Abstract
The proper expansion and contraction of hematopoietic cells requires tight regulation of cell death. BID, a “BH3-only” molecule, amplifies death receptor signals connecting the extrinsic to intrinsic pathways by triggering the mitochondrial pathway of apoptosis. Bid-deficient mice, as they age, spontaneously develop a myeloproliferative disorder, which progresses from myeloid hyperplasia to a fatal, clonal malignancy closely resembling chronic myelomonocytic leukemia (CMML). Thus, an apoptotic defect can result in myeloid leukemogenesis. Premalignant Bid−/− myeloid precursor cells are resistant to death receptor-induced apoptosis. Furthermore, a competitive reconstitution assay demonstrates that Bid-deficient long-term repopulating cells give rise to expanded myelomonocytic cells in vivo. Surprisingly, a single BH3-only molecule operating in the extrinsic death receptor pathway proved essential in vivo for physiologic cell death required to maintain myeloid homeostasis. Moreover, progression to CMML indicates that an upstream BH3-only molecule, BID, is required to suppress tumorigenesis.
Keywords: BID, BCL-2, myeloid, cancer, apoptosis, Fas
Multicellular organisms have developed a highly organized and carefully regulated pathway of cell suicide to control homeostasis and ensure survival of the organism as a whole. Normal development and morphogenesis proceeds by the production of excess cells which are then removed by a genetically programmed, evolutionarily conserved process. This same program of cell death is used to maintain homeostasis of cell populations in long-lived mammals and to respond to both physiologic and pathologic death stimuli such as infectious agents.
The BCL-2 family of proteins controls a critical decisional checkpoint in the cell death pathway operating immediately upstream of mitochondria (Adams and Cory 1998; Gross et al. 1999a). Pro- as well as antiapoptotic members have been identified. The proapoptotic members can be further subdivided into “multidomain” members, such as BAX and BAK, harboring four BCL-2 homology domains (BH1-4), and “BH3-only” members such as BID, BAD, BIM, BIK, NOXA, HRK, and PUMA. Cells doubly deficient in Bax and Bak are profoundly resistant to multiple apoptotic stimuli that signal through the intrinsic pathway, indicating that multidomain BAX, BAK are essential downstream death effectors required for the mitochondrial pathway of apoptosis (Wei et al. 2001). In contrast, BH3-only molecules appear to operate upstream, connecting proximal death signals to the “multidomain” antiapoptotic and proapoptotic BCL-2 members at the mitochondria (Cheng et al. 2001; Zong et al. 2001). BID was the first identified member of the BH3-only subset and is capable of binding multidomain proapoptotic BAX, BAK as well as antiapoptotic BCL-2 (Wang et al. 1996). BID appears unique among the BH3-only members as it interconnects the extrinsic pathway initiated by death receptors to the intrinsic pathway of mitochondria-based cell death. Following death receptor signaling, caspase-8 is activated, which cleaves the inactive p22 conformer within an unstructured loop (Li et al. 1998; Luo et al. 1998; Gross et al. 1999b), which exposes a glycine that is N-myristoylated (Zha et al. 2000). This p7/myr–p15 BID complex targets mitochondria with increased efficiency to trigger cell death. BID establishes a proapoptotic cascade where the BH3 domain of BID induces BAX, BAK to undergo an allosteric activation, including their homo-oligomerization at the mitochondrion, resulting in permeabilization of the outer membrane and the release of cytochrome c (Wei et al. 2000). In cells protected by adequate amounts of BCL-2, BCL-XL, activated BH3-only molecules including tBID are sequestered by these antiapoptotic members, preventing the activation of BAX, BAK (Cheng et al. 2001).
In contrast to the pervasive defect in apoptosis displayed by BAX, BAK doubly deficient cells, Bid-deficient cells display a very focused deficit following death receptor activation. Bid-deficient mice are viable and develop normally, and at birth display balanced homeostasis in all examined tissues. However, when challenged with pathologic Fas activation in vivo, Bid-deficient livers proved resistant to Fas-mediated hepatic apoptosis and hemorrhagic necrosis that kills wild-type mice (Yin et al. 1999). Moreover, other BH3-only molecules also function as upstream connectors that participate in a signal-specific and lineage-restricted fashion. BAD is activated and inactivated through its differential phosphorylation in response to extracellular survival factors (Zha et al. 1996). PUMA and NOXA are induced by p53 in response to DNA damage (Nakano and Vousden 2001). BIM associates with a dynein light chain, suggesting that it could prove to monitor aberrations of this microtubule complex (Puthalakath et al. 1999). Bim-deficient mice demonstrate persistence of autoreactive thymocytes and plasma cell expansions (Bouillet et al. 1999, 2002). The focused roles of each BH3-only molecule and the selective defects in cells deficient for a BH3-only molecule contrast with the global defect in BAX, BAK doubly deficient cells, suggesting a model in which BH3-only molecules often work in concert. BH3 domain peptides were recently subdivided into “activators” and “sensitizers” and were shown to be synergistic in inducing apoptosis (Letai et al. 2002).
