Abstract
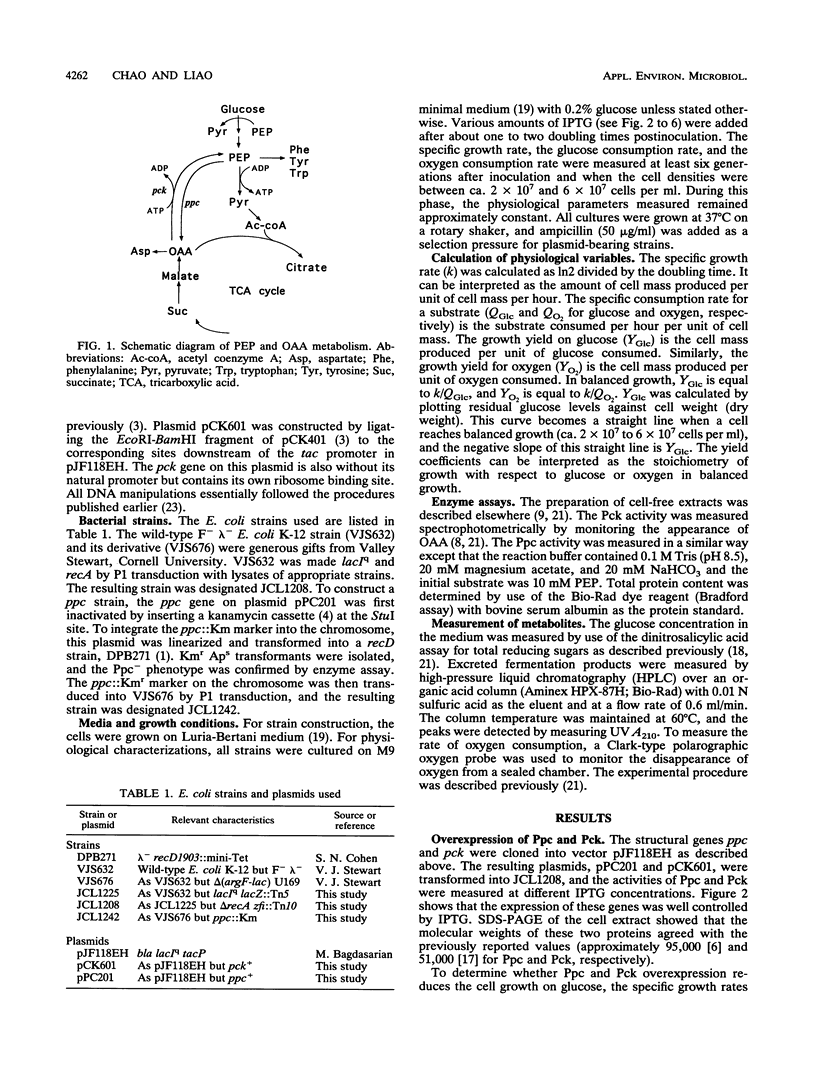
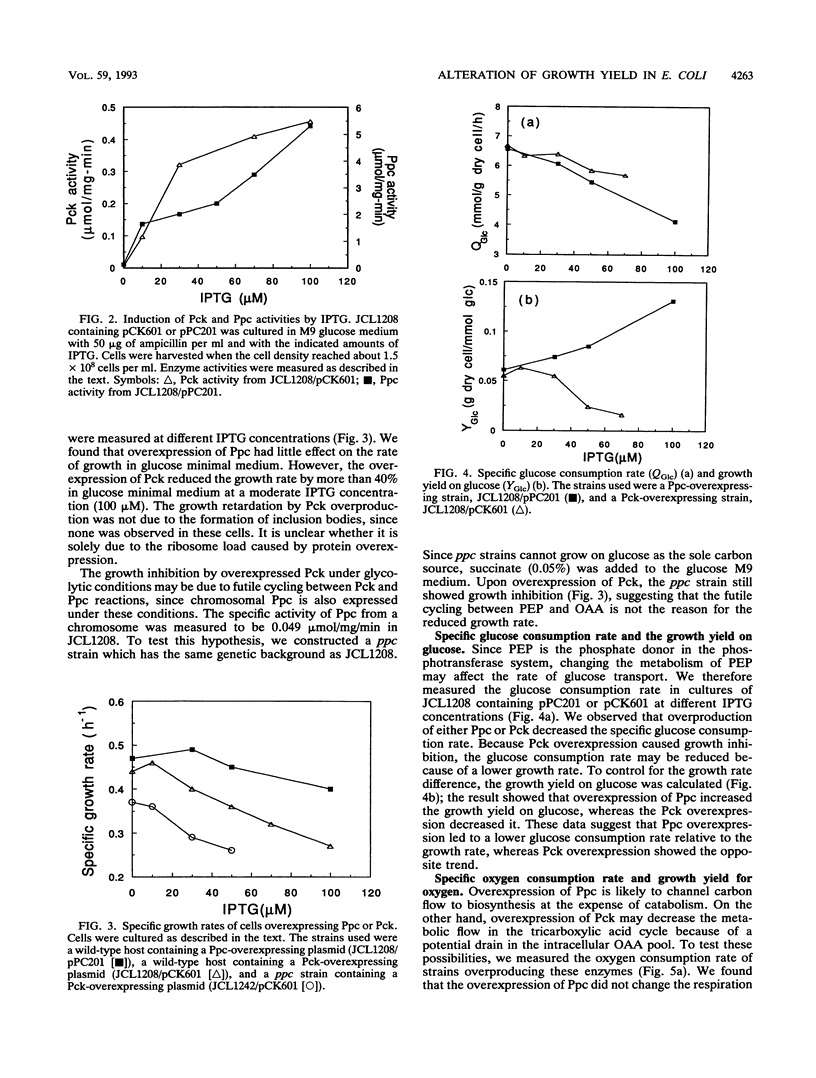
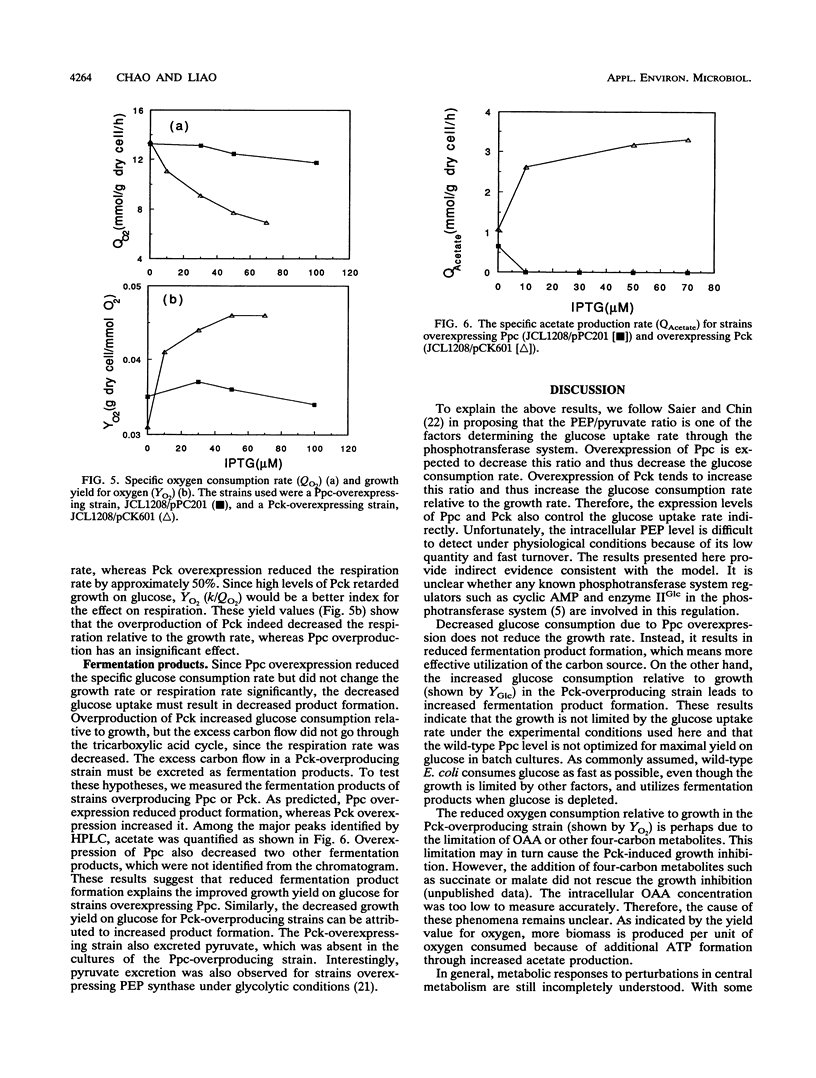
Phosphoenolpyruvate and oxaloacetate are key intermediates at the junction between catabolism and biosynthesis. Alteration of carbon flow at these branch points will affect the growth yield and the formation of products. We attempted to modulate the metabolic flow between phosphoenolpyruvate and oxaloacetate by overexpressing phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase from a multicopy plasmid under the control of the tac promoter. It was found that overexpression of phosphoenolpyruvate carboxylase decreased the rates of glucose consumption and organic acid excretion, but the growth and respiration rates remained unchanged. Consequently, the growth yield on glucose was improved. This result indicates that the wild-type level of phosphoenolpyruvate carboxylase is not optimal for the most efficient glucose utilization in batch cultures. On the other hand, overexpression of phosphoenolpyruvate carboxykinase increased glucose consumption and decreased oxygen consumption relative to those levels required for growth. Therefore, the growth yield on glucose was reduced because of a higher rate of fermentation product excretion. These data provide useful insights into the regulation of central metabolism and facilitate further manipulation of pathways for metabolite production.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biek D. P., Cohen S. N. Identification and characterization of recD, a gene affecting plasmid maintenance and recombination in Escherichia coli. J Bacteriol. 1986 Aug;167(2):594–603. doi: 10.1128/jb.167.2.594-603.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y. P., Patnaik R., Roof W. D., Young R. F., Liao J. C. Control of gluconeogenic growth by pps and pck in Escherichia coli. J Bacteriol. 1993 Nov;175(21):6939–6944. doi: 10.1128/jb.175.21.6939-6944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Gross L., Joho K. E., McAllister W. T. A modified kanamycin-resistance cassette to facilitate two-codon insertion mutagenesis. Gene. 1992 Feb 1;111(1):143–144. doi: 10.1016/0378-1119(92)90617-x. [DOI] [PubMed] [Google Scholar]
