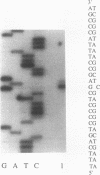
Abstract
Cytochrome aa3 is one of two terminal oxidases expressed in free-living Bradyrhizobium japonicum but not symbiotically in bacteroids. Difference spectra (dithionite reduced minus ferricyanide oxidized) for membranes from cells incubated with progressively lower O2 concentrations showed a concomitant decrease in the A603, the absorption peak characteristic of cytochrome aa3. The level of N,N,N',N'-tetramethyl-p-phenylenediamine oxidase activity, a measure of cytochrome aa3 activity, was also found to depend on the O2 level. Dot blots of total RNA isolated from cells grown at various O2 levels were probed with a fragment of the coxA gene from B. japonicum; a sixfold reduction in transcription from the highest (250 microM) to the lowest (12.5 microM) O2 concentration was observed. Bacteroids had even less coxA message, approximately 19% that in the 12.5 microM O2-incubated cells. Primer extension analysis established the transcription initiation site of the coxA gene at 72 bases upstream of the putative translational start codon. Sequence analysis of the region upstream of the transcription initiation site revealed no homology with previously reported B. japonicum promoters.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. Bacterial electron transport chains. Annu Rev Biochem. 1988;57:101–132. doi: 10.1146/annurev.bi.57.070188.000533. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- Bott M., Bolliger M., Hennecke H. Genetic analysis of the cytochrome c-aa3 branch of the Bradyrhizobium japonicum respiratory chain. Mol Microbiol. 1990 Dec;4(12):2147–2157. doi: 10.1111/j.1365-2958.1990.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Daniel R. M., Appleby C. A. Anaerobic-nitrate, symbiotic and aerobic growth of Rhizobium japonicum: effects on cytochrome P 450 , other haemoproteins, nitrate and nitrite reductases. Biochim Biophys Acta. 1972 Sep 20;275(3):347–354. doi: 10.1016/0005-2728(72)90215-0. [DOI] [PubMed] [Google Scholar]
- Gabel C., Maier R. J. Nucleotide sequence of the coxA gene encoding subunit I of cytochrome aa3 of Bradyrhizobium japonicum. Nucleic Acids Res. 1990 Oct 25;18(20):6143–6143. doi: 10.1093/nar/18.20.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Horsman J. A., Barquera B., Gonzalez-Halphen D., Escamilla J. E. Purification and characterization of two-subunit cytochrome aa3 from Bacillus cereus. Mol Microbiol. 1991 Jan;5(1):197–205. doi: 10.1111/j.1365-2958.1991.tb01840.x. [DOI] [PubMed] [Google Scholar]
- Gubler M., Hennecke H. Regulation of the fixA gene and fixBC operon in Bradyrhizobium japonicum. J Bacteriol. 1988 Mar;170(3):1205–1214. doi: 10.1128/jb.170.3.1205-1214.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka M., Machida K., Shimada S., Mogi A., Tsuchiya T., Ohmori T., Souma Y., Gonda M., Sone N. Nucleotide sequence of the gene coding for four subunits of cytochrome c oxidase from the thermophilic bacterium PS3. J Biochem. 1990 Nov;108(5):866–873. doi: 10.1093/oxfordjournals.jbchem.a123294. [DOI] [PubMed] [Google Scholar]
- Jones C. W. Cytochrome patterns in classification and identification including their relevance to the oxidase test. Soc Appl Bacteriol Symp Ser. 1980;8:127–138. [PubMed] [Google Scholar]
- Keister D. L., Marsh S. S., El Mokadem M. T. Cytochromes of Rhizobium japonicum 61A76 Bacteroids from Soybean Nodules. Plant Physiol. 1983 Jan;71(1):194–196. doi: 10.1104/pp.71.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. L., Marsh S. S. Hemoproteins of Bradyrhizobium japonicum Cultured Cells and Bacteroids. Appl Environ Microbiol. 1990 Sep;56(9):2736–2741. doi: 10.1128/aem.56.9.2736-2741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A. J., Hontelez J. G., Roozendaal B., van Kammen A. On the operon structure of the nitrogenase genes of Rhizobium leguminosarum and Azotobacter vinelandii. Nucleic Acids Res. 1982 Jul 24;10(14):4147–4157. doi: 10.1093/nar/10.14.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepo J. E., Hanus F. J., Evans H. J. Chemoautotrophic growth of hydrogen-uptake-positive strains of Rhizobium japonicum. J Bacteriol. 1980 Feb;141(2):664–670. doi: 10.1128/jb.141.2.664-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Mutaftschiev S. Reconstitution of H2 oxidation activity from H2 uptake-negative mutants of Rhizobium japonicum bacteroids. J Biol Chem. 1982 Feb 25;257(4):2092–2096. [PubMed] [Google Scholar]