Thus, a considerable question exists as to whether any single BH3-only protein, and especially BID, which functions as an upstream amplifier in the death receptor pathways, would be essential to control homeostasis of a cell lineage in vivo. BCL-2 was discovered at the t(14;18) chromosomal breakpoint of human follicular lymphoma. Transgenic mice bearing a BCL-2-Ig minigene that recapitulated this breakpoint developed B-cell follicular hyperplasia that progressed to monoclonal, large cell lymphoma (McDonnell et al. 1989; McDonnell and Korsmeyer 1991). BCL-2 functions by extending cell survival and conferring resistance to apoptosis, which initiated a new category of oncogenes: regulators of cell death (Vaux et al. 1988; McDonnell et al. 1989; Hockenbery et al. 1990). Similarly, proapoptotic BCL-2 members might be expected to serve as tumor suppressors and their loss-of-function to contribute to malignancy. Choroid plexus tumors were accelerated by introducing Bax deficiency together with a truncated SV40 T antigen transgene (Yin et al. 1997). However, singly deficient Bax or Bak null mice have not developed a high incidence of spontaneous tumors (Knudson et al. 1995; Lindsten et al. 2000). Adding an adenoviral E1A oncogene to the profound apoptotic block of Bax, Bak doubly deficient cells is sufficient to transform them (Degenhardt et al. 2002). However, surviving mice doubly deficient for Bax, Bak have not to date spontaneously developed tumors (Lindsten et al. 2000).
Here we extensively examined Bid-deficient mice and discovered that the myelomonocyte lineage is singularly dependent on BID for physiologic cell death. Bid-deficient bone marrow initially displays increased clonogenic potential in vitro and enhanced regenerative potential in competitive repopulation assays. Over time, Bid null mice display a myeloid hyperplasia which progresses to a fatal clonal disorder resembling human chronic myelomonocytic leukemia (CMML). Thus, BID, a single BH3-only molecule, plays an essential role in maintenance of myeloid homeostasis and tumor suppression.
Results
Bid-deficient mice display altered myeloid homeostasis
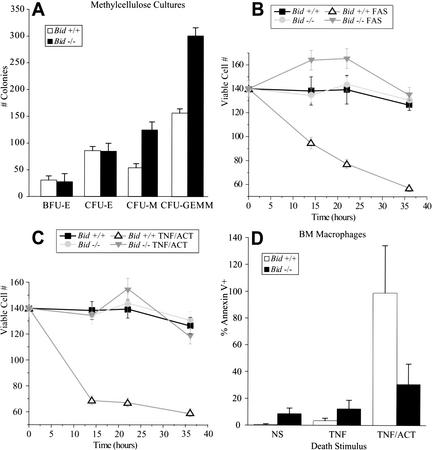
Analysis of aging Bid-deficient mice revealed an elevated absolute neutrophil count at age 18–24 mo accompanied by mild hepatosplenomegaly due to infiltration of myeloid cells (Table 1). Hematopoietic cell populations in younger Bid−/− mice (<12 mo old) were indistinguishable from age- and sex-matched wild-type controls. This prompted a detailed analysis of bone marrow in which young (6–12-wk-old) Bid-deficient mice demonstrated increased colony-forming units of macrophage and mixed colonies when plated in methylcellulose with recombinant growth factors that stimulate both precursor cells and committed myeloid cells (SCF, IL-6, IL-3, GCSF, GMCSF; Fig. 1A). This enhanced clonogenic potential revealed by in vitro culture may have uncovered a defect that is compensated for in vivo in young mice, but appears to manifest with age.
Table 1.
Myeloid expansion in premalignant and leukemic Bid-deficient mice
| Wild-type and Bid-deficient mice, age 18–24 months
| |||
|---|---|---|---|
|
|
Bid +/+
|
Premalignant Bid −/−
|
Leukemic Bid −/−
|
| WBC (×1000 cells/ml) | 11.37 ± 5.28 | 6.04 ± 4.11 | 16.11 ± 18.23 |
| Absolute Neutrophil Count | 1.48 ± 0.54 | 2.13 ± 2.5 | 11.18 ± 15.96 |
| Hb (g/dL) | 13.24 ± 1.87 | 11.27 ± 2.95 | 8.59 ± 2.92 |
| Platelets (<1000 cells/ml) | 1951 ± 611 | 1361 ± 555 | 549 ± 420 |
| Liver (g) | 1.5 ± 0.27 | 1.9 ± 0.69 | 5.7 ± 2.13 |
| Spleen (g) | 0.08 ± 0.04 | 0.36 ± 0.27 | 0.59 ± 0.44 |
Figure 1.
(A) Premalignant Bid-deficient bone marrow displays increased clonogenic potential in methylcellulose. For myeloid colonies, 10,000 cells were resuspended in Methylcult H4100 supplemented with cytokines: GCSF (2 ng/mL), GMCSF (10 ng/mL), Il3 (20 ng/mL), Il6 (4 ng/mL), Il11 (10 ng/mL), SCF (20 ng/mL). For erythroid colonies, cells were supplemented with cytokines SCF (20 ng/mL) and erythropoietin (4 U/mL). Colonies were counted on day 7. Appropriate assessment of colony morphology was verified by May Grunwald-Giemsa stained cytospins from representative colonies. (B,C) Bid-deficient myeloid precursor cells are resistant to anti-Fas Ab (Jo2)- and TNFα/actinomycin D-induced death. Lin-SCA1+ myeloid precursor cells from 8–12-wk-old mice were cultured in the presence of SCF, GCSF, and Il3. Cultures containing 80%–90% promyelocytes and myelocytes were treated with anti-Fas Ab (Jo2, μg/mL; B) or TNFα + actinomycin D (20 ng/mL; C), as TNFα alone was not effective. Viable cells were determined over a 36-h time course by trypan blue exclusion and annexin V staining. (D) Bid-deficient macrophages are resistant to TNF-α/actinomycin D-induced death. Primary bone marrow cells from 8–12-wk-old mice were cultured for 1 wk in MCSF and IL3. Cells were treated with TNFα (10 ng/mL) or TNFα+ actinomycin D (20 ng/mL). After 72 h, cells were stained with annexin V and analyzed by flow cytometry.
