Abstract
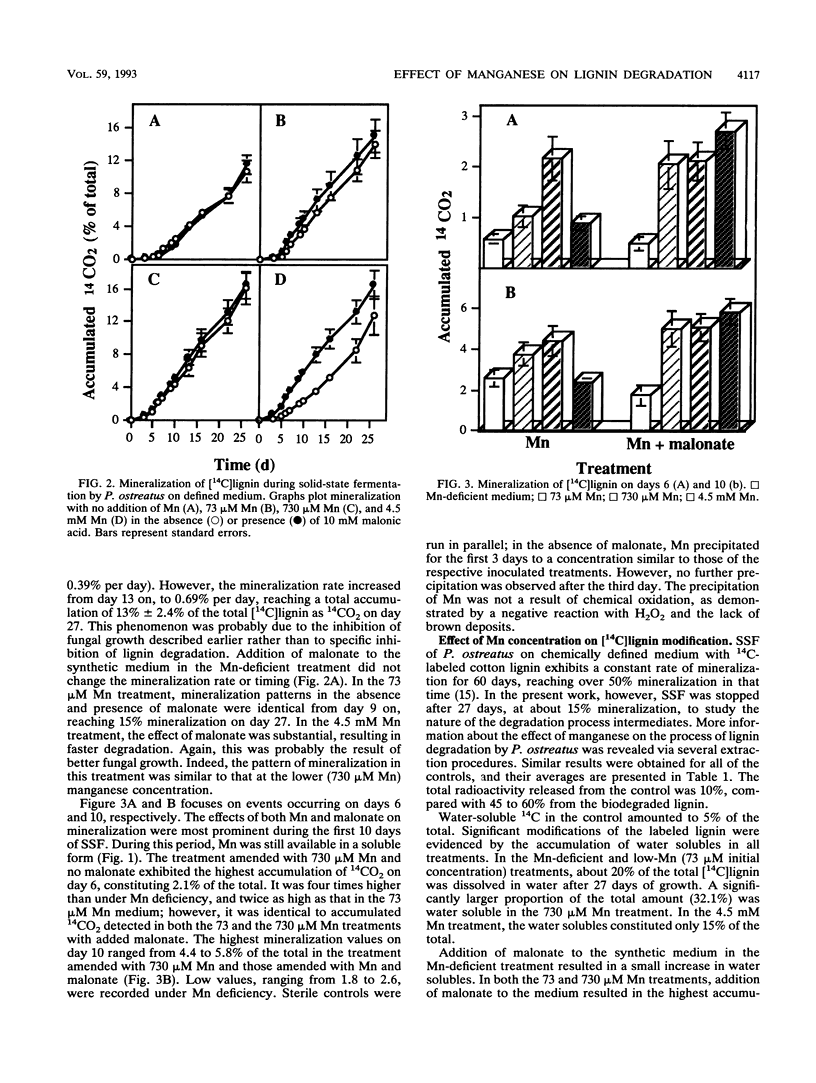
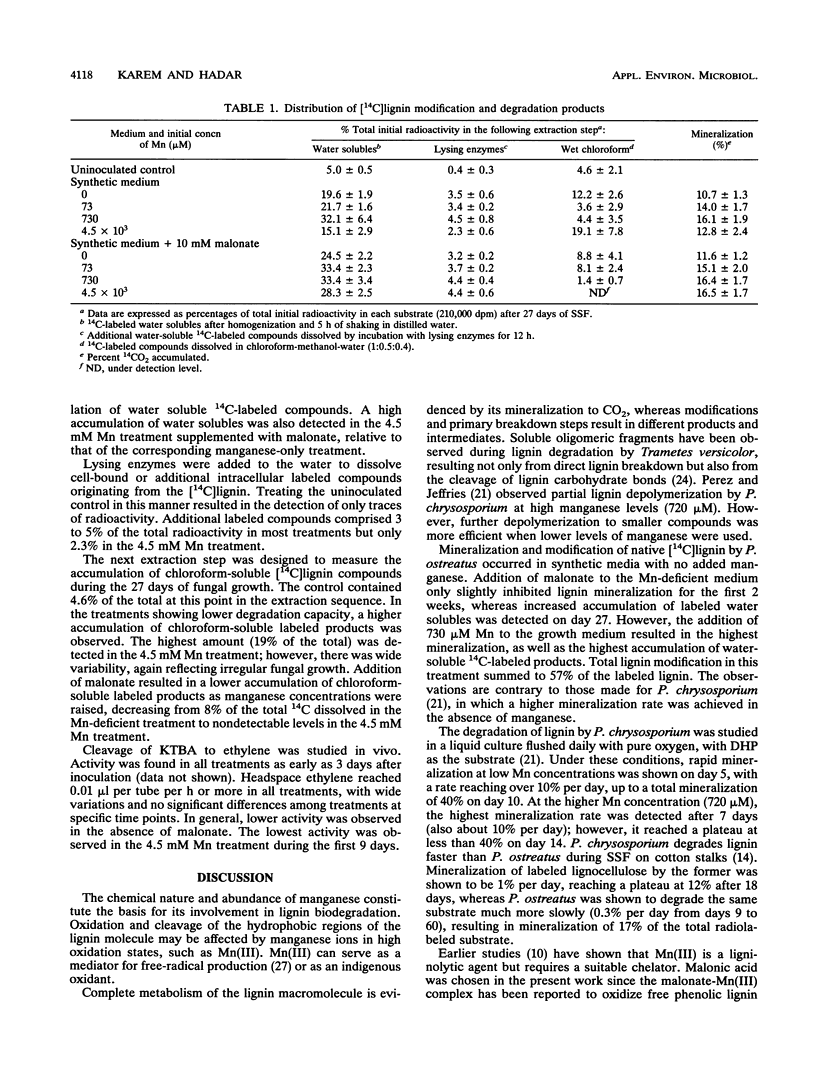
Lignin degradation by Pleurotus ostreatus was studied under solid-state fermentation (SSF) in chemically defined medium containing various levels of Mn. Degradation of [14C]lignin prepared from cotton branches to soluble products, as well as its mineralization to 14CO2, was enhanced by the addition of Mn. The effect of malonate on lignin mineralization was most marked during the first 10 days of SSF, in a treatment amended with 73 μM Mn. A high concentration of Mn (4.5 mM) caused inhibition of both fungal growth and mineralization rates during the first 2 weeks of incubation. Addition of malonate reversed this effect because of chelation of Mn. Mn was found to precipitate in all treatments, with or without the addition of malonate. α-Keto-γ-methiolbutyric acid cleavage to ethylene, an indication of . OH production, was observed as early as 3 days of incubation in all treatments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S. Lignin Peroxidase Activity Is Not Important in Biological Bleaching and Delignification of Unbleached Kraft Pulp by Trametes versicolor. Appl Environ Microbiol. 1992 Sep;58(9):3101–3109. doi: 10.1128/aem.58.9.3101-3109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg P. J., Young L. Y. Anaerobic degradation of soluble fractions of [C-lignin]lignocellulose. Appl Environ Microbiol. 1985 Feb;49(2):345–349. doi: 10.1128/aem.49.2.345-349.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Crawford R. L. Microbial degradation of lignocellulose: the lignin component. Appl Environ Microbiol. 1976 May;31(5):714–717. doi: 10.1128/aem.31.5.714-717.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester I. T., Grabski A. C., Burgess R. R., Leatham G. F. Manganese, Mn-dependent peroxidases, and the biodegradation of lignin. Biochem Biophys Res Commun. 1988 Dec 30;157(3):992–999. doi: 10.1016/s0006-291x(88)80972-0. [DOI] [PubMed] [Google Scholar]
- Guillén F., Martínez A. T., Martínez M. J. Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur J Biochem. 1992 Oct 15;209(2):603–611. doi: 10.1111/j.1432-1033.1992.tb17326.x. [DOI] [PubMed] [Google Scholar]
