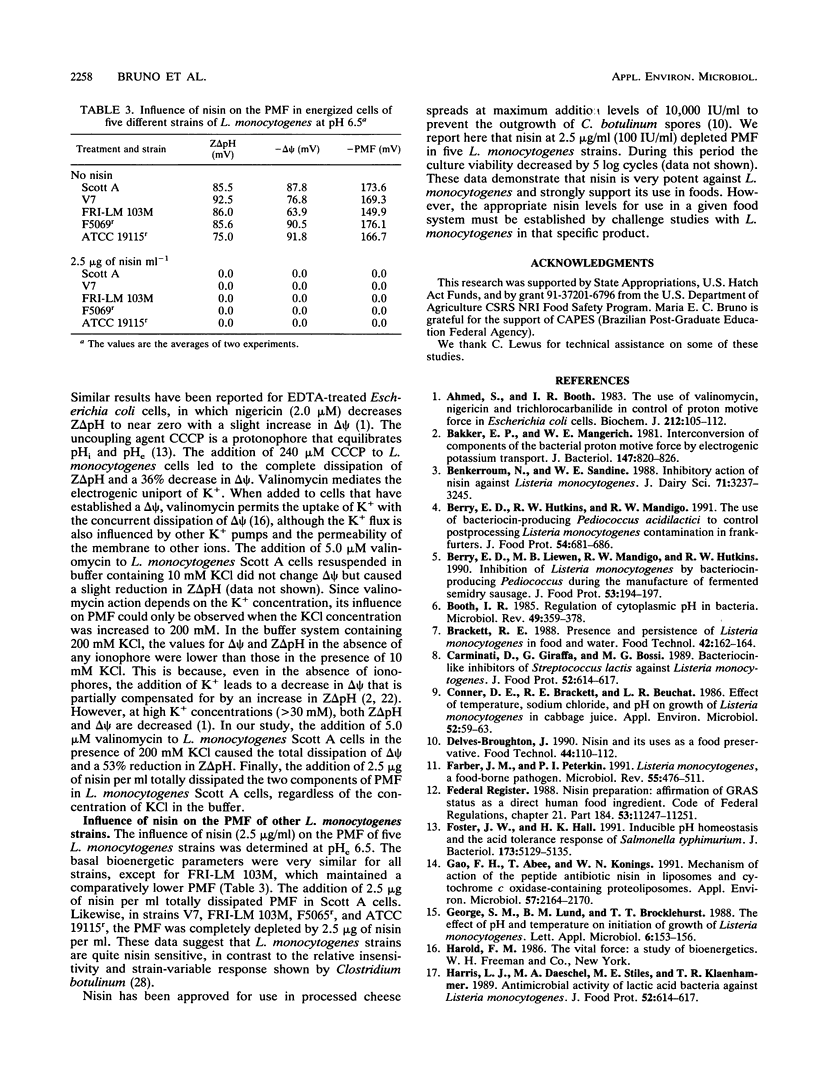
The basal proton motive force (PMF) levels and the influence of the bacteriocin nisin on the PMF were determined in Listeria monocytogenes Scott A. In the absence of nisin, the interconversion of the pH gradient (Z delta pH) and the membrane potential (delta psi) led to the maintenance of a fairly constant PMF at -160 mV over the external pH range 5.5 to 7.0. The addition of nisin at concentrations of greater than or equal to 5 micrograms/ml completely dissipated PMF in cells at external pH values of 5.5 and 7.0. With 1 microgram of nisin per ml, delta pH was completely dissipated but delta psi decreased only slightly. The action of nisin on PMF in L. monocytogenes Scott A was both time and concentration dependent. Valinomycin depleted only delta pH, whereas nigericin and carbonyl cyanide m-chlorophenylhydrazone depleted only delta psi, under conditions in which nisin depleted both. Four other L. monocytogenes strains had basal PMF parameters similar to those of strain Scott A. Nisin (2.5 micrograms/ml) also completely dissipated PMF in these strains.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Booth I. R. The use of valinomycin, nigericin and trichlorocarbanilide in control of the protonmotive force in Escherichia coli cells. Biochem J. 1983 Apr 15;212(1):105–112. doi: 10.1042/bj2120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Mangerich W. E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J Bacteriol. 1981 Sep;147(3):820–826. doi: 10.1128/jb.147.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkerroum N., Sandine W. E. Inhibitory action of nisin against Listeria monocytogenes. J Dairy Sci. 1988 Dec;71(12):3237–3245. doi: 10.3168/jds.S0022-0302(88)79929-4. [DOI] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner D. E., Brackett R. E., Beuchat L. R. Effect of temperature, sodium chloride, and pH on growth of Listeria monocytogenes in cabbage juice. Appl Environ Microbiol. 1986 Jul;52(1):59–63. doi: 10.1128/aem.52.1.59-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol. 1991 Aug;173(16):5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F. H., Abee T., Konings W. N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991 Aug;57(8):2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Barker S. L. Effects of potassium ions on the electrical and pH gradients across the membrane of Streptococcus lactis cells. J Bacteriol. 1977 Jun;130(3):1017–1023. doi: 10.1128/jb.130.3.1017-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Lewus C. B., Kaiser A., Montville T. J. Inhibition of food-borne bacterial pathogens by bacteriocins from lactic acid bacteria isolated from meat. Appl Environ Microbiol. 1991 Jun;57(6):1683–1688. doi: 10.1128/aem.57.6.1683-1688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugikura S., Nishikawa M., Igarashi K., Kobayashi H. Maintenance of a neutral cytoplasmic pH is not obligatory for growth of Escherichia coli and Streptococcus faecalis at an alkaline pH. J Biochem. 1990 Jul;108(1):86–91. doi: 10.1093/oxfordjournals.jbchem.a123168. [DOI] [PubMed] [Google Scholar]
- RAMSEIER H. R. [The effect of nisin on Clostridium butyricum Prazm]. Arch Mikrobiol. 1960;37:57–94. doi: 10.1007/BF00414627. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. J., Archer P., Banks J. G. Growth of Listeria monocytogenes at refrigeration temperatures. J Appl Bacteriol. 1990 Feb;68(2):157–162. doi: 10.1111/j.1365-2672.1990.tb02561.x. [DOI] [PubMed] [Google Scholar]
- Yousef A. E., Luchansky J. B., Degnan A. J., Doyle M. P. Behavior of Listeria monocytogenes in wiener exudates in the presence of Pediococcus acidilactici H or pediocin AcH during storage at 4 or 25 degrees C. Appl Environ Microbiol. 1991 May;57(5):1461–1467. doi: 10.1128/aem.57.5.1461-1467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


