Abstract
Responses by marine top predators to environmental variability have previously been almost impossible to observe directly. By using animal-mounted instruments simultaneously recording movements, diving behavior, and in situ oceanographic properties, we studied the behavioral and physiological responses of southern elephant seals to spatial environmental variability throughout their circumpolar range. Improved body condition of seals in the Atlantic sector was associated with Circumpolar Deep Water upwelling regions within the Antarctic Circumpolar Current, whereas High-Salinity Shelf Waters or temperature/salinity gradients under winter pack ice were important in the Indian and Pacific sectors. Energetic consequences of these variations could help explain recently observed population trends, showing the usefulness of this approach in examining the sensitivity of top predators to global and regional-scale climate variability.
Keywords: body condition, ocean observation, oceanography, elephant seals
The Southern Ocean (SO) is one of the most productive of the world's oceans, mainly a result of short, intensive spring phytoplankton blooms (1). Because of restrictions on land–ocean–atmosphere interactions by the Antarctic ice cap, nutrient supply via rivers and dust is generally small or absent. Input of sedimentary nutrients is limited to coastal shelves, whereas pelagic waters over deep basins can be enriched via nutrient release from melting sea ice (2), advection of nutrient-rich water masses from upstream shelf regions (3), or upwelling from distant sediment sources (4). The spatial and temporal distribution of nutrients is therefore highly influenced by interactions between bottom topography, water mass properties, ocean currents, and sea–ice dynamics. Significant phytoplankton blooms occur mostly on continental or island shelves, in the wake of the retreating sea ice or along frontal systems within the Antarctic Circumpolar Current (ACC) (5). Understanding the responses of higher trophic levels to such spatial and temporal variability is fundamental to the effective management of living resources in the SO, and for predicting how animals may respond to climate change and the consequent changes in ocean circulation, ice dynamics, and biogeochemistry.
It is often difficult or impossible to observe directly how marine predators interact with their environment and the prey within it. It is especially challenging to obtain information on diet and the distribution of potential prey for long-ranging migrating species. Stomach contents and fecal remains are rarely available, and sufficiently detailed surveys of prey distribution are often lacking. Most studies of foraging ecology of marine predators have instead attempted to correlate habitat use or movement patterns to environmental characteristics (6–8). Such studies do not adequately examine prey choice or food web interactions, but can characterize critical habitats for conservation and management purposes. Relating movement and behavioral data from animal tracking to specific local environmental features is also challenging. For instance, data on ocean surface properties may not be good indicators of environmental conditions relevant to deep-diving species, and subsurface data are often not available at relevant spatial and temporal scales. It is not surprising that some studies have found strong correlations between behavioral patterns and environmental characteristics (8, 9), whereas other results have been more ambiguous (10).
To understand the effects of environmental variability on foraging success and, ultimately, reproductive performance requires not only direct measurements of reproductive output coupled with studies of movement patterns while at sea, but also some method of identifying where and when animals actually improve their body condition. Appropriate feeding indices are often difficult to obtain, and most studies instead use proxies such as changes in movement patterns and time spent within discrete areas. Although these patterns may indicate high search effort, they do not necessarily relate to foraging success or, even more importantly, changes in animal condition.
Southern elephant seals (Mirounga leonina) represent a unique opportunity for studying links between environmental variability, individual physiology, behavior, and population dynamics across a range of scales in space and time. They are long-ranging (6, 11) and deep-diving (12) predators that can potentially access a wide range of geographic and oceanographic regimes in the SO, from benthic shelf areas to midwater pelagic water masses. They require ample stored reserves obtained at sea to fuel their reproductive efforts on land. Declines occurred at key colonies in the Indian and Pacific sectors during the 1950s– 1970s (13–15), whereas populations in the Atlantic sector remained stable (15–17). A recent reexamination concluded that changes in the marine environment were the most plausible cause of the observed declines (18).
The diet of elephant seals is not well known. Because of their long migrations, stomach contents are almost entirely digested by the time seals return to land where prey remains can be studied. Cephalopods (squid and octopus) probably constitute their main diet (19–21), but new methods for diet study suggest that fish may, at least seasonally, make up a significant proportion (22). The spatial and vertical distribution of potential prey species is also poorly understood, especially during the winter season. It is, nevertheless, critically important to describe seal movements and habitat use, and to describe the environmental features that characterize their feeding habitats. Movements, habitat use, and diving behavior have been intensively studied at some colonies (6, 10, 11, 23–34), but there has been no comprehensive description of these across the entire range of the species. Attempts to physically characterize their feeding habitats have been limited to remotely sensed surface water properties.