- Moshiri F., Chawla A., Maier R. J. Cloning, characterization, and expression in Escherichia coli of the genes encoding the cytochrome d oxidase complex from Azotobacter vinelandii. J Bacteriol. 1991 Oct;173(19):6230–6241. doi: 10.1128/jb.173.19.6230-6241.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal C. S., van Berkum P., Sadowsky M. J., Keister D. L. Cytochrome mutants of bradyrhizobium induced by transposon tn5. Plant Physiol. 1989 Jun;90(2):553–559. doi: 10.1104/pp.90.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Kirshbom P. M., Maier R. J. Tn5-induced cytochrome mutants of Bradyrhizobium japonicum: effects of the mutations on cells grown symbiotically and in culture. J Bacteriol. 1987 Mar;169(3):1089–1094. doi: 10.1128/jb.169.3.1089-1094.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Electron transport components involved in hydrogen oxidation in free-living Rhizobium japonicum. J Bacteriol. 1982 Oct;152(1):422–430. doi: 10.1128/jb.152.1.422-430.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Expression of cytochrome o in hydrogen uptake constitutive mutants of Rhizobium japonicum. J Bacteriol. 1985 Feb;161(2):507–514. doi: 10.1128/jb.161.2.507-514.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Involvement of cytochromes and a flavoprotein in hydrogen oxidation in Rhizobium japonicum bacteroids. J Bacteriol. 1983 Aug;155(2):481–487. doi: 10.1128/jb.155.2.481-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Molecular aspects of the energetics of nitrogen fixation in Rhizobium-legume symbioses. Biochim Biophys Acta. 1989 May 30;974(3):229–246. doi: 10.1016/s0005-2728(89)80239-7. [DOI] [PubMed] [Google Scholar]
- O'brian M. R., Maier R. J. Isolation of a cytochrome aa(3) gene from Bradyrhizobium japonicum. Proc Natl Acad Sci U S A. 1987 May;84(10):3219–3223. doi: 10.1073/pnas.84.10.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Metso T., Nakari T., Jalli T., Lauraeus M., Van der Oost J. The Bacillus subtilis cytochrome-c oxidase. Variations on a conserved protein theme. Eur J Biochem. 1991 Jan 30;195(2):517–525. doi: 10.1111/j.1432-1033.1991.tb15732.x. [DOI] [PubMed] [Google Scholar]
- Shapleigh J. P., Gennis R. B. Cloning, sequencing and deletion from the chromosome of the gene encoding subunit I of the aa3-type cytochrome c oxidase of Rhodobacter sphaeroides. Mol Microbiol. 1992 Mar;6(5):635–642. doi: 10.1111/j.1365-2958.1992.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B., Appleby C. A., Wittenberg B. A. The kinetics of the reactions of leghemoglobin with oxygen and carbon monoxide. J Biol Chem. 1972 Jan 25;247(2):527–531. [PubMed] [Google Scholar]
- Yoshida T., Fee J. A. Studies on cytochrome c oxidase activity of the cytochrome c1aa3 complex from Thermus thermophilus. J Biol Chem. 1984 Jan 25;259(2):1031–1036. [PubMed] [Google Scholar]
- de Vrij W., Konings W. N. Kinetic characterization of cytochrome c oxidase from Bacillus subtilis. Eur J Biochem. 1987 Aug 3;166(3):581–587. doi: 10.1111/j.1432-1033.1987.tb13553.x. [DOI] [PubMed] [Google Scholar]
- van der Oost J., Haltia T., Raitio M., Saraste M. Genes coding for cytochrome c oxidase in Paracoccus denitrificans. J Bioenerg Biomembr. 1991 Apr;23(2):257–267. doi: 10.1007/BF00762221. [DOI] [PubMed] [Google Scholar]