Abstract
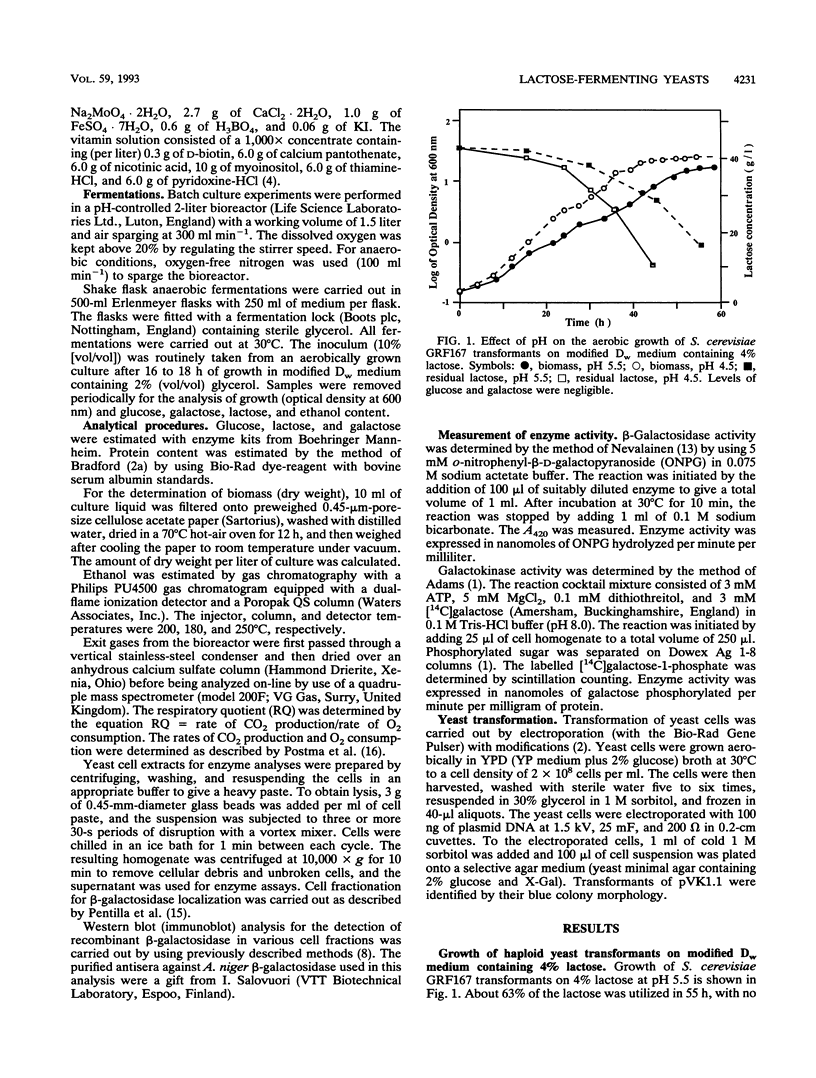
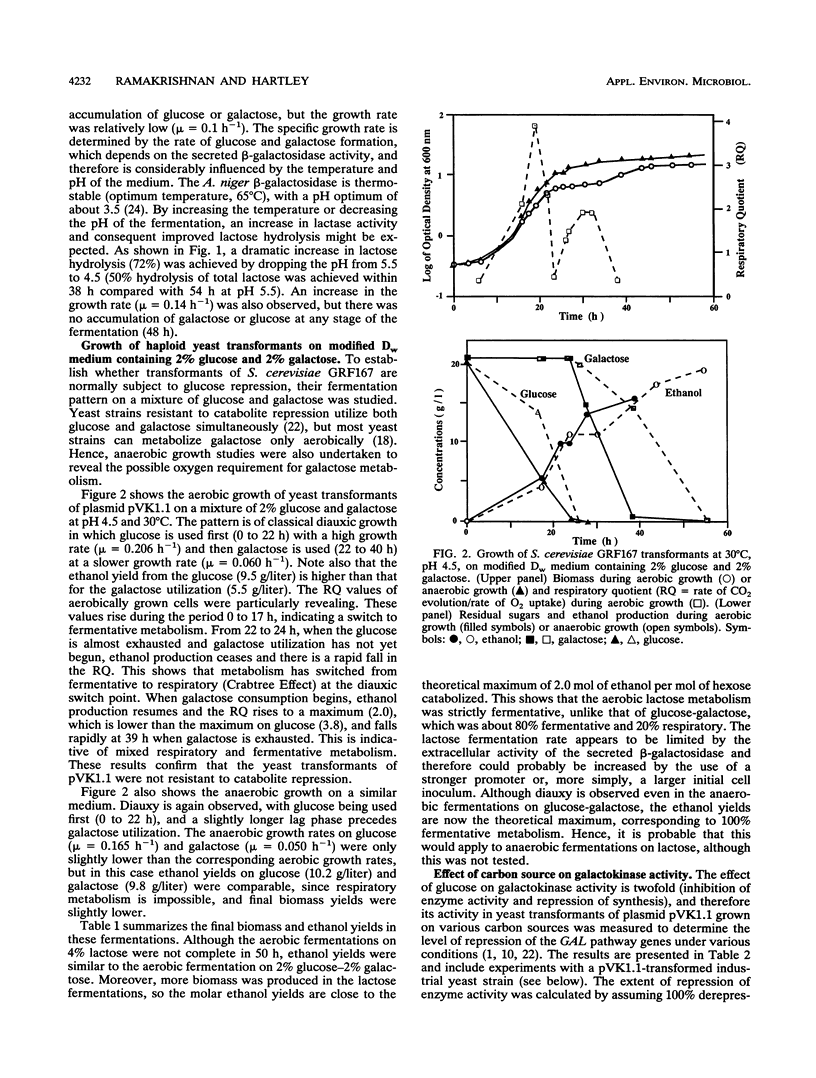
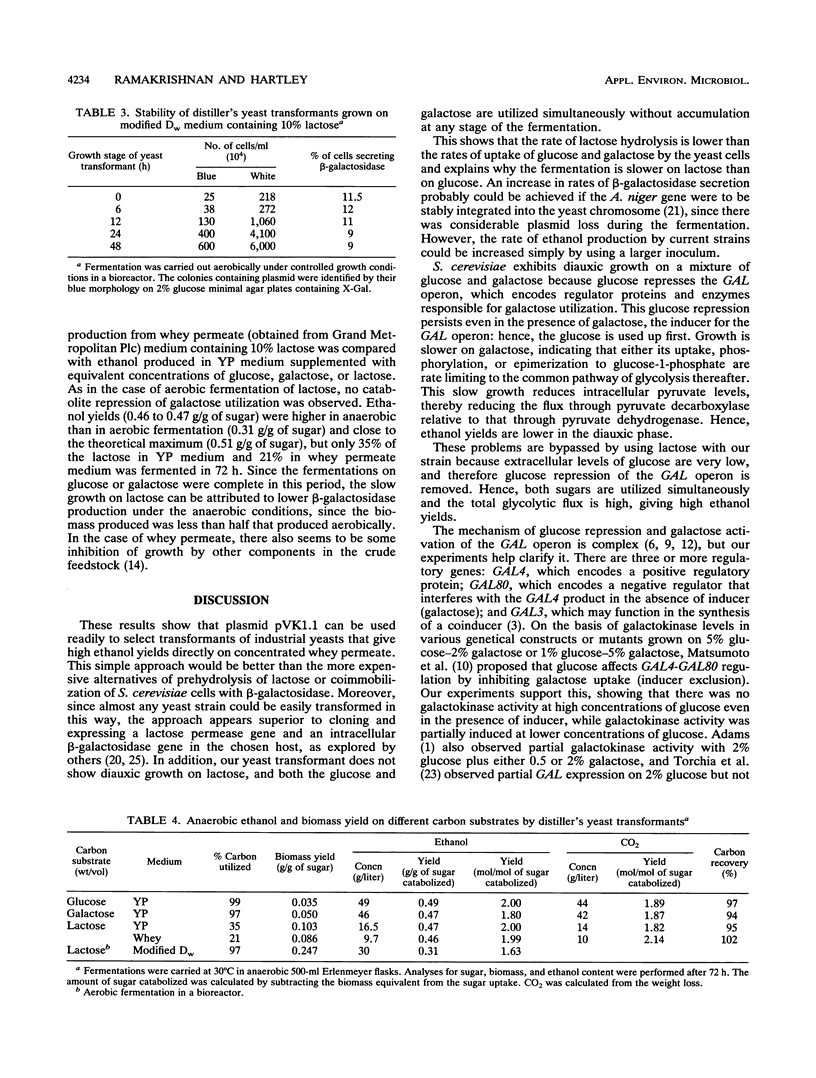
Strains of Saccharomyces cerevisiae transformed with a yeast multicopy expression vector carrying the cDNA for Aspergillus niger secretory beta-galactosidase under the control of ADH1 promoter and terminator were studied for their fermentation properties on lactose (V. Kumar, S. Ramakrishnan, T. T. Teeri, J. K. C. Knowles, and B. S. Hartley, Biotechnology 10:82-85, 1992). Lactose was hydrolyzed extracellularly into glucose and galactose, and both sugars were utilized simultaneously. Diauxic growth patterns were not observed. However, a typical biphasic growth was observed on a mixture of glucose and galactose under aerobic and anaerobic conditions with transformants of a haploid S. cerevisiae strain, GRF167. Polyploid distiller's yeast (Mauri) transformants were selected simply on the basis of the cloned gene expression on X-Gal (5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside) plates. Rapid and complete lactose hydrolysis and higher ethanol (0.31 g/g of sugar) and biomass (0.24 g/g of sugar) production were observed with distiller's yeast grown under aerobic conditions. A constant proportion (10%) of the population retained the plasmid throughout the fermentation period (48 h). Nearly theoretical yields of ethanol were obtained under anaerobic conditions on lactose, glucose, galactose, and whey permeate media. However, the rate and the amount of lactose hydrolysis were lower under anaerobic than aerobic conditions. All lactose-grown cells expressed partial galactokinase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. G. Induction of galactokinase in Saccharomyces cerevisiae: kinetics of induction and glucose effects. J Bacteriol. 1972 Aug;111(2):308–315. doi: 10.1128/jb.111.2.308-315.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D. M., Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlson M. Regulation of sugar utilization in Saccharomyces species. J Bacteriol. 1987 Nov;169(11):4873–4877. doi: 10.1128/jb.169.11.4873-4877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiechter A., Fuhrmann G. F., Käppeli O. Regulation of glucose metabolism in growing yeast cells. Adv Microb Physiol. 1981;22:123–183. doi: 10.1016/s0065-2911(08)60327-6. [DOI] [PubMed] [Google Scholar]