Recent developments in animal-borne sensors and data loggers have resulted in an oceanographic instrument: the conductivity-temperature-depth satellite relay data logger (CTD-SRDL) (35), capable of providing high-accuracy vertical temperature/salinity (T-S) profiles relayed via satellite. See Materials and Methods and supporting information (SI) for details on instrument specifications, sensor accuracy, data compression, and transmission strategies, etc. When deployed on deep-diving and long-ranging marine vertebrates, these instruments provide extensive spatial coverage and high temporal resolution of key physical oceanographic variables while simultaneously resolving the spatial and temporal scales of importance to the behavior and physiology of individuals. Species returning regularly to breathe at the surface (such as seals, whales, and turtles) allow data to be relayed via satellite in near-real time. This approach complements traditional oceanographic data collection methods, especially in logistically difficult regions such as the SO. Many seals inhabit seasonally ice-covered seas, and regularly dive beneath the ice where most ocean-observing techniques cannot operate effectively. Here, we present results from Southern Elephant Seals as Oceanographic Samplers (SEaOS), a circumpolar study demonstrating how this unique approach can be used to study interactions between large marine predators and their environment.
Results and Discussion
We deployed CTD-SRDLs on 85 southern elephant seals from key colonies throughout the SO (Fig. 1). Instruments were deployed in January and February at the end of the annual molt, and lasted throughout most of the Antarctic winter season (mean ± 1 standard deviation, 160.9 ± 83.3 days). The longest track (326 days, highlighted in Fig. 1) covered the entire winter migration of a South Georgia female (February 14 to October 9, 2005). The tag remained attached during the ≈30-day breeding period at South Georgia, and covered almost the entire subsequent summer trip (November 8 to January 1). Overall, seals delivered 2.3 ± 0.4 complete temperature (T)-S profiles per day, at an average spacing of 21.3 km (95% less than 62 km apart; see SI).
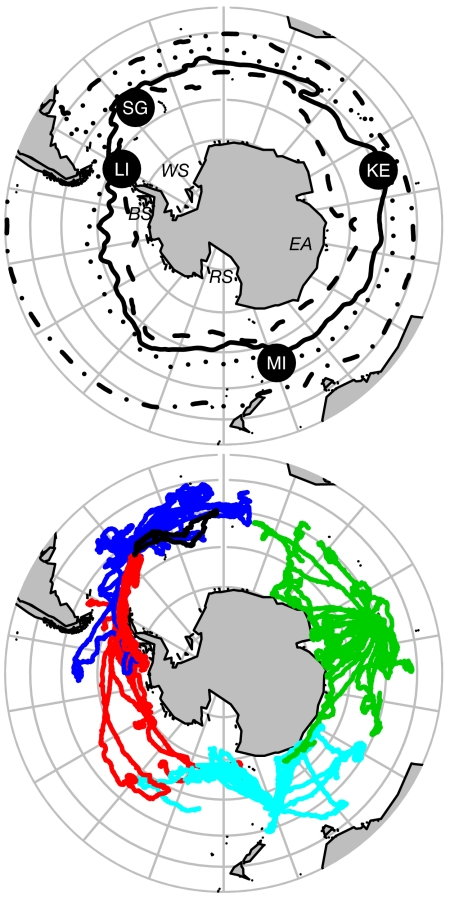
Fig. 1.
Orthographic view of the SO. (Upper) The four sites of instrument deployments are indicated by the filled black circles (SG, South Georgia; KE, Kerguelen Islands; MI, Macquarie Island; LI, Livingston Island), whereas the black lines represent mean locations of the major ACC fronts. From north to south, these include the Subtropical Front (dotted-dashed line) and SAF (dotted line) taken from Orsi et al. (36), followed by the PF (solid line) and SACCF (dashed line), taken from Moore and Abbott (51), the latter modified in the Scotia Sea region by data from Argo floats and CTD-SRDLs deployed on seals in this study (L.B., S.T., M.M., M.B., and M.A.F., unpublished work). The Weddell Sea (WS), East Antarctica (EA), Ross Sea (RS), and Bellingshausen Sea (BS) are also indicated. (Lower) The circumpolar movements of 85 southern elephant seals between January 2004 and April 2006. Colors represent tracks from South Georgia (dark blue), Kerguelen (green), Macquarie (light blue), and the South Shetlands, Antarctica (red). Note the contrast between seals in the Atlantic sector showing a preference for ACC waters compared with the rapid southerly migrations by most Kerguelen and Macquarie seals across ACC waters toward the continental margin of East Antarctica or into the Ross Sea. The longest track (326 days) is shown in black.
Movements and Distribution.
