Abstract
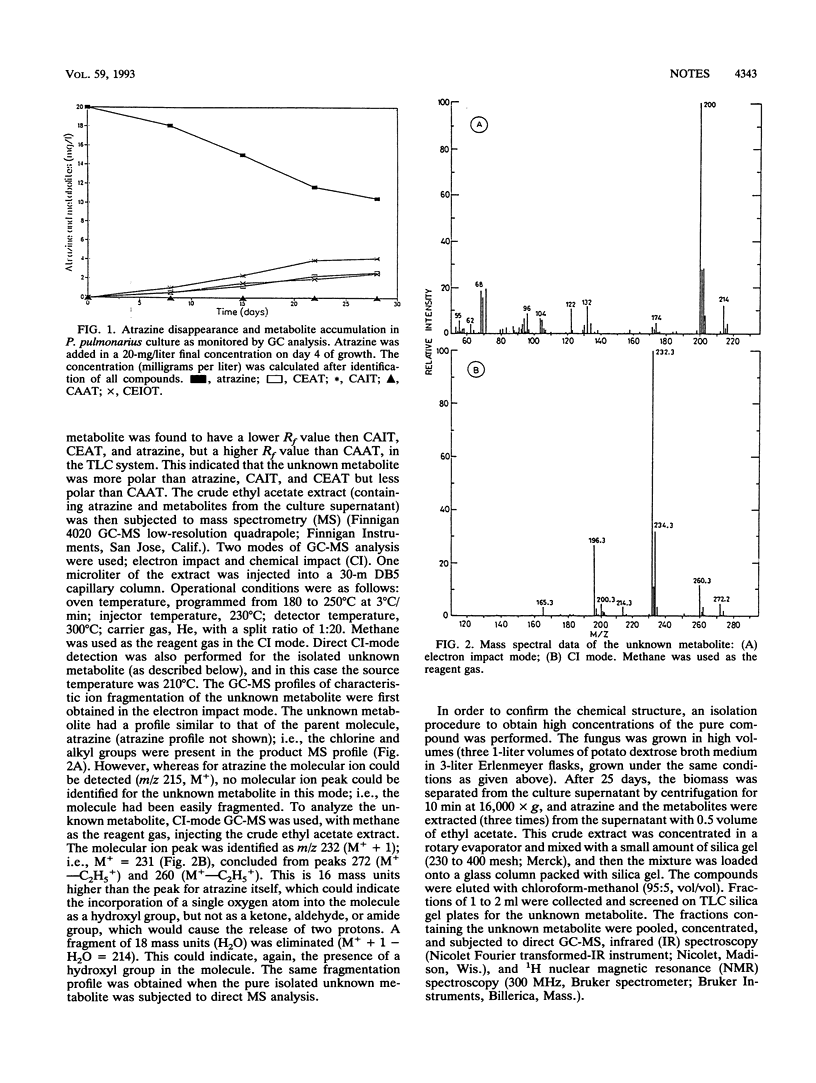
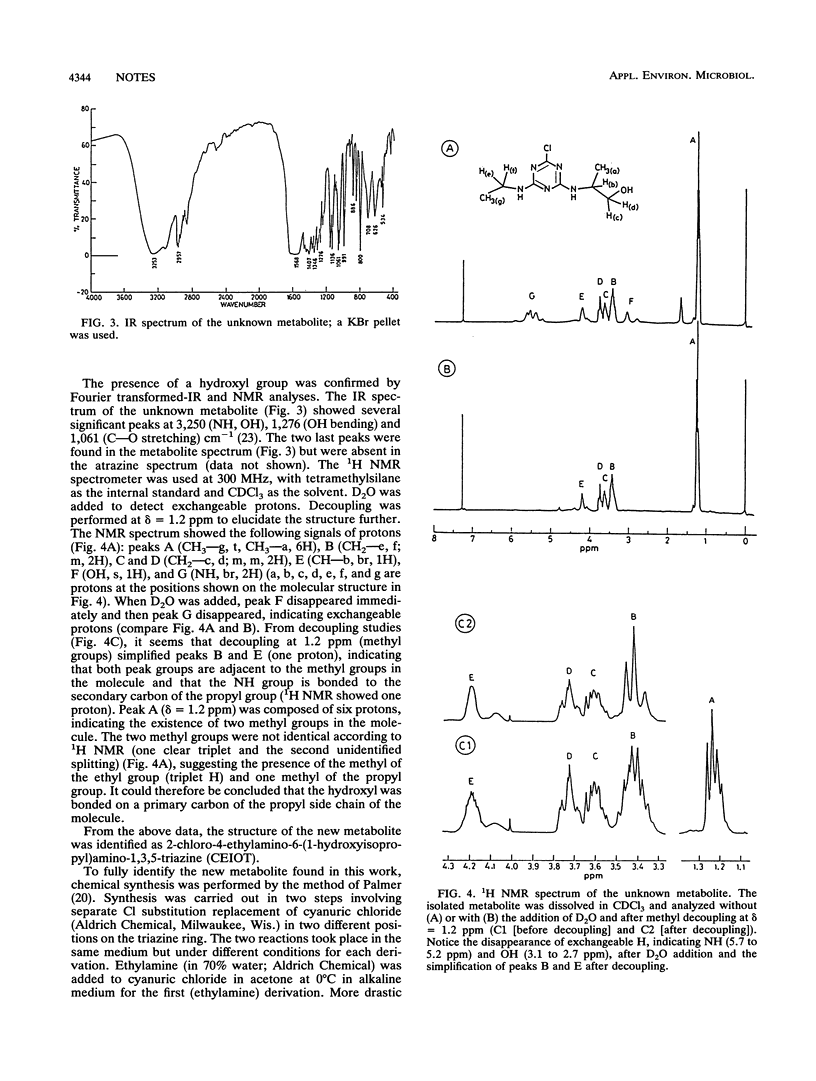
The white rot fungus Pleurotus pulmonarius exhibited metabolism of atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine) in liquid culture, producing the dealkylated metabolites desethylatrazine, desisopropylatrazine, and desethyl-desisopropylatrazine. A fourth, unknown metabolite was also produced. It was isolated and was identified as 2-chloro-4-ethylamino-6-(1-hydroxyisopropyl)amino-1,3,5-triazine by gas chromatography-mass spectrometry, Fourier transformed infrared spectroscopy, and 1H nuclear magnetic resonance analysis. The structure of this metabolite was confirmed by chemical synthesis of the compound and comparison with the fungally produced metabolite.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollag J. M. Biochemical transformation of pesticides by soil fungi. CRC Crit Rev Microbiol. 1972 Nov;2(1):35–58. doi: 10.3109/10408417209108382. [DOI] [PubMed] [Google Scholar]
- Bollag J. M., Liu S. Y. Hydroxylations of carbaryl by soil fungi. Nature. 1972 Mar 24;236(5343):177–178. doi: 10.1038/236177a0. [DOI] [PubMed] [Google Scholar]
- Jordan L. S., Farmer W. J., Goodin J. R., Day B. E. Nonbiological detoxication of the s-triazine herbicides. Residue Rev. 1970;32:267–286. doi: 10.1007/978-1-4615-8464-3_10. [DOI] [PubMed] [Google Scholar]
- Kaufman D. D., Kearney P. C. Microbial degradation of s-triazine herbicides. Residue Rev. 1970;32:235–265. doi: 10.1007/978-1-4615-8464-3_9. [DOI] [PubMed] [Google Scholar]
- Okazaki O., Guengerich F. P. Evidence for specific base catalysis in N-dealkylation reactions catalyzed by cytochrome P450 and chloroperoxidase. Differences in rates of deprotonation of aminium radicals as an explanation for high kinetic hydrogen isotope effects observed with peroxidases. J Biol Chem. 1993 Jan 25;268(3):1546–1552. [PubMed] [Google Scholar]
- Pothuluri J. V., Freeman J. P., Evans F. E., Cerniglia C. E. Fungal metabolism of acenaphthene by Cunninghamella elegans. Appl Environ Microbiol. 1992 Nov;58(11):3654–3659. doi: 10.1128/aem.58.11.3654-3659.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


