Abstract
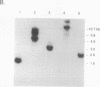
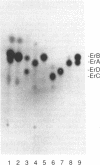
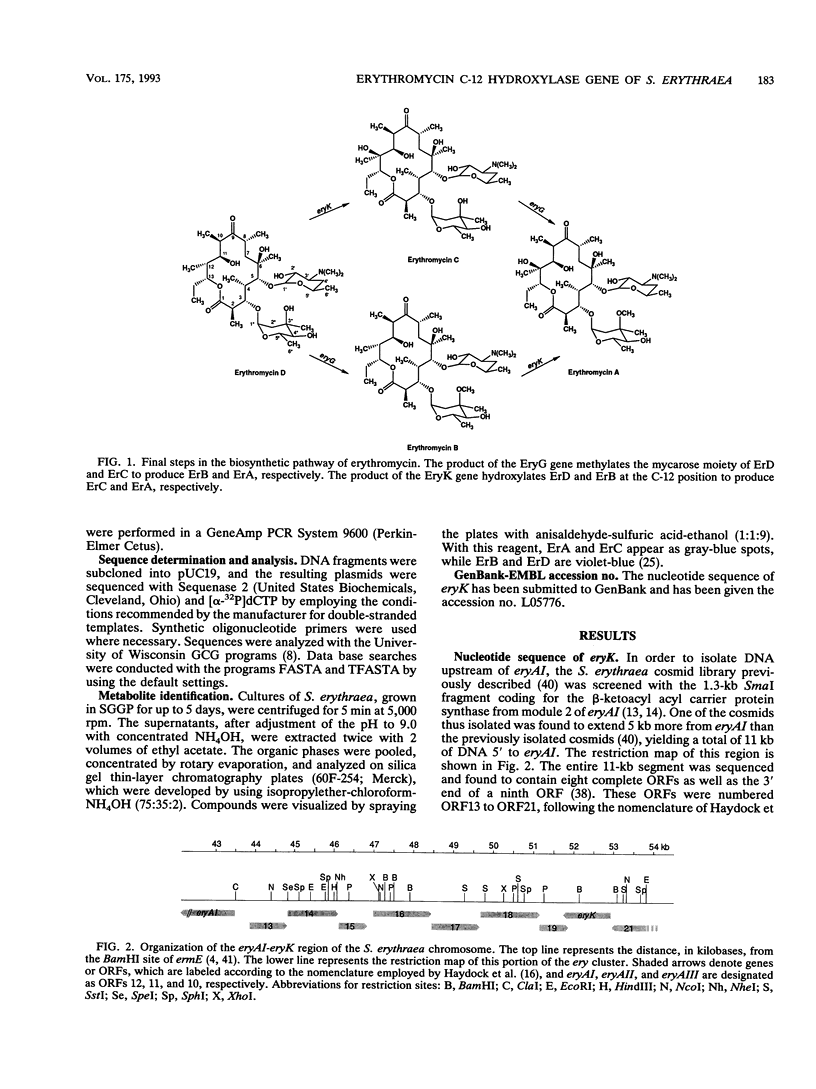
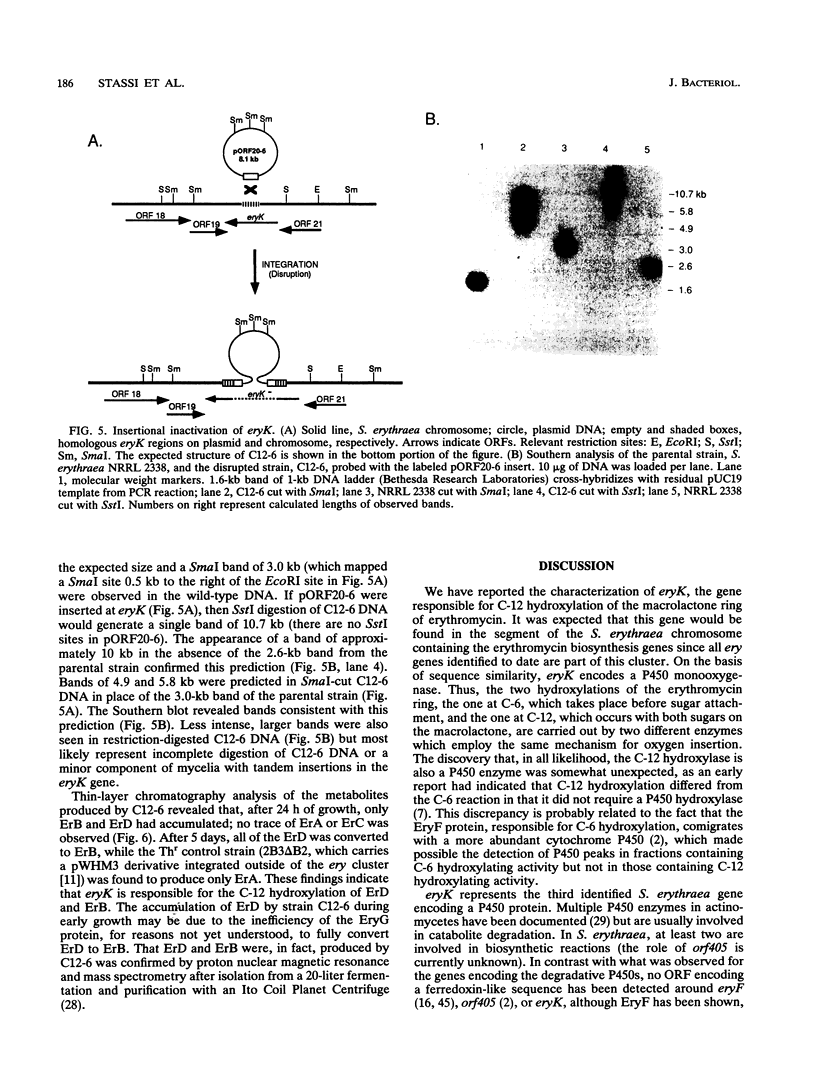
In analyzing the region of the Saccharopolyspora erythraea chromosome responsible for the biosynthesis of the macrolide antibiotic erythromycin, we identified a gene, designated eryK, located about 50 kb downstream of the erythromycin resistance gene, ermE. eryK encodes a 44-kDa protein which, on the basis of comparative analysis, belongs to the P450 monooxygenase family. An S. erythraea strain disrupted in eryK no longer produced erythromycin A but accumulated the B and D forms of the antibiotic, indicating that eryK is responsible for the C-12 hydroxylation of the macrolactone ring, one of the last steps in erythromycin biosynthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn K. S., Wake R. G. Variations and coding features of the sequence spanning the replication terminus of Bacillus subtilis 168 and W23 chromosomes. Gene. 1991 Feb 1;98(1):107–112. doi: 10.1016/0378-1119(91)90111-n. [DOI] [PubMed] [Google Scholar]
- Andersen J. F., Hutchinson C. R. Characterization of Saccharopolyspora erythraea cytochrome P-450 genes and enzymes, including 6-deoxyerythronolide B hydroxylase. J Bacteriol. 1992 Feb;174(3):725–735. doi: 10.1128/jb.174.3.725-735.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Janssen G. R., Ward J. M. Cloning and analysis of the promoter region of the erythromycin resistance gene (ermE) of Streptomyces erythraeus. Gene. 1985;38(1-3):215–226. doi: 10.1016/0378-1119(85)90220-3. [DOI] [PubMed] [Google Scholar]