The movements of the 85 seals extended the documented range of the species, and demonstrated the circumpolar coverage of their migrations, from subtropical waters in the north to continental polar waters in the south (Fig. 1). One conspicuous exception is the apparent avoidance of the Weddell Sea. Seals in the Atlantic sector did not cross the Weddell/Scotia Confluence into Antarctic waters. Instead, the majority remained within the ACC, and a few individuals migrated into waters to the north of the Subantarctic Front (SAF). Similarly, seals tagged at the South Shetland Islands that migrated east into the Atlantic Sector stayed north of the South Scotia Ridge marking the border between the Scotia and Weddell Seas.
The only South Georgia seals to reach Antarctic waters did so along the shelf and shelf break west of the Antarctic Peninsula or in the Bellingshausen Sea during late summer before moving north into the ACC as the ice expanded in the autumn (Fig. 1). These patterns are consistent with previous studies (6) suggesting limited use of Antarctic waters. Most seals from the South Shetlands remained on or close to the western Antarctic Peninsula shelf. However, some of the South Shetland seals undertook very long migrations to the west, either along the Antarctic Polar Front (PF) or the ice edge, overlapping with seals from the Macquarie Island population (Fig. 1). Migration patterns of seals from Kerguelen and Macquarie Island were less variable. Generally, a rapid southerly transit across the ACC frontal systems into Antarctic waters was followed by meandering movements, either in relatively confined, seasonally ice-covered shelf waters along the East Antarctic coastline, or within the pack ice in the northern part of the Ross Sea (Fig. 1). Few seals from Kerguelen and Macquarie remained within the ACC during the entire tracking period or moved northward into the ACC when the ice expanded in winter. In contrast to South Georgia, seals from these two populations spent very little time north of the SAF.
Diurnal Variations in Diving.
Elephant seals from all colonies displayed strong diurnal patterns, presumably reflecting diurnal vertical migrations of prey. But day and night dive depths were not uniform across the SO. Overall, dives were deeper in the northern parts of the SO and became shallower toward the south. This was especially evident for nighttime dives, which were substantially deeper (>500 m) north of the PF than further south (≈200–400 m; Fig. 2). The greatest diurnal differences were found between the SAF and the Southern ACC Front (SACCF), where seals typically dived to ≈200–300 m at night and 400–600 m or more during daylight hours. South of the SACCF, diurnal variations gradually diminished, in some regions because of the continental shelf where seals typically dived to or near the seafloor during both day and night. Interestingly, a similar pattern was observed over deep water in the northern Ross Sea, where dive depths were relatively consistent between 200 and 400 m, regardless of the time of day. Small-scale variations in dive depth were observed in some areas, such as within the main ACC system in the Atlantic sector (Fig. 2), possibly indicating association with high eddy activity and increased vertical mixing processes at these frontal regions.
Fig. 2.
Circumpolar interpolated surface map of weighted mean nighttime dive depths of southern elephant seals.
Drift Rate and Relative Body Condition.
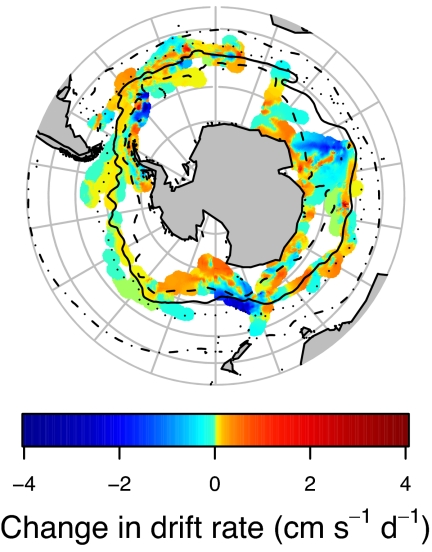
Changes in animal buoyancy (i.e., relative fat content; see ref. 33, Materials and Methods, and SI) varied substantially across the SO (Fig. 3). For most South Georgia seals, the largest positive changes occurred within the ACC, especially between the SAF and the SACCF, but seals migrating to the west of the Antarctic Peninsula also displayed substantial positive changes while on the shelf break and in the Bellingshausen Sea. Most seals from Kerguelen and Macquarie showed strong negative changes while migrating across the ACC, especially between the PF and SACCF. In the Indian Ocean sector, this zone broadens meridionally around the Kerguelen Plateau (36), representing a large region of apparently unfavorable foraging conditions. For these two populations, positive changes in drift rate were observed mainly south of the SACCF, either along the continental margin and shelf break along East Antarctica or within the marginal ice zone in the Ross Sea. Kerguelen and Macquarie seals remaining within the ACC showed positive changes mainly associated with the PF. There were many smaller-scale variations in the change in buoyancy, particularly in the Atlantic sector. These small-scale variations likely reflect the patchy distribution of prey resulting from the high eddy activity and small-scale dynamics of these frontal ACC regions.
