Abstract
This study was conducted to determine the persistence of Giardia lamblia cysts in mixed septic tank effluent and swine manure slurry and to correlate fluorescein diacetate-propidium iodide staining of G. lamblia cysts with their morphology under low-voltage scanning electron microscopy. Under field conditions, G. lamblia cysts were degraded more rapidly in the mixed waste than in the control Dulbecco's phosphate-buffered saline (PBS). For total and viable cysts, the mixed waste had D values (time for a 90% reduction in number of cysts) of 18.3 and 15.5 days, and the Dulbecco's PBS control had D values of 41.6 and 26.8 days. The rates of cyst degradation in septic tank effluent and in Dulbecco's PBS were similar. Increasing the proportion of swine manure slurry in the mixed waste favored degradation of the parasite. These results indicate that the mixed waste treatment was the predominant factor affecting the cyst persistence and that it was swine manure slurry that played the role of degrading the parasite. Visualization of viable and nonviable Giardia cysts with low-voltage scanning electron microscopy revealed an excellent correlation between the viability of the cysts determined by fluorescein diacetate-propidium iodide staining and their electron microscopic morphology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbaszadegan M., Gerba C. P., Rose J. B. Detection of Giardia cysts with a cDNA probe and applications to water samples. Appl Environ Microbiol. 1991 Apr;57(4):927–931. doi: 10.1128/aem.57.4.927-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M., MacLean J. D., Law C., Croll N. A. Giardia lamblia infections in Mongolian gerbils: an animal model. J Infect Dis. 1983 Feb;147(2):222–226. doi: 10.1093/infdis/147.2.222. [DOI] [PubMed] [Google Scholar]