Bid-deficient myeloid precursors are resistant to death receptor signaling
Since BID is activated downstream of death receptor signaling, we investigated the susceptibility of Bid-deficient myeloid precursor cells to death receptor engagement. Linneg, Sca-1pos precursor cells from 8–12-wk-old mice were cultured in the presence of SCF, GCSF, and IL-3 (McLemore et al. 2001), yielding 80%–90% promyelocytes and myelocytes. Viability assays indicated that Bid-deficient myeloid precursors were markedly resistant to death induced by anti-Fas antibody (Ab) and by TNFα (Fig. 1B,C). A small but reproducible increase in viable total cell numbers in anti-Fas-treated versus untreated Bid−/− cells leaves open the possibility of an unopposed proliferation signal, as suggested in other cell types (Kataoka et al. 2000). Resistance to TNFα-induced apoptosis was also noted in mature Bid-deficient macrophages (Fig. 1D). Wild-type as well as Bid-deficient macrophages were resistant to anti-Fas Ab (data not shown), consistent with prior reports (Perlman et al. 1999).
BID regulates homeostasis of long-term repopulating myeloid cells
We next examined the progenitor cell populations in Bid−/− animals at 12 wk, 14 mo, and 16 mo. Myeloid progenitor populations are characterized by isolation of Lin-ckit+sca1 cells by FACS sorting, and staining for FcγII/III and CD34. This protocol allows the separation of megakaryocyte-erythrocyte progenitors, which are FcγII/IIIlow and CD34+; granulocyte/macrophage progenitors (GMPs), which are FcγII/IIIhigh and CD34+; and common myeloid progenitors (CMPs), which are FcγII/IIIlow and CD34low. We found that the progenitor populations in the Bid−/− animals were not increased. Similarly, the relative number of cells marking as hematopoietic stem cells (HSCs) defined as lineage-negative (lineage = Il-7R, B220, CD19, IgM, Thy1.1, Ter119, Gr1), Sca1+, Ckit+ is equivalent in wild-type and Bid−/− animals. This contrasts with enforced overexpression of BCL-2 in which the HSC population is increased (Traver et al. 1998).
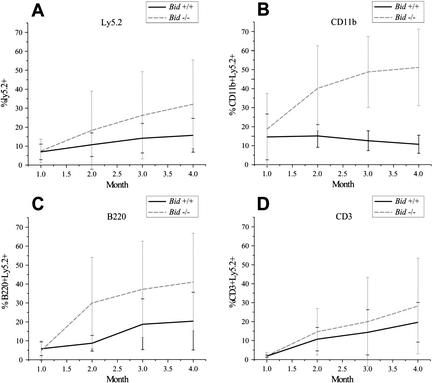
A stringent test of hematopoietic cells is to compare the competitive ability of genetically altered versus wild-type bone marrow cells to repopulate a marrow compartment that has been depleted by lethal γ-irradiation (Szilvassy et al. 1990). Similar frequencies of competitive repopulating units (CRUs) were noted for Ly5.2+marked wild-type [1:16919 (1 CRU per number transferred cells), 95% confidence interval: 12141–23578] and Bid-deficient (1:17483, 95% confidence interval: 13492–22653) bone marrow 1 mo after reconstitution. The frequency of CRU remained similar at 4 mo (Bid+/+ 1:21566; 95% confidence interval: 15664–29692, and Bid−/− 1:29497; 95% confidence interval: 22074–39418), indicating that the absolute numbers of cells capable of long-term repopulation cells do not obviously differ between the groups. However, over a 4-mo time course, the Ly5.2+-marked Bid-deficient cells comprised a significantly increasing percentage of peripheral blood, indicating a competitive advantage for Bid−/− cells (Fig. 2A). This was most prominent for CD11b+ cells, which include myeloid precursor and mature monocytic and granulocytic cells (p = 0.0069; 4 mo; Fig. 2B). A trend was also noted in B220+ cells (precursor and mature B cells); however, it did not reach statistical significance (p = 0.112; 4 mo; Fig. 2C). In contrast, the repopulation of CD3+ T cells did not vary, indicating a lineage restriction for this particular role of BID, in which the different kinetics of T lymphopoiesis could be a factor (Fig. 2D). Although Bid deficiency did not alter the number of stem cells or early progenitors, defective death receptor killing as manifested in the myeloid precursors does appear to provide a competitive advantage, which is evidenced by expanded myelomonocytic cells presumably derived from long-term repopulating cells in vivo.
Figure 2.
(A–D) Bid-deficient myeloid cells display a competitive advantage in repopulating assays. Eight thousand Ly5.2+ wild-type or Bid knockout bone marrow cells along with 1.2 × 105 Ly5.1+ bone marrow cells (1:20 ratio) were injected intravenously into the tail vein of lethally irradiated Ly5.1+ wild-type congenic mice. Percentages of donor repopulation as assessed by percent Ly5.2+ cells (A), percent Ly5.2+CD11b+ cells (B), percent Ly5.2+B220+ cells (C), and percent Ly5.2+CD3+ cells (D) were determined by flow cytometric analysis of peripheral blood.
