Abstract
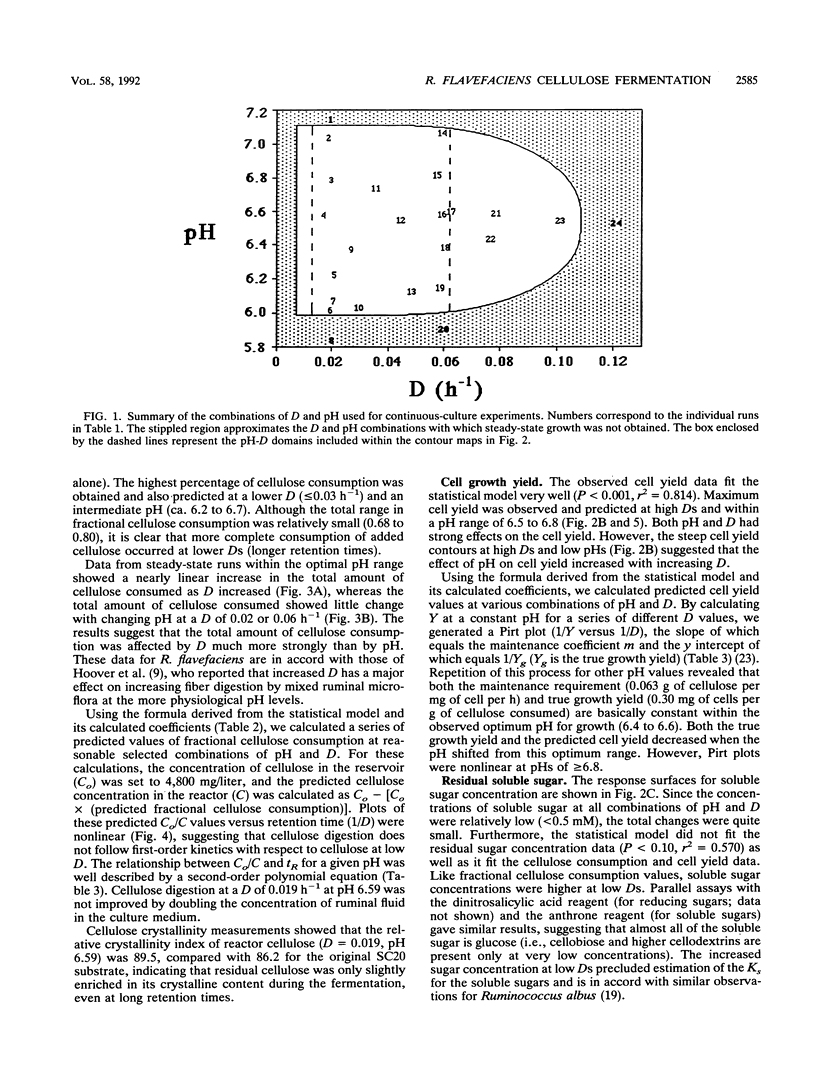
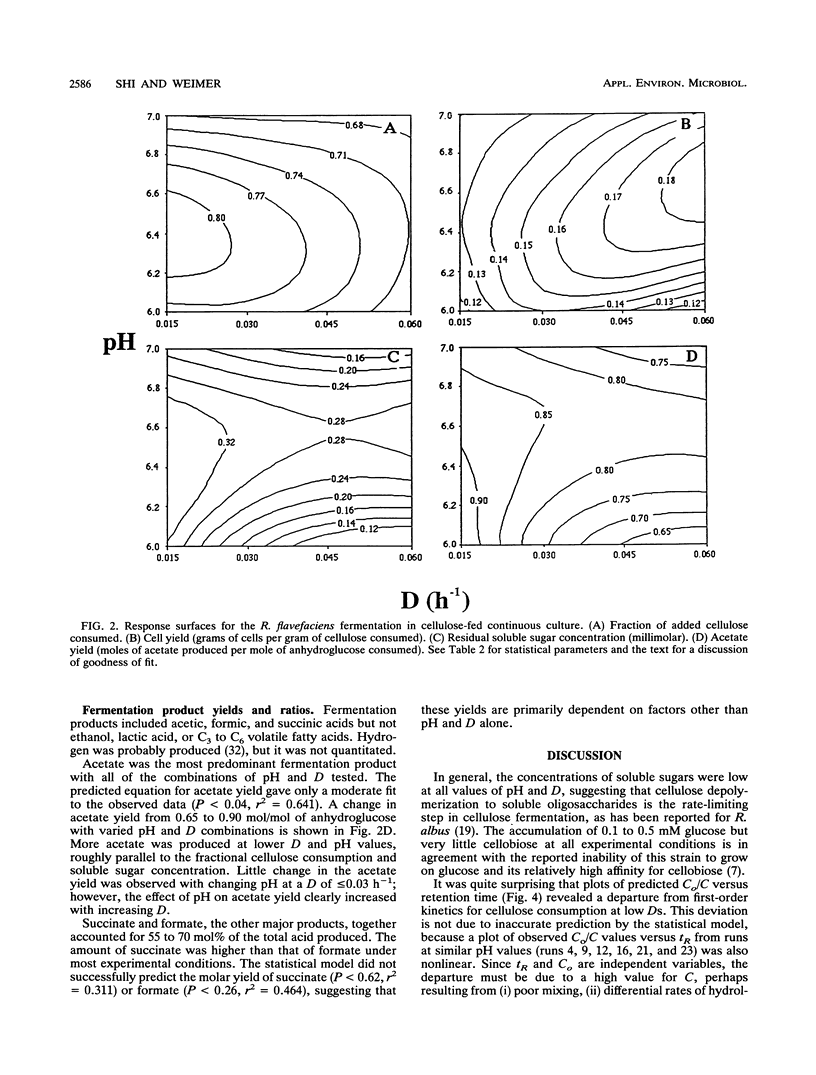
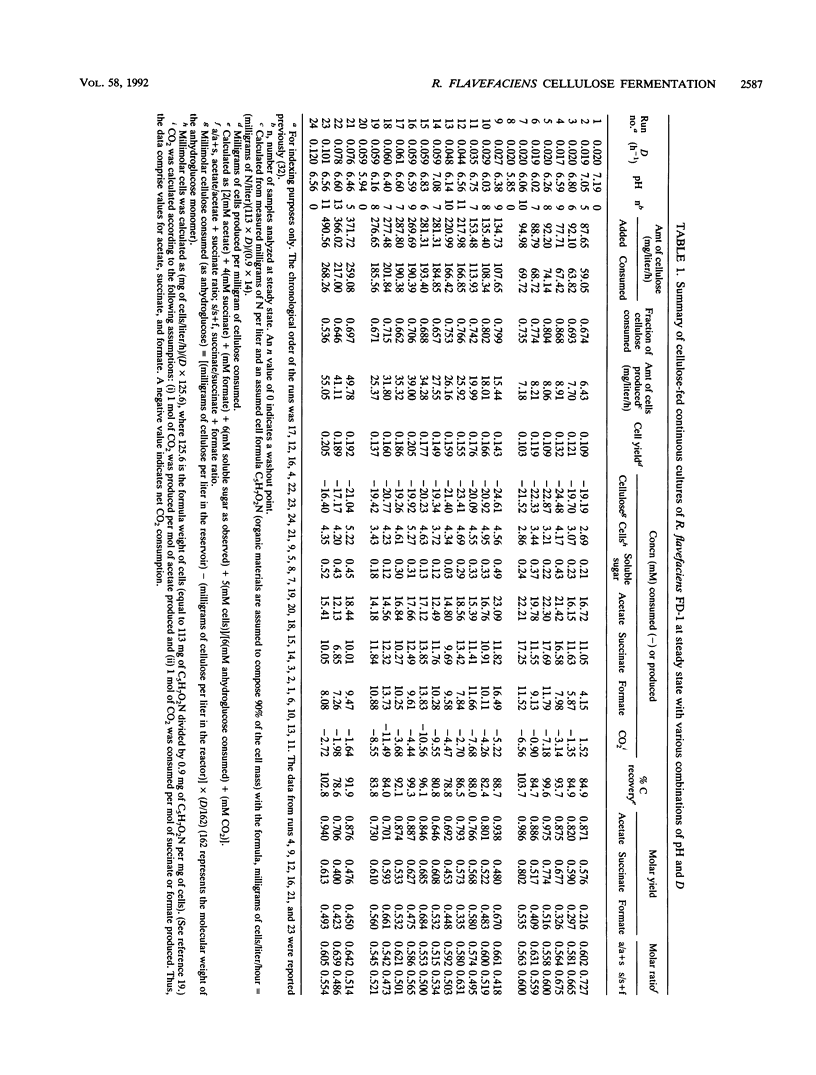
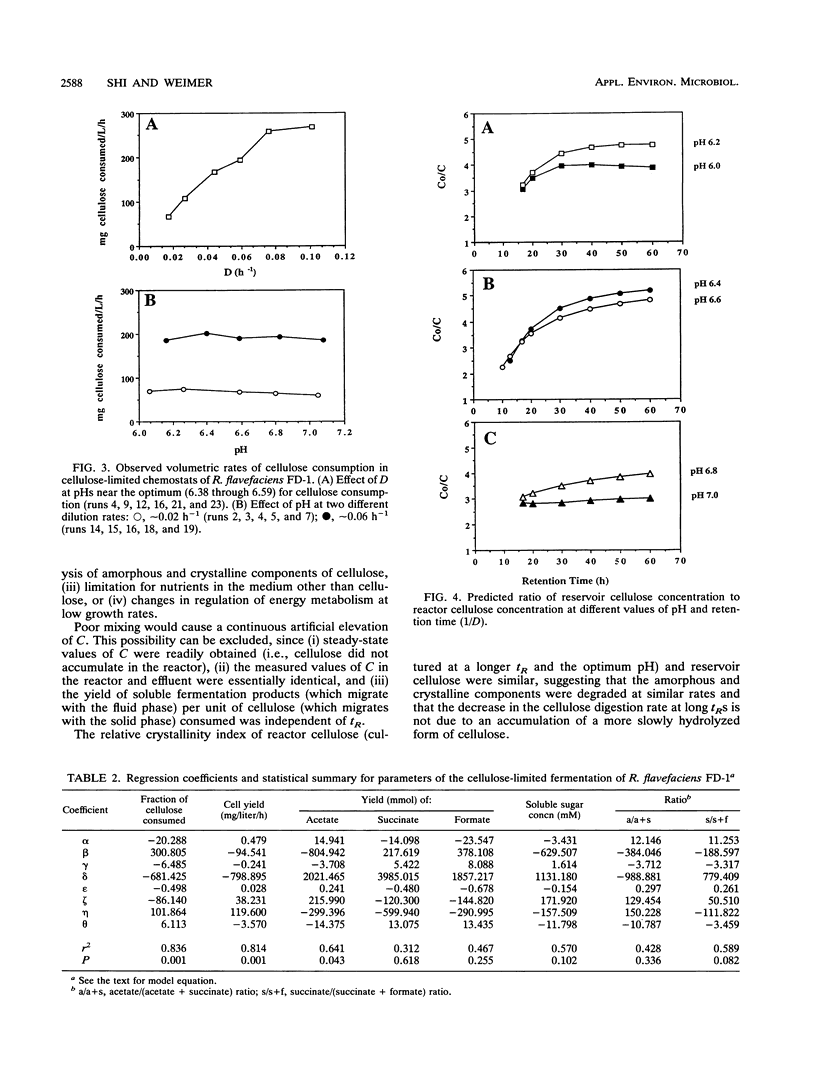
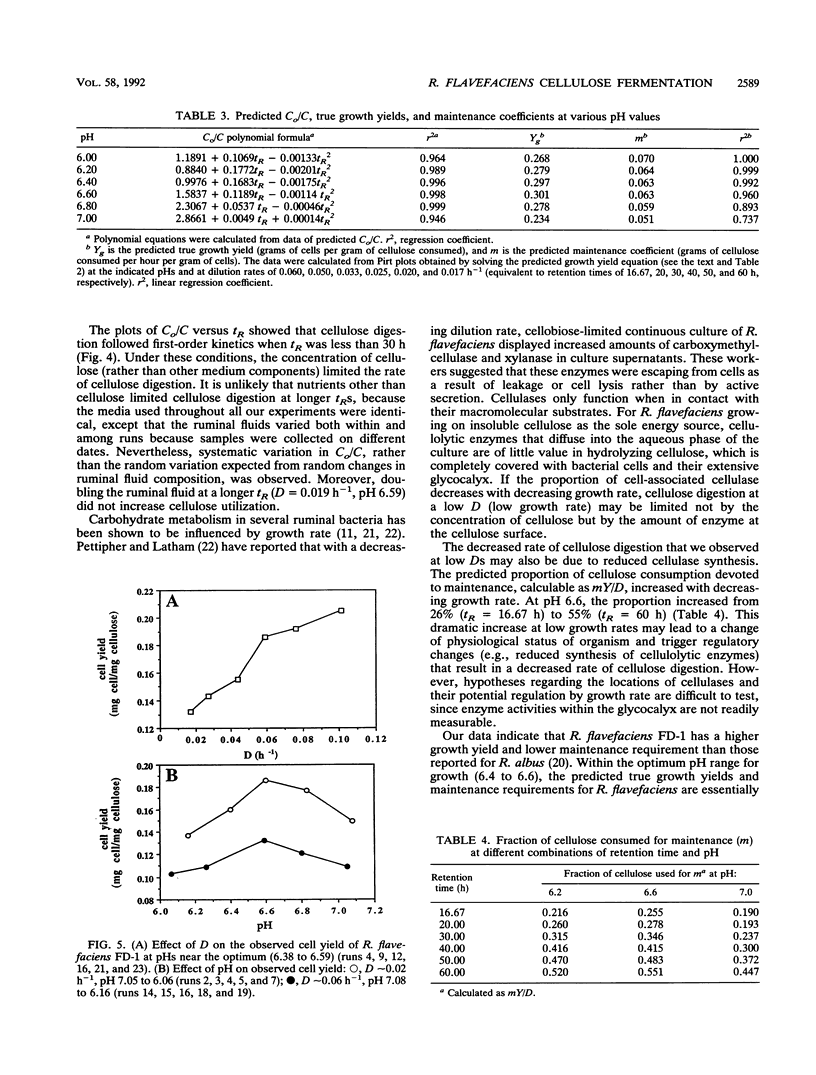
The ruminal cellulolytic bacterium Ruminococcus flavefaciens FD-1 was grown in cellulose-fed continuous culture with 20 different combinations of pH and dilution rate (D); the combinations were selected according to the physiological pH range of the organism (6.0 to 7.1) and growth rate of the organism on cellulose (0.017 to 0.10 h-1). A response surface analysis was used to characterize the effects of pH and D on the extent of cellulose consumption, growth yield, soluble sugar concentration, and yields of fermentation products. The response surfaces indicate that pH and D coordinately affect cellulose digestion and growth yield in this organism. As expected, the net cellulose consumption increased with increasing D while the fraction of added cellulose that was utilized decreased with increasing D. The effect of changes in pH within the physiological range on cellulose consumption