Abstract
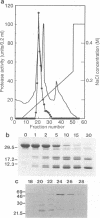
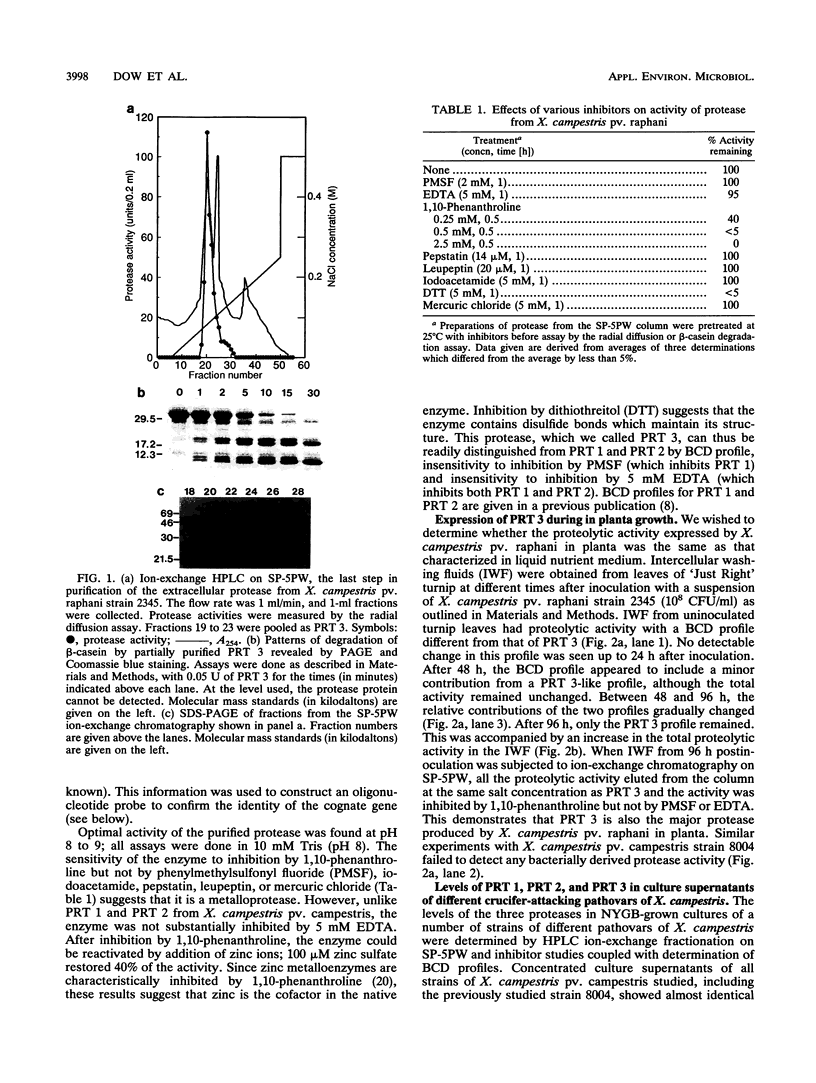
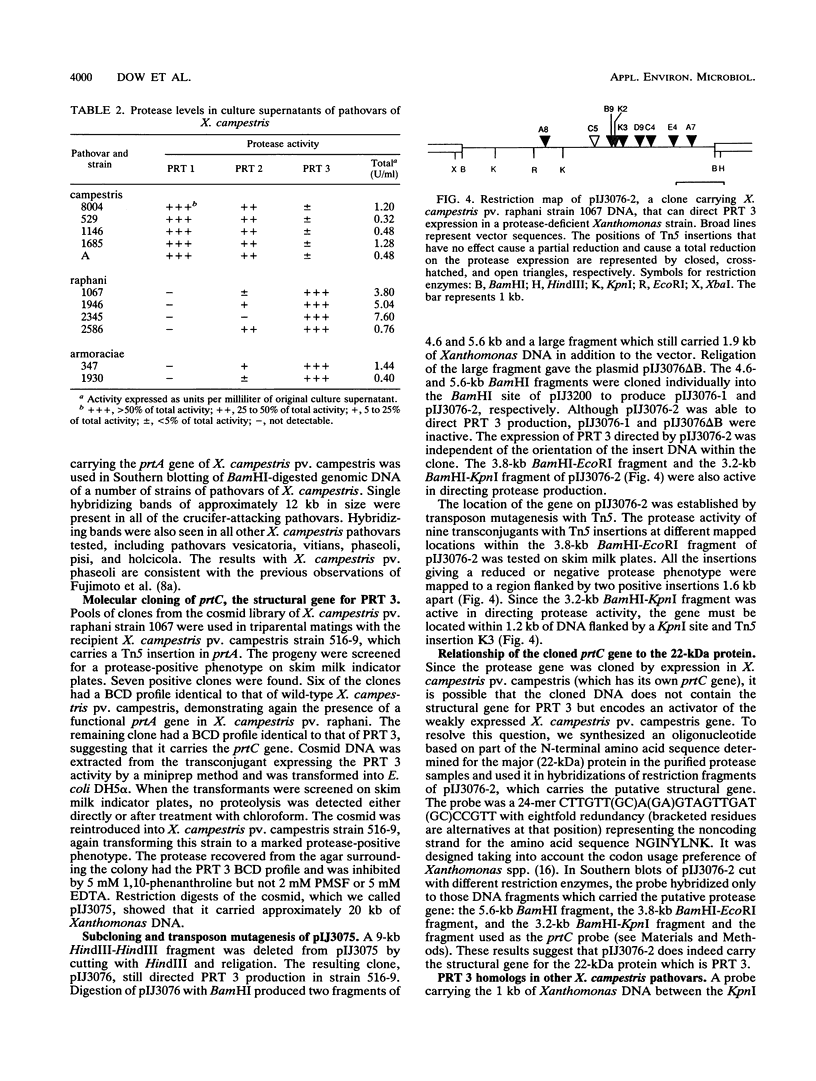
Strains of Xanthomonas campestris pathovars armoraciae and raphani, which cause leaf spotting diseases in brassicas, produce a major extracellular protease in liquid culture which was partially purified. The protease (PRT 3) was a zinc-requiring metalloenzyme and was readily distinguishable from the two previously characterized proteases (PRT 1 and PRT 2) of X. campestris pv. campestris by the pattern of degradation of beta-casein and sensitivity to inhibitors. PRT 3 was produced at a low level in the vascular brassica pathogen X. campestris pv. campestris (five strains tested), in which PRT 1 and PRT 2 predominate. In contrast, expression of PRT 1, a serine protease, could not be detected in the six tested strains of the leaf spotting mesophyll pathogens. However, all these strains had DNA fragments which hybridized to a prtA probe and which probably carry a functional prtA (the structural gene for PRT 1). The structural gene for PRT 3 (prtC) was cloned by screening a genomic library of X. campestris pv. raphani in a protease-deficient X. campestris pv. campestris strain. Subcloning and Tn5 mutagenesis located the structural gene to 1.2 kb of DNA. DNA fragments which hybridized to the structural gene were found in all strains of the crucifer-attacking X. campestris pathovars tested as well as in a number of other pathovars. Experiments in which the pattern of protease production of the pathovars was manipulated by introduction of cloned genes into heterologous pathovars suggested that no determinative relationship exists between the pattern of protease gene expression and the (vascular or mesophyllic) mode of pathogenesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahler G. S., Barras F., Keen N. T. Cloning of genes encoding extracellular metalloproteases from Erwinia chrysanthemi EC16. J Bacteriol. 1990 Oct;172(10):5803–5815. doi: 10.1128/jb.172.10.5803-5815.