Bid-null mice progress to CMML
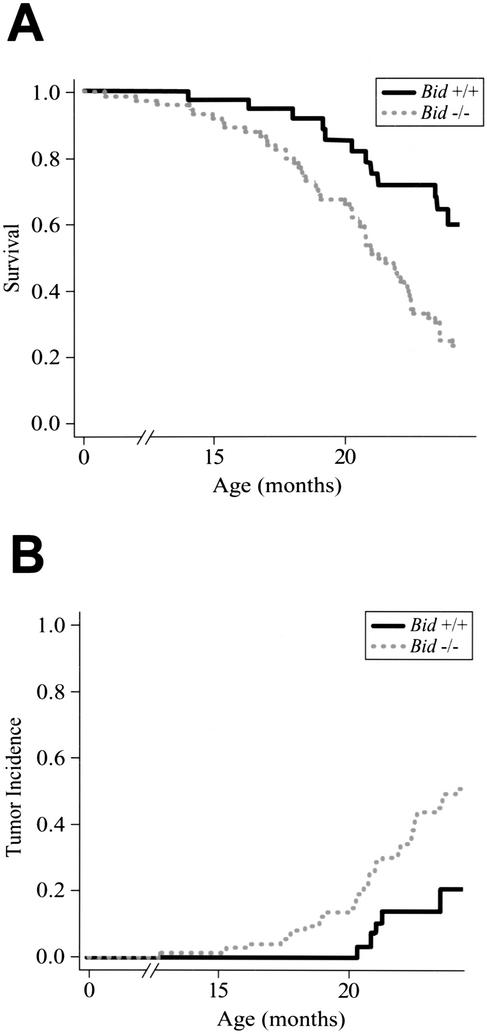
Given the disordered cellular homeostasis, we monitored a cohort of Bid−/− and Bid+/+ mice over a 2-year period. Kaplan-Meier analysis demonstrated that Bid-deficient mice display increased mortality (p = 0.003; Fig. 3A), increased tumor incidence (Fig. 3B; Table 2), and a shortened time to death from tumor (p = 0.01). The most prominent malignancy of Bid−/− mice by 2 years of age was a pathology that closely resembled human CMML (53%; p = 0.002; Table 2). The incidence of CMML increased to 73% by 27 mo of age. Moreover, Bid heterozygous mice displayed an intermediate incidence in which 4 of 12 Bid+/− mice followed developed CMML.
Figure 3.
Bid-deficient mice display increased mortality and increased tumor incidence. (A) Kaplan-Meier survival plot for 38 wild-type and 74 Bid-deficient mice monitored over 24 mo. Bid−/− mice show increased mortality (p = 0.003), and a shortened time to death from tumor (p = 0.01). (B) Tumor incidence. Among mice that died of tumor, the presence of CMML between the Bid-deficient and wild type was highly significant (p = 0.002).
Table 2.
Bid-deficient mice develop a fatal myeloproliferative malignancy
| Pathology of aged wild-type and Bid-deficient animals
| ||
|---|---|---|
|
|
Bid +/+ (n = 38)
|
Bid −/− (n = 74)
|
| CMML | 1 (2.6%) | 39 (53%) |
| Macrophage tumors | 2 (5.2%) | 4 (5%) |
| Macrophage hyperplasia | 0 | 8 (10%) |
| Lymphoma | 1 (2.6%) | 4 (5%) |
| Dermatitis | 5 (13%) | 9 (12%) |
| Angiosarcoma | 1 (2.6%) | 2 (2.5) |
| Lung cancer | 1 (2.6%) | 1 (1%) |
| Polyarteritis | 0 | 3 (4%) |
| Hydronephosis | 1 (2.6%) | 1 (1%) |
| Bullous emphysema | 1 (1%) | |
| Hepatoma | 2 (5.2%) | 1 (1%) |
| Normal (sacrificed at 24 mos) | 24 (63%) | 3 (4%) |
Two mice had both CMML and lymphoma.
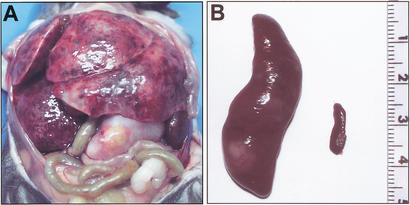
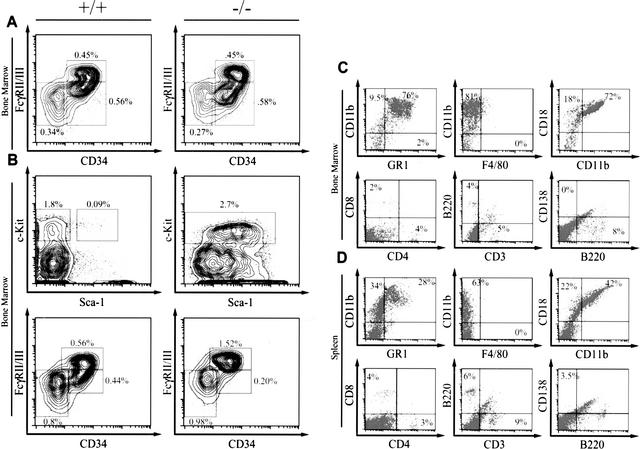
Necropsy of leukemic Bid-deficient mice revealed massive hepatosplenomegaly and variable lymphadenopathy (Fig. 4A; Table 1) in which the spleen was particularly enlarged (Fig. 4B). Peripheral blood displayed anemia, thrombocytopenia, and leukocytosis, with a predominance of monocytes (∼21%) and neutrophils (Fig. 5A,C; Table 1). Bone marrow revealed a myeloproliferative disorder with predominantly mature monocytes and granulocytes in which blasts were <15% (Fig. 5B,D). Both liver and spleen displayed a myeloid infiltrate and prominent extramedullary hematopoiesis (Fig. 5E,F). Flow cytometric analysis of cells from bone marrow and spleen of leukemic Bid-deficient mice confirmed the myeloid immunophenotype of this proliferative disorder (Fig. 6C,D). A marked increase in CD11b+Gr1+ cells was indicative of expanded immature myeloid cells and mature neutrophils, whereas increased CD11b+Gr1− cells indicated expanded monocytes. A relative decrease in the percentage of T cells (CD4+ and CD8+) and B cells (B220+) was noted in bone marrow and spleen. Neither macrophages (F4/80+) nor plasma cells (B220−, CD138+) were increased (Fig. 6C,D).