Fig. 3.
Circumpolar map of physiological changes during winter migrations of elephant seals. Daily change in drift rate was calculated for 36 individuals during their winter migrations in 2004 and 2005. Blue shading represents a decrease in vertical change in depth during passive drifts, indicating reduced relative lipid content, whereas green–red shading indicates increased vertical depth change and increasing relative lipid content. Interpolated surfaces were created by using the same mapping as that used for Fig. 2. Differences in coverage between here and Fig. 2 are a result of the fact that drift dives (for which vertical change of depth during passive drifts can be calculated) represent only ≈8–10% of all dives. Thus, this surface is calculated based on a smaller data set than those in Fig. 2.
Physical Ocean Properties.
The following analyses focus on the seawater properties at the deepest point of each dive (see Materials and Methods). The South Shetland data set was excluded from these analyses because of the limited number of seals for which the entire set of physical and behavioral data were available.
Seals from South Georgia encountered a wide range of physical water properties but tended to target waters typical of the ACC. Potential temperatures were relatively uniform at 2.02 ± 0.27°C, whereas salinities showed two clusters at 34.38 ± 0.05 and 34.58 ± 0.09. These values correspond either to Upper Circumpolar Deep Water or, in the case of the lower salinities, the boundary between Upper Circumpolar Deep Water and Antarctic Intermediate Water (AAIW) (37). A smaller cluster was also observed at significantly higher temperatures and lower salinities (5.34 ± 0.12°C and 34.16 ± 0.08, respectively). These characteristics were observed mainly from one seal spending ≈2 months in a well defined area at ≈20° west of Drake Passage north of the SAF, and are consistent with deep-reaching highly mixed surface waters in late winter indicative of the formation of Subantarctic Mode Water and AAIW in this region (38).
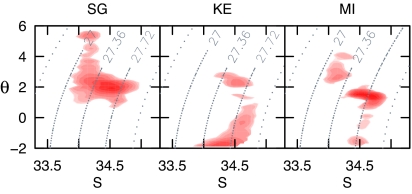
Physical water properties encountered by Kerguelen and Macquarie seals were strikingly different from those for South Georgia seals, but were consistent with their geographic distributions. Although some seals remained in ACC waters similar to those of South Georgia seals [two potential temperature/salinity (θ-S) clusters at 1.98 ± 0.56°C and 34.38 ± 0.13 or 34.60 ± 0.06, indicating waters between the PF and the SAF], most used colder and/or more saline waters. The main cluster showed θ-S characteristics typical of waters of the Antarctic Slope Front and shelf waters, with temperatures approaching the seawater surface freezing point (−1.70 ± 0.16°C) and salinities ranging from ≈34.0 to 34.6. Some of these profiles measured along the continental margin of East Antarctica had θ-S characteristics consistent with High-Salinity Shelf Water, a precursor of Antarctic Bottom Water. These preferences were even more obvious when θ-S values corresponding to positive changes in drift rate were highlighted. Most of the positive changes for Kerguelen seals occurred in these extremely cold water masses associated with the Antarctic shelf regions (Fig. 4), although θ-S characteristics associated with positive changes in drift rate for the few individuals remaining within the ACC were similar to those of the majority of the South Georgia seals. Although temperatures close to the freezing point were also observed in profiles from Macquarie seals over deep water in the Ross Sea pack ice, seals in this region appeared to favor warmer waters (1.47 ± 0.13°C) with salinities of 34.70 ± 0.52. This corresponds to the subsurface θ and S maxima at depths of ≈200–300 m, immediately below the permanent thermocline at the interface between cold winter surface waters and the Circumpolar Deep Water. This is consistent with the lack of diurnal pattern in this region and the restricted vertical depth coverage, suggesting that elephant seals feeding within the pack ice over deep basins dive through the cold surface mixed layer to target sharp discontinuities that may represent important overwintering areas for mesopelagic fauna (39). Most of the observed positive changes in drift rate for Macquarie seals occurred within these well defined θ-S and depth ranges, although some also displayed positive changes in waters encountered within the ACC with characteristics typical for the SAF (2.52 ± 0.20°C and 34.17 ± 0.13).
Fig. 4.
In situ θ-S measurements collected by instruments deployed on southern elephant seals at three of the main locations (South Georgia, Kerguelen, and Macquarie Island). The curved dotted lines indicate the water density corresponding to these θ-S properties. The red surfaces represent kernel densities of θ-S properties at the bottom of dives. Initially, two density surfaces were created for each location: one using only those dives occurring during periods of positive change in drift rate (i.e., periods of increasing relative lipid content) and the other based on dives during periods of negative change. The displayed surfaces represent the positive minus negative density surfaces, and the color intensity therefore highlights areas of predominantly increasing lipid content. Kernel surfaces were created by using a 50 × 50 grid over the range of θ and S, yielding a resolution of 0.056 × 0.199 for θ and S, respectively.