- Hage-van Noort M., Puijk W. C., Plasman H. H., Kuperus D., Schaaper W. M., Beekman N. J., Grootegoed J. A., Meloen R. H. Synthetic peptides based upon a three-dimensional model for the receptor recognition site of follicle-stimulating hormone exhibit antagonistic or agonistic activity at low concentrations. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3922–3926. doi: 10.1073/pnas.89.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae. Microbiol Rev. 1987 Dec;51(4):458–476. doi: 10.1128/mr.51.4.458-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Ramakrishnan S., Teeri T. T., Knowles J. K., Hartley B. S. Saccharomyces cerevisiae cells secreting an Aspergillus niger beta-galactosidase grow on whey permeate. Biotechnology (N Y) 1992 Jan;10(1):82–85. doi: 10.1038/nbt0192-82. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Yoshimatsu T., Oshima Y. Recessive mutations conferring resistance to carbon catabolite repression of galactokinase synthesis in Saccharomyces cerevisiae. J Bacteriol. 1983 Mar;153(3):1405–1414. doi: 10.1128/jb.153.3.1405-1414.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlin J. O., Carlberg M., Ronne H. Control of yeast GAL genes by MIG1 repressor: a transcriptional cascade in the glucose response. EMBO J. 1991 Nov;10(11):3373–3377. doi: 10.1002/j.1460-2075.1991.tb04901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen K. M. Induction, isolation, and characterization of aspergillus niger mutant strains producing elevated levels of beta-galactosidase. Appl Environ Microbiol. 1981 Mar;41(3):593–596. doi: 10.1128/aem.41.3.593-596.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttilä M. E., André L., Saloheimo M., Lehtovaara P., Knowles J. K. Expression of two Trichoderma reesei endoglucanases in the yeast Saccharomyces cerevisiae. Yeast. 1987 Sep;3(3):175–185. doi: 10.1002/yea.320030305. [DOI] [PubMed] [Google Scholar]