Figure 4.
Massive hepatosplenomegaly typifies CMML of Bid-deficient mice. (A) Bid-deficient mouse with massive hepatosplenomegaly and lymphadenopathy. (B) Spleen of same Bid-deficient mouse (left) shown relative to an age-matched wild-type spleen (right).
Figure 5.
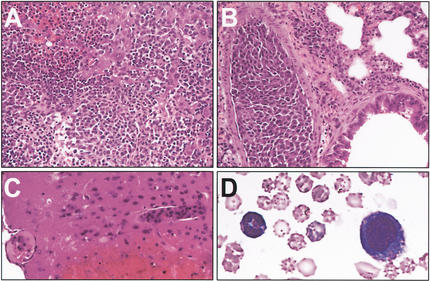
(A) Leukemic Bid-deficient mice reveal increased mature monocytes and granulocytes. Peripheral blood May Grunwald-Giemsa stain 20× reveals leukocytosis comprised predominantly of maturing myeloid elements. (B) Bone marrow, May Grunwald-Giemsa stain 20× reveals features of a myeloproliferative disorder with marked myeloid hyperplasia consisting of predominantly mature granulocytes. (C) Peripheral blood, May Grunwald-Giemsa stain 100×. (D) Bone marrow, May Grunwald-Giemsa stain 100×. (E) Liver, hematoxylin & eosin (H&E) 20×. (F) Spleen, H&E 20×. Liver and spleen reveal a myeloid infiltrate and extramedullary hematopoiesis.
Figure 6.
(A) Bid−/− bone marrow displays normal numbers of progenitor cells. FACS-staining profiles of progenitor populations from littermate Bid+/+ or Bid−/− bone marrow (14–16 mo of age). The lineage (Lin) cocktail consists of: CD3, CD4, CD8, B220, CD19, Ter119, Mac-1, Gr-1. Lin−, c-kit+, Sca-1− cells were collected by flow sorting, and stained for CD34 and FcγII/III to delineate subpopulations of progenitor cells. HSCs were defined as Lin−, c-kithigh, Sca-1high. Myeloid-erythroid progenitors were defined as Lin− c-kithighCD34− FcγII/IIIlo, GMPs were defined as Lin− c-kithighCD34+FcγII/IIIhigh, and CMPs were defined as Lin− c-kithighCD34+FcγII/IIIlo. (B) Transferred Bid−/− leukemic bone marrow demonstrates an expansion of GMP. FACS-staining profiles of progenitor populations of bone marrow from a secondary transplant of leukemic Bid−/− bone marrow reveals increased linneg, Sca1pos, c-kitpos cells consistent with expansion of GMP cells. Immunophenotype of bone marrow (C) and spleen (D) cells from a representative, primary leukemic Bid-deficient mouse. Flow cytometric analysis revealed an expansion of myeloid precursor cells and mature monocytes. There is a corresponding relative depletion of T cells (CD3+, CD4+, CD8+) and B cells (B220+). Plasma cells (CD138+B220−) and macrophages (F4/80+) were not expanded. Samples were gated on live cells based on forward and side scatter profiles.
The myeloproliferation of Bid−/− mice pursues a chronic course in which mice demonstrate a myeloid expansion but little impairment for months. Eventually, nearly all mice display prominent hepatosplenomegaly and subsequently progress to life-threatening complications secondary to organomegaly (Figs. 4A,B; 5E,F), tumor thrombi, or bone marrow replacement (Fig. 5B,D). This compilation of findings, including the chronic course, monocytosis, anemia, thrombocytopenia, low percentage of blast forms, myelocytosis, and extramedullary leukemic infiltrates, most closely resembles the pathology of human CMML, a heterogeneous disorder with features that vary along a spectrum from predominantly myelodysplastic to mainly myeloproliferative in nature.
Bid-deficient leukemia is transplantable
To test the malignant potential of Bid-deficient leukemic cells, we transferred three independent primary tumors (one spleen, two bone marrow) into three sets of nine syngeneic lethally irradiated mice. All recipient mice developed pathology that recapitulated the phenotype of the donors, but displayed substantially decreased latency, showing evidence of tumor by day 77 (Fig. 7). Microscopic analysis revealed extensive tumor infiltrates in spleen, liver (Fig. 7A), and lung (Fig. 7B), as well as the brain of several mice, showing evidence of hemorrhagic cerebral infarcts due to tumor thrombi (Fig. 7C). Peripheral blood also displayed expanded myeloid lineage cells. The bone marrow of recipients, following transplantation of Bid−/− leukemic bone marrow, developed an expanded cell population bearing surface markers characteristic of GMPs (Fig. 6B). This provides evidence that the Bid-deficient leukemia involves a progenitor cell population.
Figure 7.
(A) Leukemic cells from Bid-deficient animals display malignant potential. Microscopic analysis of secondary bone marrow transplant animals demonstrated aggressive tumor infiltrate in multiple organs. (A) Liver, H&E-stained paraffin section (20×) reveals a diffuse infiltrate of mature monocytic cells. (B) Lung, H&E-stained paraffin section (20×) reveals tumor infiltrate filling blood vessels. (C) Brain, H&E-stained paraffin section (20×) reveals tumor infiltrate obstructing blood vessels with associated hemorrhagic infarct. (D) May Grunwald-Giemsa stained peripheral blood (100×) shows mature myeloid elements.