Global Snapshot of Elephant Seal Habitats.
This study of southern elephant seal migrations from some key breeding and moulting sites during their long-ranging winter feeding migrations provides a unique simultaneous circumpolar view of the habitat use of any SO predator. The in situ hydrographic measurements of water masses across both their horizontal and vertical ranges also provide a direct description of the detailed environmental conditions experienced by them. Because our information about the distribution and abundance of potential prey is sparse at best, this study does not attempt to correlate seal movements, behavior, and changes in condition to prey fields and diet per se. Our approach instead attempts to characterize the foraging habits of elephant seals in terms of the physical environment processes that influence nutrient availability and biological productivity. In the highly dynamic three-dimensional marine environment where geography alone is a poor descriptor, the in situ oceanographic measurements obtained by the approach presented in this paper provide a detailed description of these physical characteristics at spatial and temporal scales relevant to the animals.
The most obvious emerging pattern is the substantial basin-scale difference in habitat use by seals in the Atlantic sector in contrast with those in the Indian and Pacific sectors. The ACC frontal systems in the Atlantic are known for their comparatively high primary productivity (40), presumably driven by a combination of iron enrichment from nearby shelf areas (3) and possibly upwelling of Circumpolar Deep Water enriched in nutrients from sources in, for example, the North Atlantic. ACC frontal systems in the Atlantic sector may therefore represent an accessible and predictable resource for South Georgia seals. Although similar processes of nutrient enrichment have also been described from island shelf regions in other sectors of the SO, such as around Crozet (41) and Kerguelen (42), these regions are substantially smaller than those in the Atlantic sector. The high upwelling and diffusivity rates reported for the Scotia Sea (43) may also cause higher nutrient enrichment and hence support higher primary production than in other regions. These differences may explain the high usage of the ACC frontal system by South Georgian seals, whereas this strategy was much less common among seals from Macquarie and Kerguelen. During the shorter summer migrations between breeding and molt, seals from Macquarie and Kerguelen may be restricted to more northern regions closer to their colonies (44), leading to migration patterns more similar to those from South Georgia. Nevertheless, it seems clear that the South Georgia population operates within different oceanographic regimes from other populations, at least during the winter migration.
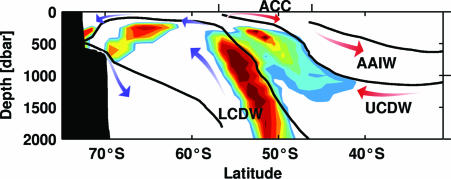
Our findings are summarized schematically in Fig. 5, which compares hydrographic properties (θ-S) measured by seals with historical data along a representative SO vertical section. It is clear from this figure that areas in which elephant seals show positive changes in body condition can be characterized by specific hydrographic properties, and that these properties follow the general horizontal and vertical circulation regimes of the SO. In general, within the ACC, regions of upwelling of nutrient-rich Circumpolar Deep Water are clearly favored, whereas there is an almost total avoidance of AAIW. South of the ACC, favorable conditions are mainly found beneath the seasonally mixed layer, in waters that feature temperature and salinity maxima derived from the Lower Circumpolar Deep Water. In the subpolar gyres, this water is often termed Warm Deep Water. Another region of positive change in body condition corresponds to the sinking and spreading of mixed waters to the south of the Antarctic Slope Front. The predicted depth ranges correspond well with those observed and presented in Fig. 4, including the gradual deepening to the north within the ACC.
Fig. 5.
Generalized section of the SO, highlighting areas where southern elephant seals are predicted to change their relative body fat stores. We used typical sections of temperature and salinity from the Levitus 1° data set (52). The derived potential density values were matched to those in table 2 in Heywood and King (37) to highlight the main water mass boundaries between AAIW, Upper Circumpolar Deep Water (UCDW), and Lower Circumpolar Deep Water (LCDW). Colored contours represent the accumulated number of matches between temperature, salinity, and derived density values obtained from seals, and corresponding values in the schematic hydrographic section. We used only values at the deepest point of each profile, and only those profiles obtained during periods of positive change in drift rate. The arrows indicate the main circulation pathways as summarized by Toggweiler et al. (53). Note the preference for upwelling regions of Circumpolar Deep Water and water mass transformation regions adjacent to the Antarctic continent, and the avoidance of regions of AAIW subduction. The gradual deepening of predicted regions to the north agrees well with the patterns of daytime and nighttime dive depths shown in Fig. 2.
Ecological Implications.