- De Reuse H., Danchin A. Positive regulation of the pts operon of Escherichia coli: genetic evidence for a signal transduction mechanism. J Bacteriol. 1991 Jan;173(2):727–733. doi: 10.1128/jb.173.2.727-733.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N., Miwa T., Ishijima S., Izui K., Katsuki H. The primary structure of phosphoenolpyruvate carboxylase of Escherichia coli. Nucleotide sequence of the ppc gene and deduced amino acid sequence. J Biochem. 1984 Apr;95(4):909–916. doi: 10.1093/oxfordjournals.jbchem.a134718. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Goldie A. H., Sanwal B. D. Allosteric control by calcium and mechanism of desensitization of phosphoenolpyruvate carboxykinase of Escherichia coli. J Biol Chem. 1980 Feb 25;255(4):1399–1405. [PubMed] [Google Scholar]
- Goldie A. H., Sanwal B. D. Genetic and physiological characterization of Escherichia coli mutants deficient in phosphoenolpyruvate carboxykinase activity. J Bacteriol. 1980 Mar;141(3):1115–1121. doi: 10.1128/jb.141.3.1115-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie H. Regulation of transcription of the Escherichia coli phosphoenolpyruvate carboxykinase locus: studies with pck-lacZ operon fusions. J Bacteriol. 1984 Sep;159(3):832–836. doi: 10.1128/jb.159.3.832-836.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui K. Kinetic studies on the allosteric nature of phosphoenolpyruvate carboxylase from Escherichia coli. J Biochem. 1970 Aug;68(2):227–238. doi: 10.1093/oxfordjournals.jbchem.a129350. [DOI] [PubMed] [Google Scholar]
- Izui K., Nishikido T., Ishihara K., Katsuki H. Studies on the allosteric effectors and some properties of phosphoenolpyruvate carboxylase from Escherichia coli. J Biochem. 1970 Aug;68(2):215–226. doi: 10.1093/oxfordjournals.jbchem.a129349. [DOI] [PubMed] [Google Scholar]