- Bingham A. K., Jarroll E. L., Jr, Meyer E. A., Radulescu S. Giardia sp.: physical factors of excystation in vitro, and excystation vs eosin exclusion as determinants of viability. Exp Parasitol. 1979 Apr;47(2):284–291. doi: 10.1016/0014-4894(79)90080-8. [DOI] [PubMed] [Google Scholar]
- Bingham A. K., Meyer E. A. Giardia excystation can be induced in vitro in acidic solutions. Nature. 1979 Jan 25;277(5694):301–302. doi: 10.1038/277301a0. [DOI] [PubMed] [Google Scholar]
- Buret A., denHollander N., Wallis P. M., Befus D., Olson M. E. Zoonotic potential of giardiasis in domestic ruminants. J Infect Dis. 1990 Jul;162(1):231–237. doi: 10.1093/infdis/162.1.231. [DOI] [PubMed] [Google Scholar]
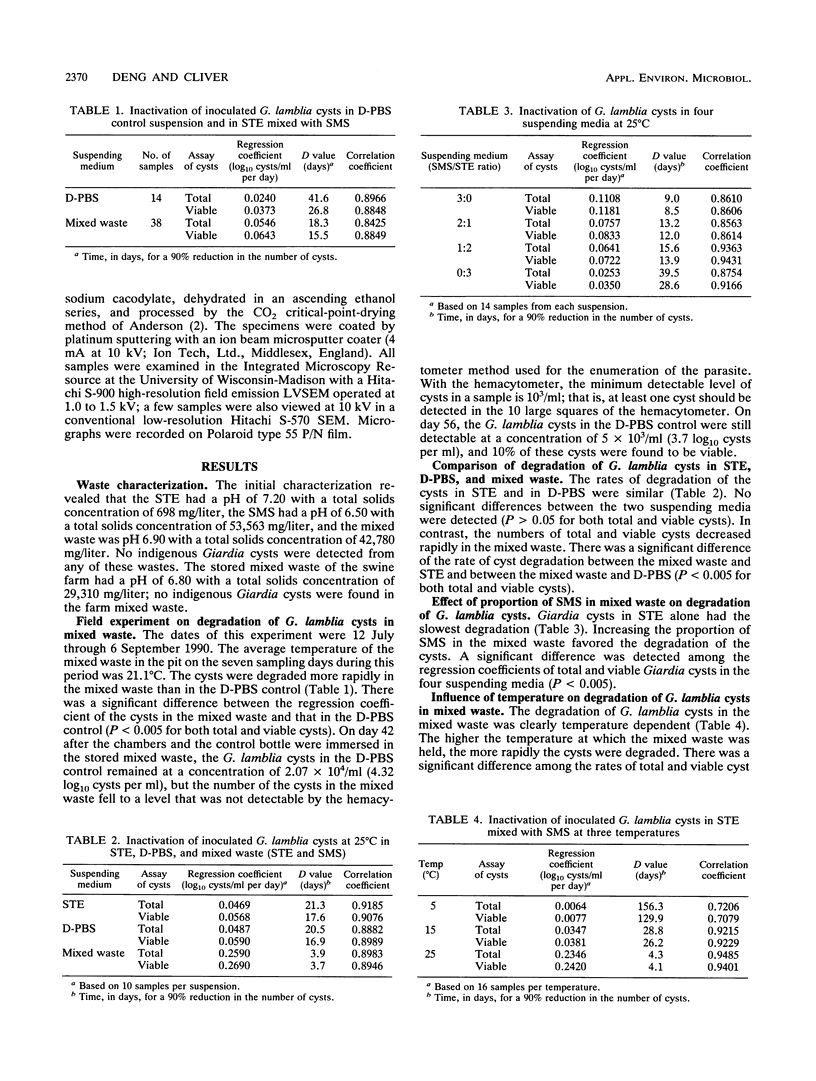
- Erlandsen S. L., Bemrick W. J., Pawley J. High-resolution electron microscopic evidence for the filamentous structure of the cyst wall in Giardia muris and Giardia duodenalis. J Parasitol. 1989 Oct;75(5):787–797. [PubMed] [Google Scholar]
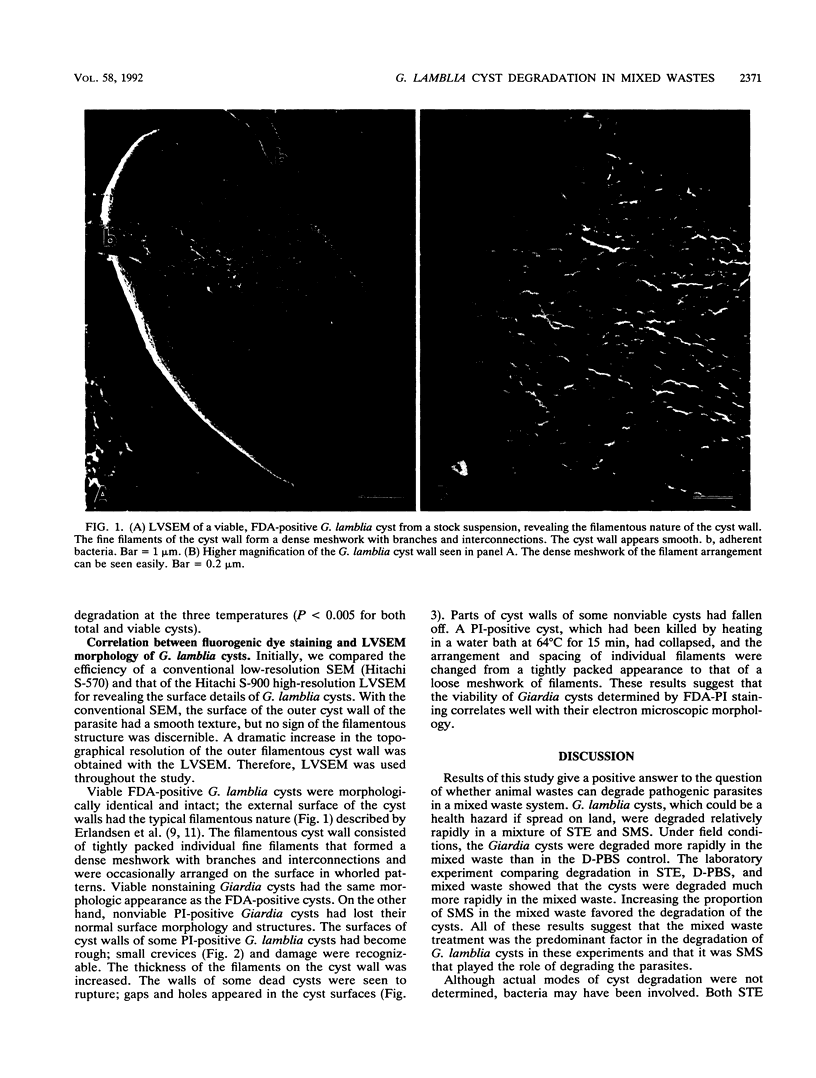
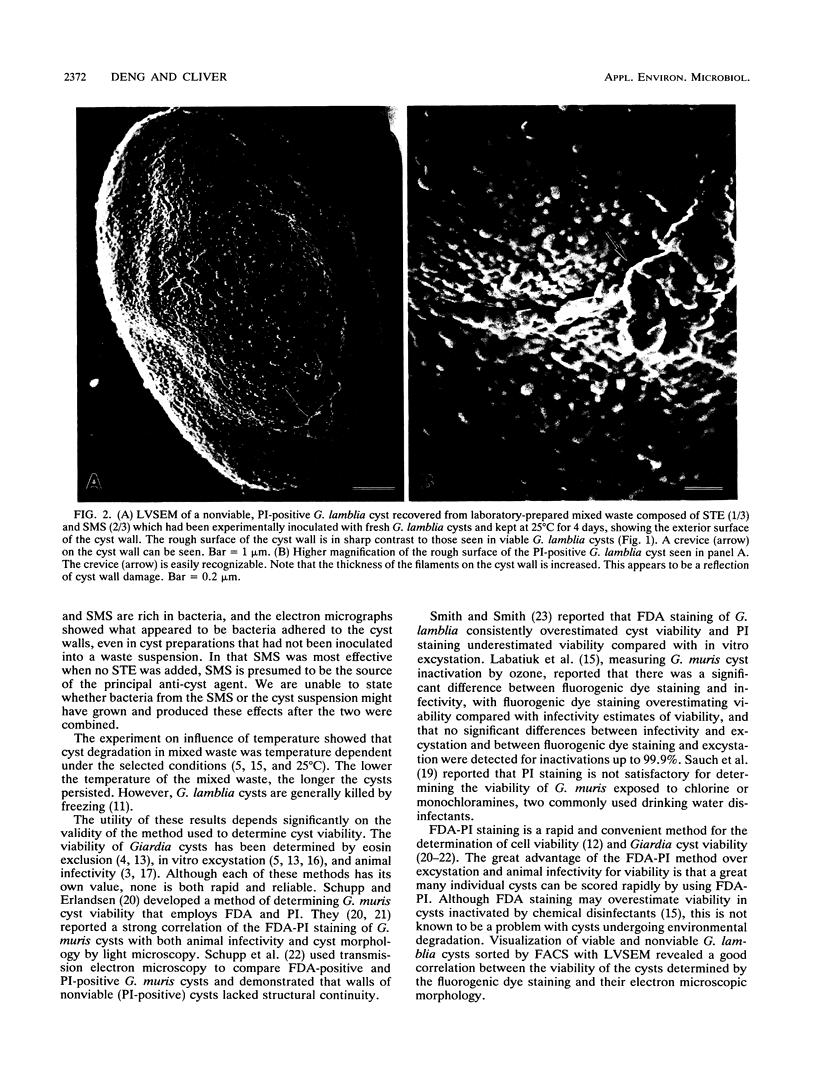
- Erlandsen S. L., Bemrick W. J., Schupp D. E., Shields J. M., Jarroll E. L., Sauch J. F., Pawley J. B. High-resolution immunogold localization of Giardia cyst wall antigens using field emission SEM with secondary and backscatter electron imaging. J Histochem Cytochem. 1990 May;38(5):625–632. doi: 10.1177/38.5.2332623. [DOI] [PubMed] [Google Scholar]
- Erlandsen S. L., Sherlock L. A., Bemrick W. J. The detection of Giardia muris and Giardia lamblia cysts by immunofluorescence in animal tissues and fecal samples subjected to cycles of freezing and thawing. J Parasitol. 1990 Apr;76(2):267–271. [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Kasprzak W., Majewska A. C. Infectivity of Giardia sp. cysts in relation to eosin exclusion and excystation in vitro. Tropenmed Parasitol. 1983 Mar;34(1):70–72. [PubMed] [Google Scholar]