The observed differences in the occurrence of positive changes in drift rate between populations could have important consequences for the energy budgets of these animals. As an example, our results suggest that seals from Kerguelen and Macquarie may spend four times longer in transit compared with their South Georgia counterparts before they show signs of improved body condition. Seals from Kerguelen and Macquarie may therefore spend as much as one extra month in transit during a roundtrip winter migration and travel an extra distance of >1,000 km. Using equations of the diving metabolic rate of grey seals (45) scaled up to the body size of elephant seals, we estimate that this corresponds to the expenditure of four to five times more energy, which must be recouped by a higher net energy gain while on the feeding grounds to ensure an unchanged overall net energy balance over the entire trip. Based on estimates of reproductive expenditure of female elephant seals (see, e.g., ref. 46), a breeding female at Kerguelen or Macquarie that is unable to recoup this additional expenditure at sea would have to reduce her reproductive expenditure on land by ≈10–20% compared with her South Georgia counterpart, either by reducing the energy and material transferred to her pup or by somehow reducing her metabolic overheads. This could have a negative effect on the subsequent survival of their pups, particularly in years of low or uncertain summer food abundance.
Although these estimates are relatively imprecise and do not take individual variation in energy budgets into account, they nevertheless suggest a simple mechanism that may contribute to the different population trends observed for these populations during the 1950s–1970s. There is evidence for a significant decline in Antarctic sea ice extent through this period along the coast of East Antarctica (47), whereas some other regions (notably the Weddell Sea) remained relatively unchanged (48). The hydrographic properties we identified as key features of favorable feeding regions depend partially on sea ice dynamics. It is likely that such changes in circulation patterns and ice conditions may affect prey abundance and/or distribution and, ultimately, the net energy gain of seals while at sea. This discussion suggests possible mechanisms by which environmental variation can affect individual behavior and ability to obtain sufficient energy stores to allow them to reproduce successfully, and exemplifies a promising approach for studying these interactions. As pointed out in ref. 49, such detailed individual studies are also crucial for understanding population changes and how they are affected by environmental variability.
Materials and Methods
CTD-SRDLs [designed and manufactured by the Sea Mammal Research Unit (St. Andrews, U.K.), incorporating a CTD sensor built by Valeport Ltd. (Totnes, U.K.); see SI for detailed specifications] were deployed on southern elephant seals in the Austral summers of 2003–2006 at four locations: Macquarie Island (n = 16), Kerguelen Islands (n = 29), South Georgia (n = 21), and South Shetland Islands of the Antarctic Peninsula (n = 19), representing the main breeding populations around the SO (Fig. 1). We assessed spatial and temporal changes in diurnal diving behavior by using weighted mean daytime and nighttime dive depths. These were calculated by using weights defined by Gaussian density curves centered at local noon and midnight, respectively, with the 5–95% interval extending to 3 h in either direction. Values therefore represent the weighted mean dive depths over 6-h day and night periods. We estimated changes in body condition of seals from their buoyancy, measured by the vertical rate of passive descent or ascent during so-called “drift dives.” This method has previously been described by Biuw et al. (33), and provides a qualitative, indirect measure of changes in relative fat content. Briefly, buoyancy of seals at depth is almost entirely determined by relative amounts of lipid and lean tissue, and important feeding habitats can be inferred by mapping the change in relative lipid content (i.e., buoyancy as measured by drift rate) across the animals' range. Behavioral and physiological data were linked with the contemporaneous in situ measured physical properties collected by the seals, allowing us to study their responses to oceanographic features. See SI for details of sensor accuracy and compression algorithm. To define the most important seawater properties encountered by seals, Gaussian mixture models (50) were fitted to the θ and S properties measured at the deepest point of each dive, where the entire range of a given property can be described by a mixture of several normal distributions. The individual mixture components (clusters) can then be conveniently defined by their means and standard deviations.
Supplementary Material
Acknowledgments
This work was carried out under the international Southern Elephant Seals as Oceanographic Samplers (SEaOS) program (http://biology.st-andrews.ac.uk/seaos/). Funding for instrument development was provided by the National Oceanographic Partnership Program–Office of Naval Research. The Sea Mammal Research Unit telemetry team helped develop hardware and analytical tools. The United Kingdom component was supported by the Natural Environment Research Council. Support for the Australian component was provided by the Australian Research Council, the Australian Antarctic Science Grants Scheme, Commonwealth Scientific and Industrial Research Organisation's Wealth from Oceans National Research Flagship, and the Australian Government's Cooperative Research Centre Scheme through the Antarctic Climate and Ecosystems Cooperative Research Centre. The French component was funded by Terre-Océan-Surface Continentale-Atmosphère–Centre National d'Études Spatiales, the Premier Groupe de Mission Mercator Coriolis, and the Institut Polaire Français Paul Emile Victor, whereas the U.S. work was funded by the National Science Foundation, National Oceanographic Partnership Program–Office of Naval Research, and the National Undersea Research Program–National Oceanic and Atmospheric Administration.