Bid-deficient leukemia demonstrates clonal chromosomal aberrations
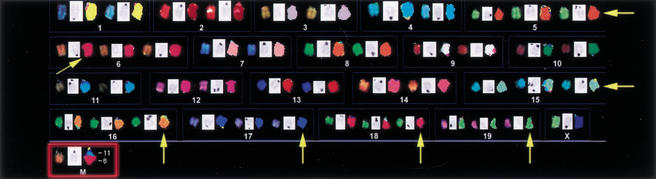
To assess whether the leukemic cells showed evidence of clonality, we performed spectral karyotypes (SKY) on eight tumor specimens. Six showed evidence of chromosomal abnormalities. Two tumors displayed multiple chromosomal trisomies, and the other four showed a translocation or an amplicon. All metaphase spreads from a representative leukemia displayed trisomy of chromosomes 5, 6, 15, 18, 19), and 4/11 metaphase spreads examined contained a t(6:11). These findings support a clonal/oligoclonal origin of the tumor (Fig. 8).
Figure 8.
Bid-deficient tumors are clonal, displaying chromosomal aberrations. A representative SKY of Bid-deficient leukemia. Pseudocolored chromosomes are on the left, inverted DAPI-banded chromosomes are in the middle, and classification-colored chromosomes are on the right. Tumor displays a t(11;6) (red box) and trisomy of multiple chromosomes (yellow arrows).
Discussion
BID maintains myeloid homeostasis
These studies establish that a single BH3-only molecule can be essential to maintain long-term cellular homeostasis within a lineage. Loss of BID leads to an accumulation of myeloid cells over time, demonstrating a critical role for this BH3-only molecule that amplifies death receptor signals in physiologic deaths that assure balanced cell numbers in the myeloid lineage. Consistent with this, enforced overexpression of BCL-2 in the myeloid lineage was noted to extend neutrophil survival (Lagasse and Weissman 1997). Overexpression of an antiapoptotic member rather globally blocks apoptosis through the intrinsic pathway, indicating the participation of a mitochondrial pathway in myeloid homeostasis. However, this loss-of-function model indicates that developmentally appropriate levels of BID, which connects the extrinsic to the intrinsic death pathways, are specifically used to control myeloid homeostasis.
Lpr mice, which harbor a mutation in the Fas receptor, develop extramedullary myelopoiesis, which first suggested a role for Fas in the control of myeloid homeostasis (Schneider et al. 1999). An examination of cell lines suggested that certain lineages do not (Type I) and do (Type II) require a mitochondrial amplification loop to execute death signals initiated by death receptors (Scaffidi et al. 1999). Liver as a representative Type II cell did require BID to undergo apoptosis following a pathological Fas stimulus (Yin et al. 1999). However, the need for this mitochondrial amplification loop in vivo to control cellular homeostasis had not been established. For example, the liver developed normally and remained normal-sized as our unchallenged Bid-deficient mice aged. However, both mature macrophages and myeloid progenitor cells from Bid-deficient mice demonstrated profound resistance to death receptor-induced apoptosis. The myeloproliferative disorder that ensues in Bid-deficient mice demonstrates that a BID-mediated amplification loop is critical to maintain cellular homeostasis in vivo, specifically in the myeloid lineage.
Of note is that Bid-deficient bone marrow, when transferred, demonstrates a competitive advantage in select cell lineages that persists long-term in repopulation assays in vivo. This implicates BID in regulating homeostasis of the myeloid and macrophage compartments derived from long-term repopulating cells. Broadly blocking apoptosis by overexpression of BCL-2 has been shown to result in increased HSCs by surface marker analysis, as well as in increased competitive repopulating ability (Domen et al. 2000). Our data indicate that proapoptotic BID as a singular member regulates homeostasis in the myeloid compartment at physiologic levels during normal development.
BID is essential to suppress tumorigenesis
Also of note is that BID deficiency, a proximal defect in the death receptor pathway, results in a clonal malignancy, CMML. Chronic myeloid leukemia (CML) syndromes in human patients are associated with chromosomal translocations resulting in constitutive activation of tyrosine kinases, most notably the BCR/ABL t(9;22) fusion in CML (for review, see Kelly and Gilliland 2002). Dysregulation of these tyrosine kinases is thought to result in a proliferative and perhaps survival advantage for cells. One model for myeloid leukemogenesis holds that abnormalities in differentiative cell fates contributed by transcription factors are complimented by proliferative signals from activated tyrosine kinases (Deguchi and Gilliland 2002). The development of CMML in the Bid-deficient mouse underscores that loss of apoptosis can be a key component of myeloproliferative disorders.
The long latency for development of CMML indicates the acquisition of secondary oncogenic events, as is characteristic of tumor progression (Land et al. 1983). Acquired secondary oncogenic events often attempt to trigger apoptosis. In this instance it is possible that such an apoptotic signal might be mediated via death receptors, as Bid deficiency could be argued to have protected the cell. This would be consistent with other models in which oncogenes such as myc induce death receptor pathways (Klefstrom et al. 2002). An additional possibility is that loss of apoptotic BID might unmask a FAS proliferative pathway (Fig. 1B), thus simulating two-hit oncogenesis. When mice overexpressing BCL-2 were crossed to FASlpr/lpr, acute myeloid leukemia resulted, suggesting a synergy between the extrinsic death receptor and intrinsic BCL-2 mitochondrial pathways. Bid deficiency may constitute a dual defect, as BID is both activated by caspase 8 and inhibited by mitochondrial BCL-2; thus, loss of BID could reset susceptibility in both extrinsic and intrinsic pathways.