- Rice E. W., Schaefer F. W., 3rd Improved in vitro excystation procedure for Giardia lamblia cysts. J Clin Microbiol. 1981 Dec;14(6):709–710. doi: 10.1128/jcm.14.6.709-710.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Stevens D. P., Mahmoud A. A., Warren K. S. Giardiasis in the mouse: an animal model. Gastroenterology. 1976 Jul;71(1):57–61. [PubMed] [Google Scholar]
- Sauch J. F., Flanigan D., Galvin M. L., Berman D., Jakubowski W. Propidium iodide as an indicator of Giardia cyst viability. Appl Environ Microbiol. 1991 Nov;57(11):3243–3247. doi: 10.1128/aem.57.11.3243-3247.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauch J. F. Use of immunofluorescence and phase-contrast microscopy for detection and identification of Giardia cysts in water samples. Appl Environ Microbiol. 1985 Dec;50(6):1434–1438. doi: 10.1128/aem.50.6.1434-1438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp D. G., Erlandsen S. L. A new method to determine Giardia cyst viability: correlation of fluorescein diacetate and propidium iodide staining with animal infectivity. Appl Environ Microbiol. 1987 Apr;53(4):704–707. doi: 10.1128/aem.53.4.704-707.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp D. G., Erlandsen S. L. Determination of Giardia muris cyst viability by differential interference contrast, phase, or brightfield microscopy. J Parasitol. 1987 Aug;73(4):723–729. [PubMed] [Google Scholar]
- Smith A. L., Smith H. V. A comparison of fluorescein diacetate and propidium iodide staining and in vitro excystation for determining Giardia intestinalis cyst viability. Parasitology. 1989 Dec;99(Pt 3):329–331. doi: 10.1017/s0031182000059035. [DOI] [PubMed] [Google Scholar]