Abbreviations
- SO
Southern Ocean
- CTD-SRDL
conductivity-temperature-depth satellite relay data logger
- ACC
Antarctic Circumpolar Current
- SACCF
Southern ACC Front
- SAF
Subantarctic Front
- PF
Polar Front
- AAIW
Antarctic Intermediate Water
- θ
potential temperature
- S
salinity.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.E.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701121104/DC1.
References
- 1.Smetacek V, Nicol S. Nature. 2005;437:362–368. doi: 10.1038/nature04161. [DOI] [PubMed] [Google Scholar]
- 2.Brierley AS, Thomas DN. Adv Mar Biol. 2002;43:171–276. doi: 10.1016/s0065-2881(02)43005-2. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan CW, Arrigo KR, McClain CR, Comiso JC, Firestone J. Science. 1993;262:1832–1837. doi: 10.1126/science.262.5141.1832. [DOI] [PubMed] [Google Scholar]
- 4.Prézelin BB, Hofmann EE, Mengelt C, Klinck JM. J Mar Res. 2000;58:165–202. [Google Scholar]
- 5.Moore JK, Abbott MR. J Geophys Res C. 2000;105:28709–28722. [Google Scholar]
- 6.McConnell BJ, Chambers C, Fedak MA. Antarct Sci. 1992;4:393–398. [Google Scholar]
- 7.Pinaud D, Weimerskirch H. J Anim Ecol. 2005;74:852–863. [Google Scholar]
- 8.Lea MA, Dubroca L. ICES J Mar Sci. 2003;60:990–1002. [Google Scholar]
- 9.Guinet C, Dubroca L, Lea MA, Goldsworthy S, Cherel Y, Duhamel G, Bonadonna F, Donnay JP. Mar Ecol Prog Ser. 2001;219:251–264. [Google Scholar]
- 10.Bradshaw CJA, Higgins J, Michael KJ, Wotherspoon SJ, Hindell MA. ICES J Mar Sci. 2004;61:1014–1027. [Google Scholar]
- 11.Hindell MA, Bradshaw CJA, Sumner MD, Michael KJ, Burton HR. J Appl Ecol. 2003;40:703–715. [Google Scholar]
- 12.Hindell MA, Slip DJ, Burton HR, Bryden MM. Can J Zool. 1992;70:370–379. [Google Scholar]
- 13.Hindell MA, Burton HR. J Zool. 1987;213:365–380. [Google Scholar]
- 14.Guinet C, Jouventin P, Weimerskirch H. Antarct Sci. 1999;11:193–197. [Google Scholar]
- 15.Laws RM. In: Elephant Seals. LeBoeuf BJ, Laws RM, editors. Los Angeles: Univ of California Press; 1994. pp. 49–65. [Google Scholar]
- 16.Boyd IL, Walker TR, Poncet J. Antarct Sci. 1996;8:237–244. [Google Scholar]
- 17.McCann TS. In: Studies of Sea Mammals in South Latitudes. Ling JK, Bryden MM, editors. Sydney: South Australian Museum; 1985. pp. 1–17. [Google Scholar]
- 18.McMahon CR, Bester MN, Burton HR, Hindell MA, Bradshaw CJA. Mamm Rev. 2005;35:82–100. [Google Scholar]
- 19.Clarke MR, MacLeod N. Br Antarct Surv Bull. 1982;57:27–31. [Google Scholar]
- 20.Rodhouse PG, Arnbom TR, Fedak MA, Yeatman J, Murray AWA. Can J Zool. 1992;70:1007–1015. [Google Scholar]
- 21.van den Hoff J, Burton H, Davies R. Polar Biol. 2003;26:27–31. [Google Scholar]
- 22.Bradshaw CJA, Hindell MA, Best NJ, Phillips KL, Wilson G, Nichols PD. Proc R Soc London Ser B. 2003;270:1283–1292. doi: 10.1098/rspb.2003.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hindell MA, Slip DJ, Burton HR. Austr J Zool. 1991;39:595–619. [Google Scholar]
- 24.Hindell MA, Burton HR, Slip DJ. Austr J Mar Fresh Res. 1991;42:115–128. [Google Scholar]
- 25.Campagna C, Le Boeuf BJ, Blackwell SB, Crocker DE, Quintana F. J Zool. 1995;236:55–71. [Google Scholar]