- Izui K., Taguchi M., Morikawa M., Katsuki H. Regulation of Escherichia coli phosphoenolpyruvate carboxylase by multiple effectors in vivo. II. Kinetic studies with a reaction system containing physiological concentrations of ligands. J Biochem. 1981 Nov;90(5):1321–1331. doi: 10.1093/oxfordjournals.jbchem.a133597. [DOI] [PubMed] [Google Scholar]
- Jensen P. R., Westerhoff H. V., Michelsen O. Excess capacity of H(+)-ATPase and inverse respiratory control in Escherichia coli. EMBO J. 1993 Apr;12(4):1277–1282. doi: 10.1002/j.1460-2075.1993.tb05772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs A., Bridger W. A. The kinetic properties of phosphoenolpyruvate carboxykinase of Escherichia coli. Can J Biochem. 1980 Apr;58(4):309–318. doi: 10.1139/o80-041. [DOI] [PubMed] [Google Scholar]
- Medina V., Pontarollo R., Glaeske D., Tabel H., Goldie H. Sequence of the pckA gene of Escherichia coli K-12: relevance to genetic and allosteric regulation and homology of E. coli phosphoenolpyruvate carboxykinase with the enzymes from Trypanosoma brucei and Saccharomyces cerevisiae. J Bacteriol. 1990 Dec;172(12):7151–7156. doi: 10.1128/jb.172.12.7151-7156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M., Izui K., Taguchi M., Katsuki H. Regulation of Escherichia coli phosphoenolpyruvate carboxylase by multiple effectors in vivo. Estimation of the activities in the cells grown on various compounds. J Biochem. 1980 Feb;87(2):441–449. doi: 10.1093/oxfordjournals.jbchem.a132764. [DOI] [PubMed] [Google Scholar]
- Patnaik R., Roof W. D., Young R. F., Liao J. C. Stimulation of glucose catabolism in Escherichia coli by a potential futile cycle. J Bacteriol. 1992 Dec;174(23):7527–7532. doi: 10.1128/jb.174.23.7527-7532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi M., Izui K., Katsuki H. Activation of Escherichia coli phosphoenolpyruvate carboxylase by guanosine-5'-diphosphate-3'-diphosphate. FEBS Lett. 1977 May 15;77(2):270–272. doi: 10.1016/0014-5793(77)80249-4. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