- Postma E., Verduyn C., Scheffers W. A., Van Dijken J. P. Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989 Feb;55(2):468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding A., Tuite M. F. Host-plasmid interactions in Saccharomyces cerevisiae: effect of host ploidy on plasmid stability and copy number. J Gen Microbiol. 1989 Apr;135(4):1037–1045. doi: 10.1099/00221287-135-4-1037. [DOI] [PubMed] [Google Scholar]
- Sreekrishna K., Dickson R. C. Construction of strains of Saccharomyces cerevisiae that grow on lactose. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7909–7913. doi: 10.1073/pnas.82.23.7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Ma H., Botstein D. Manipulating yeast genome using plasmid vectors. Methods Enzymol. 1990;185:280–297. doi: 10.1016/0076-6879(90)85025-j. [DOI] [PubMed] [Google Scholar]
- Terrell S. L., Bernard A., Bailey R. B. Ethanol from Whey: Continuous Fermentation with a Catabolite Repression-Resistant Saccharomyces cerevisiae Mutant. Appl Environ Microbiol. 1984 Sep;48(3):577–580. doi: 10.1128/aem.48.3.577-580.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchia T. E., Hamilton R. W., Cano C. L., Hopper J. E. Disruption of regulatory gene GAL80 in Saccharomyces cerevisiae: effects on carbon-controlled regulation of the galactose/melibiose pathway genes. Mol Cell Biol. 1984 Aug;4(8):1521–1527. doi: 10.1128/mcb.4.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer F., Leuba J. L. beta-Galactosidase from Aspergillus niger. Separation and characterization of three multiple forms. Eur J Biochem. 1979 Oct 15;100(2):559–567. doi: 10.1111/j.1432-1033.1979.tb04202.x. [DOI] [PubMed] [Google Scholar]
- Yocum R. R., Johnston M. Molecular cloning of the GAL80 gene from Saccharomyces cerevisiae and characterization of a gal80 deletion. Gene. 1984 Dec;32(1-2):75–82. doi: 10.1016/0378-1119(84)90034-9. [DOI] [PubMed] [Google Scholar]