- Kerem Z., Friesem D., Hadar Y. Lignocellulose Degradation during Solid-State Fermentation: Pleurotus ostreatus versus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Apr;58(4):1121–1127. doi: 10.1128/aem.58.4.1121-1127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern D. H., Weisenthal L. M. Highly specific prediction of antineoplastic drug resistance with an in vitro assay using suprapharmacologic drug exposures. J Natl Cancer Inst. 1990 Apr 4;82(7):582–588. doi: 10.1093/jnci/82.7.582. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Asada Y., Kuwahara M. Screening of basidiomycetes for lignin peroxidase genes using a DNA probe. Appl Microbiol Biotechnol. 1990 Jan;32(4):436–442. doi: 10.1007/BF00903779. [DOI] [PubMed] [Google Scholar]
- Perez J., Jeffries T. W. Mineralization of C-Ring-Labeled Synthetic Lignin Correlates with the Production of Lignin Peroxidase, not of Manganese Peroxidase or Laccase. Appl Environ Microbiol. 1990 Jun;56(6):1806–1812. doi: 10.1128/aem.56.6.1806-1812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J., Jeffries T. W. Roles of manganese and organic acid chelators in regulating lignin degradation and biosynthesis of peroxidases by Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Aug;58(8):2402–2409. doi: 10.1128/aem.58.8.2402-2409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor W. A., Tang R. H. Ethylene formation from methional. Biochem Biophys Res Commun. 1978 Mar 30;81(2):498–503. doi: 10.1016/0006-291x(78)91562-0. [DOI] [PubMed] [Google Scholar]
- Périé F. H., Gold M. H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991 Aug;57(8):2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J Biol Chem. 1992 Nov 25;267(33):23688–23695. [PubMed] [Google Scholar]
- Winterbourn C. C. The ability of scavengers to distinguish OH. production in the iron-catalyzed Haber-Weiss reaction: comparison of four assays for OH. Free Radic Biol Med. 1987;3(1):33–39. doi: 10.1016/0891-5849(87)90037-2. [DOI] [PubMed] [Google Scholar]