Apoptosis has been shown to play a critical role in the tumor suppressor function of p53. A recent example includes a mouse model of myc-driven lymphoma in which disruption of apoptosis driven by BCL-2 overexpression alleviated the pressure to inactivate p53 during lymphomagenesis (Schmitt et al. 2002). Despite their aggressive phenotype, apoptosis-defective lymphomas that retain intact p53 genes do not display the checkpoint defects and gross aneuploidy that are characteristic of p53 mutant tumors. The consistent chromosomal abnormalities in Bid-deficient CMML in this context is surprising and warrants future studies to determine whether BID could play an unanticipated role in regulating genomic stability.
The multidomain proapoptotic molecules BAX and BAK are used more widely than BH3-only molecules, perhaps required for all intrinsic pathway cell death (Wei et al. 2001). Although the ratio of anti/proapoptotic BCL-2 members is often abnormal in cancer, the data on mutation of the multidomain death effectors BAX, BAK in human malignancies suggest that they are not frequently lost. A proof of principle does exist in that frameshift mutations in BAX have been noted in colon cancer and hematopoietic malignancies in the setting of mismatch repair defects (Rampino et al. 1997; Meijerink et al. 1998). The availability of four BAX plus BAK alleles, given their overlapping roles as downstream death effectors and their widespread coexpression, may limit their genetic participation in human cancers. Despite the signal-specific and lineage-restricted involvement of BID, the results of the present study indicate that the loss of a single such BH3-only molecule can result in cancer. Our observations should prompt a widened survey for defects in the BH3-only genes in human cancers.
Materials and methods
Precursor cultures
Bone marrow cells were isolated from Bid-deficient mice pretreated 48 h with 5 Fluorouracil, 150 mg/kg, by intraperitoneal injection. Myeloid precursor cells were isolated by lineage depletion, followed by positive selection of Sca-1+ cells by magnetic beads (Miltenyi). These cells were grown in the presence of SCF, Flt3, and IL-3 for 72 h and then induced to differentiate with G-SCF. Myeloid cells grown in this manner can be induced to differentiate in synchrony such that from day 1–3, the cells are predominantly promyelocytes and myelocytes, and on day 5, predominantly neutrophils (McLemore et al. 2001). Aliquots of cells were removed on days 1, 3, and 5 and treated with TNFα (10 ng/mL), TNFα (10 ng/mL)+actinomycin D (20 ng/mL), or anti-Fas AB (Jo2, 1 μg/mL). Cell viability measured by trypan blue and annexin V staining (data not shown) was assessed at 12-h intervals. Cell differentiation was assessed by May Grunwald-Giemsa stains of cytospins.
Methylcellulose
For myeloid colonies, 10,000 cells were resuspended in Methylcult H4100 (StemCell Technologies) supplemented with BSA and FCS, with the following cytokines: GCSF (2 ng/mL), GMCSF (10 ng/mL), Il3 (20 ng/mL), Il6 (4 ng/mL), Il11 (10 ng/mL), and SCF (20 ng/mL). For erythroid colonies, 100,000 cells were resuspended in Methylcult H4100 (StemCell Technologies) supplemented with BSA and FCS, and the cytokines SCF (20 ng/mL) and erythropoietin (4 U/mL). Cells were plated in 35-mm dishes and incubated in a humidified atmosphere at 37°C, 5% CO2. Colonies were counted on day 7.
Experimental design of competitive reconstitution
In brief, C57BL/6 recipients were lethally irradiated with 950 cGy given in two doses (5 h apart) from a 137Cs source. A slightly modified CRU assay was used to determine the number of HSCs in bone marrow (Szilvassy et al. 1990; Rebel et al. 1994). Three doses of 8000, 16,000, or 32,000 unseparated test cells (Ly5.2+) were injected together with 1 × 105 unseparated adult bone marrow “helper” cells (Ly5.1+) into lethally irradiated recipients (Ly5.1+) Hematopoietic reconstitution was followed 4, 8, 12, and 16 wk after the transplantation in peripheral blood. A mouse was considered to be reconstituted when, 12 wk after the transplantation, >1% of the peripheral blood cells are Ly5.2+ and include both myeloid and lymphoid cells, as determined by staining with CD3, CD11b, and B220. CRU frequencies for every type of bone marrow tested was calculated by analysis of the proportion of negative mice using Poisson statistics (Strijbosch et al. 1987). Results in Figure 1D are combined from two separate experiments. The reconstitution was performed on two separate occasions with three sets of 60 animals, and similar results were obtained.
A limiting dilution design was used to examine the peripheral white blood cell output from a single CRU and determine the percent of repopulation. Animals from groups in which >37% of the mice were negative were included in the analysis. On average, such recipients would have received 1 CRU, and <27% would have received >2 CRUs. In two experiments, this included seven wild-type animals and five Bid−/− animals.
Data analysis
The data from the tumor watch were analyzed with the help of the Department of Biostatistics, Dana-Farber Cancer Institute. The overall survival for the two groups of mice was calculated using the Kaplan-Meier estimate. Among the Bid-deficient mice, only three were deemed “within normal limits” (WNL); of the remaining 71, 50 died of tumors (or with tumors), and 21 died of other causes. Among the 39 wild-type mice, 29 were apparently WNL. Of the 10 wild-type animals whose deaths were not due to experimental design, 8 died of or with tumors, and 2 died from other causes. In the Bid-deficient group, median survival was 21 mo; at that time point, 79% of the wild-type mice remained alive, or had been censored for prior sacrifice in the WNL state or with dermatitis only. Tumor incidence was assessed using a competing risks model.
Pathology
Mice were dissected and weights taken of liver, lymph nodes, and spleen, organs known to be involved in myeloid malignancies of mice. Organs were fixed in Bouin's fixative or 10% formalin and embedded in paraffin for histological slides. Organ pathology was evaluated by Dr. Rod Bronson, a specialist in tumors of laboratory animals. Representative slides were reviewed by Drs. Jeff Kutok and John Aster in the hematopathology department at the Dana-Farber Cancer Institute.