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow J. M., Clarke B. R., Milligan D. E., Tang J. L., Daniels M. J. Extracellular proteases from Xanthomonas campestris pv. campestris, the black rot pathogen. Appl Environ Microbiol. 1990 Oct;56(10):2994–2998. doi: 10.1128/aem.56.10.2994-2998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heery D. M., Gannon F., Powell R. A simple method for subcloning DNA fragments from gel slices. Trends Genet. 1990 Jun;6(6):173–173. doi: 10.1016/0168-9525(90)90158-3. [DOI] [PubMed] [Google Scholar]
- Liu Y. N., Tang J. L., Clarke B. R., Dow J. M., Daniels M. J. A multipurpose broad host range cloning vector and its use to characterise an extracellular protease gene of Xanthomonas campestris pathovar campestris. Mol Gen Genet. 1990 Feb;220(3):433–440. doi: 10.1007/BF00391750. [DOI] [PubMed] [Google Scholar]
- Osbourn A. E., Clarke B. R., Stevens B. J., Daniels M. J. Use of oligonucleotide probes to identify members of two-component regulatory systems in Xanthomonas campestris pathovar campestris. Mol Gen Genet. 1990 Jun;222(1):145–151. doi: 10.1007/BF00283036. [DOI] [PubMed] [Google Scholar]
- Parker J. E., Barber C. E., Fan M. J., Daniels M. J. Interaction of Xanthomonas campestris with Arabidopsis thaliana: characterization of a gene from X. c. pv. raphani that confers avirulence to most A. thaliana accessions. Mol Plant Microbe Interact. 1993 Mar-Apr;6(2):216–224. doi: 10.1094/mpmi-6-216. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., de Vos W. M., Leunissen J. A., Dijkstra B. W. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Protein Eng. 1991 Oct;4(7):719–737. doi: 10.1093/protein/4.7.719. [DOI] [PubMed] [Google Scholar]
- Spungin A., Blumberg S. Streptomyces griseus aminopeptidase is a calcium-activated zinc metalloprotein. Purification and properties of the enzyme. Eur J Biochem. 1989 Aug 1;183(2):471–477. doi: 10.1111/j.1432-1033.1989.tb14952.x. [DOI] [PubMed] [Google Scholar]