- 26.Campagna C, Quintana F, Le Boeuf BJ, Blackwell S, Crocker DE. Aquat Mamm. 1998;24:1–11. [Google Scholar]
- 27.Jonker FC, Bester MN. Antarct Sci. 1998;10:21–30. [Google Scholar]
- 28.Campagna C, Fedak MA, McConnell BJ. J Mammal. 1999;80:1341–1352. [Google Scholar]
- 29.Hindell MA, McConnell BJ, Fedak MA, Slip DJ, Burton HR, Reijnders PJH, McMahon CR. Can J Zool. 1999;77:1807–1821. [Google Scholar]
- 30.Hindell MA, McMahon CR. Mar Mamm Sci. 2000;16:504–507. [Google Scholar]
- 31.Field I, Hindell MA, Slip DJ, Michael KJ. Antarct Sci. 2001;13:371–379. [Google Scholar]
- 32.McConnell BJ, Fedak MA, Burton HR, Englehard GH, Reijnders P. J Anim Ecol. 2002;71:65–78. [Google Scholar]
- 33.Biuw M, McConnell B, Bradshaw CJA, Burton H, Fedak M. J Exp Biol. 2003;206:3405–3423. doi: 10.1242/jeb.00583. [DOI] [PubMed] [Google Scholar]
- 34.Campagna C, Piola AR, Marin MR, Lewis M, Fernandez T. Deep Sea Res Part I Oceanogr Res Pap. 2006;53:1907–1924. [Google Scholar]
- 35.Lydersen C, Nost OA, Lovell P, McConnell BJ, Gammelsrod T, Hunter CJ, Fedak MA, Kovacs KM. Geophys Res Lett. 2002;29:2119. [Google Scholar]
- 36.Orsi AH, Whitworth T, Nowlin WD. Deep Sea Res Part I Oceanogr Res Pap. 1995;42:641–673. [Google Scholar]
- 37.Heywood KJ, King BA. J Mar Res. 2002;60:639–676. [Google Scholar]
- 38.Tsuchiya M, Talley LD. J Geophys Res C. 1998;103:12899–12918. [Google Scholar]
- 39.Lawson GL, Wiebe PH, Ashjian CJ, Gallager SM, Davis CS, Warren JD. Deep Sea Res Part II Top Stud Oceanogr. 2004;51:2041–2072. [Google Scholar]
- 40.Holm-Hansen O, Naganobu M, Kawaguchi S, Kameda T, Krasovski I, Tchernyshkov P, Priddle J, Korb R, Brandon M, Demer D. Deep Sea Res Part II Top Stud Oceanogr. 2004;51:1333–1350. [Google Scholar]
- 41.Pollard RT, Lucas MI, Read JF. Deep Sea Res Part II Top Stud Oceanogr. 2002;49:3289–3305. [Google Scholar]
- 42.Bucciarelli E, Blain S, Treguer P. Mar Chem. 2001;73:21–36. [Google Scholar]
- 43.Garabato ACN, Polzin KL, King BA, Heywood KJ, Visbeck M. Science. 2004;303:210–213. doi: 10.1126/science.1090929. [DOI] [PubMed] [Google Scholar]
- 44.Bradshaw CJA, Hindell MA, Sumner MD, Michael KJ. Anim Behav. 2004;68:1349–1360. [Google Scholar]
- 45.Sparling CE, Fedak MA. J Exp Biol. 2004;207:1615–1624. doi: 10.1242/jeb.00952. [DOI] [PubMed] [Google Scholar]
- 46.Fedak MA, Arnbom T, Boyd IL. Physiol Zool. 1996;69:887–911. [Google Scholar]
- 47.Curran MAJ, van Ommen TD, Morgan VI, Phillips KL, Palmer AS. Science. 2003;302:1203–1206. doi: 10.1126/science.1087888. [DOI] [PubMed] [Google Scholar]
- 48.Murphy EJ, Clarke A, Symon C, Priddle J. Deep Sea Res Part II Top Stud Oceanogr. 1995;42:1045–1062. [Google Scholar]
- 49.Stevenson RD, Woods WA. Integr Comp Biol. 2006;46:1169–1190. doi: 10.1093/icb/icl052. [DOI] [PubMed] [Google Scholar]
- 50.Fraley C, Raftery AE. J Am Stat Assoc. 2002;97:611–631. [Google Scholar]
- 51.Moore JK, Abbott MR, Richman JG. J Geophys Res C. 1999;104:3059–3073. [Google Scholar]
- 52.Levitus S, Burgett R, Boyer T. World Ocean Atlas 1994: Salinity. Vol 3. Washington, DC: US Government Printing Office; 1994. [Google Scholar]
- 53.Toggweiler JR, Russell JL, Carson SR. Paleoceanography. 2006;21:PA2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.