Spectral karyotyping
Tumor cells from affected bone marrow and spleen were cultured for 1 wk in MCSF to enrich for myeloid cells. Metaphase spreads were prepared by standard methods with overnight colcemid treatment (KaryoMAX colcemid solution, GIBCO-BRL). Analysis was performed on an Applied Spectral Imaging (ASI) CCD camera and interferometer attached to a Nikon Eclipse microscope equipped with 40× and 63× objective lenses. Confirmatory individual chromosome painting experiments were performed using degenerative oligonucleotide primers PCR-labeled probes generated from flow-sorted mouse chromosomes (Liyanage et al. 1996).
Malignant potential
Cell suspensions from spleen or bone marrow of affected male animals were prepared using standard methods. A range of tumor doses from 1 million to 5 million cells was injected intravenously into lethally irradiated female congenic (C57Bl6) mice. One million female bone marrow cells from a c57Bl6 mouse were injected for rescue of hematopoiesis. Mice were observed with the same protocol used for the primary tumor watch. Three independent tumors were transferred into a total of 27 animals. One animal per trial was reconstituted with wild-type bone marrow as a control.
Acknowledgments
We thank D. Neuberg for statistical analysis, J. Fisher and S. Wade for mouse colony management, E. Smith for manuscript preparation, and V. Rebel for helpful discussions. This work is supported in part by NIH grant no. R01CA50239.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL stanley_korsmeyer@dfci.harvard.edu; FAX (617) 632-6401.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1045603.
References
- Adams JM, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Chen G, Lindsten T, White E. BAX and BAK mediate p53-independent suppression of tumorigenesis. Cancer Cell. 2002;2:193–203. doi: 10.1016/s1535-6108(02)00126-5. [DOI] [PubMed] [Google Scholar]
- Deguchi K, Gilliland DG. Cooperativity between mutations in tyrosine kinases and in hematopoietic transcription factors in AML. Leukemia. 2002;16:740–744. doi: 10.1038/sj.leu.2402500. [DOI] [PubMed] [Google Scholar]
- Domen J, Cheshier SH, Weissman IL. The role of apoptosis in the regulation of hematopoietic stem cells: Overexpression of Bcl-2 increases both their number and repopulation potential. J Exp Med. 2000;191:253–264. doi: 10.1084/jem.191.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes & Dev. 1999a;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999b;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, et al. The caspase-8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- Kelly LM, Gilliland DG. Genetics of myeloid leukemias. Annu Rev Genomics Hum Genet. 2002;3:179–198. doi: 10.1146/annurev.genom.3.032802.115046. [DOI] [PubMed] [Google Scholar]
- Klefstrom J, Verschuren EW, Evan G. c-Myc augments the apoptotic activity of cytosolic death receptor signaling proteins by engaging the mitochondrial apoptotic pathway. J Biol Chem. 2002;277:43224–43232. doi: 10.1074/jbc.M206967200. [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Weissman IL. Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell. 1997;89:1021–1031. doi: 10.1016/s0092-8674(00)80290-1. [DOI] [PubMed] [Google Scholar]
- Land H, Parada LF, Weinberg RA. Cellular oncogenes and multistep carcinogenesis. Science. 1983;222:771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik M, Walensky L, Sorcinelli M, Weiler S, Korsmeyer S. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage M, Coleman A, du Manoir S, Veldman T, McCormack S, Dickson RB, Barlow C, Wynshaw-Boris A, Janz S, Wienberg J, et al. Multicolour spectral karyotyping of mouse chromosomes. Nat Genet. 1996;14:312–315. doi: 10.1038/ng1196-312. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, Korsmeyer SJ. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18) Nature. 1991;349:254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- McLemore ML, Grewal S, Liu F, Archambault A, Poursine-Laurent J, Haug J, Link DC. STAT-3 activation is required for normal G-CSF-dependent proliferation and granulocytic differentiation. Immunity. 2001;14:193–204. doi: 10.1016/s1074-7613(01)00101-7. [DOI] [PubMed] [Google Scholar]
- Meijerink JP, Mensink EJ, Wang K, Sedlak TW, Sloetjes AW, de Witte T, Waksman G, Korsmeyer SJ. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91:2991–2997. [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, Perucho M. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- Rebel VI, Dragowska W, Eaves CJ, Humphries RK, Lansdorp PM. Amplification of Sca-1+ Lin- WGA+ cells in serum-free cultures containing steel factor, interleukin-6, and erythropoietin with maintenance of cells with long-term in vivo reconstituting potential. Blood. 1994;83:128–136. [PubMed] [Google Scholar]
- Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- Schmitt CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–298. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Schneider E, Moreau G, Arnould A, Vasseur F, Khodabaccus N, Dy M, Ezine S. Increased fetal and extramedullary hematopoiesis in Fas-deficient C57BL/6-lpr/lpr mice. Blood. 1999;94:2613–2621. [PubMed] [Google Scholar]
- Strijbosch LW, Buurman WA, Does RJ, Zinken PH, Groenewegen G. Limiting dilution assays. Experimental design and statistical analysis. J Immunol Methods. 1987;97:133–140. doi: 10.1016/0022-1759(87)90115-3. [DOI] [PubMed] [Google Scholar]
- Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci. 1990;87:8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver D, Akashi K, Weissman IL, Lagasse E. Mice defective in two apoptosis pathways in the myeloid lineage develop acute myeloblastic leukemia. Immunity. 1998;9:47–57. doi: 10.1016/s1074-7613(00)80587-7. [DOI] [PubMed] [Google Scholar]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: A novel BH3 domain-only death agonist. Genes & Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes & Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes & Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]