was smaller than that of changes in D. Cellulose degradation was less sensitive to low pH than to high pH. At low Ds (longer retention times), cellulose degradation did not follow first-order kinetics. This decreased rate of cellulose digestion was not due to poor mixing, limitation by other medium components, or preferential utilization of the more amorphous fraction of the cellulose. The cell yield increased from 0.13 to 0.18 mg of cells per mg of cellulose with increasing Ds from 0.02 to 0.06 h-1 and decreased when the pH was shifted from the optimum of 6.5 to 6.8. The effect of pH on cell yield increased with increasing D. The reduced cell yield at low pH appears to be due to both an increase in maintenance energy requirements and a decrease in true growth yield.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Estell R. E., 2nd, Galyean M. L. Relationship of rumen fluid dilution rate to rumen fermentation and dietary characteristics of beef steers. J Anim Sci. 1985 Apr;60(4):1061–1071. doi: 10.2527/jas1985.6041061x. [DOI] [PubMed] [Google Scholar]
- Helaszek C. T., White B. A. Cellobiose uptake and metabolism by Ruminococcus flavefaciens. Appl Environ Microbiol. 1991 Jan;57(1):64–68. doi: 10.1128/aem.57.1.64-68.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltner P., Dehority B. A. Effect of soluble carbohydrates on digestion of cellulose by pure cultures of rumen bacteria. Appl Environ Microbiol. 1983 Sep;46(3):642–648. doi: 10.1128/aem.46.3.642-648.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti E. L., Kafkewitz D., Wolin M. J., Bryant M. P. Glucose fermentation products in Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H 2 . J Bacteriol. 1973 Jun;114(3):1231–1240. doi: 10.1128/jb.114.3.1231-1240.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson H. R., Hinds F. C., Bryant M. P., Owens F. N. Efficiency of energy utilization by mixed rumen bacteria in continuous culture. J Dairy Sci. 1975 Nov;58(11):1645–1659. doi: 10.3168/jds.S0022-0302(75)84763-1. [DOI] [PubMed] [Google Scholar]
- Kennedy P. M., Milligan L. P. Effects of cold exposure on digestion, microbial synthesis and nitrogen transformations in sheep. Br J Nutr. 1978 Jan;39(1):105–117. doi: 10.1079/bjn19780017. [DOI] [PubMed] [Google Scholar]
- Pavlostathis S. G., Miller T. L., Wolin M. J. Fermentation of Insoluble Cellulose by Continuous Cultures of Ruminococcus albus. Appl Environ Microbiol. 1988 Nov;54(11):2655–2659. doi: 10.1128/aem.54.11.2655-2659.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlostathis S. G., Miller T. L., Wolin M. J. Kinetics of Insoluble Cellulose Fermentation by Continuous Cultures of Ruminococcus albus. Appl Environ Microbiol. 1988 Nov;54(11):2660–2663. doi: 10.1128/aem.54.11.2660-2663.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirt S. J. Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch Microbiol. 1982 Dec 3;133(4):300–302. doi: 10.1007/BF00521294. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Dombrowski D. B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980 Mar;39(3):604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. S. Factors affecting the cellulolytic activity of rumen contents. Appl Environ Microbiol. 1977 Mar;33(3):497–502. doi: 10.1128/aem.33.3.497-502.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