- Caballero J. L., Martinez E., Malpartida F., Hopwood D. A. Organisation and functions of the actVA region of the actinorhodin biosynthetic gene cluster of Streptomyces coelicolor. Mol Gen Genet. 1991 Dec;230(3):401–412. doi: 10.1007/BF00280297. [DOI] [PubMed] [Google Scholar]
- DeWitt J. P. Evidence for a sex factor in Streptomyces erythreus. J Bacteriol. 1985 Nov;164(2):969–971. doi: 10.1128/jb.164.2.969-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon N., Hale R. S., Cortes J., Leadlay P. F. Molecular characterization of a gene from Saccharopolyspora erythraea (Streptomyces erythraeus) which is involved in erythromycin biosynthesis. Mol Microbiol. 1989 Oct;3(10):1405–1414. doi: 10.1111/j.1365-2958.1989.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Donadio S., Hutchinson C. R. Cloning and characterization of the Saccharopolyspora erythraea fdxA gene encoding ferredoxin. Gene. 1991 Apr;100:231–235. doi: 10.1016/0378-1119(91)90372-i. [DOI] [PubMed] [Google Scholar]
- Donadio S., Katz L. Organization of the enzymatic domains in the multifunctional polyketide synthase involved in erythromycin formation in Saccharopolyspora erythraea. Gene. 1992 Feb 1;111(1):51–60. doi: 10.1016/0378-1119(92)90602-l. [DOI] [PubMed] [Google Scholar]
- Donadio S., Staver M. J., McAlpine J. B., Swanson S. J., Katz L. Biosynthesis of the erythromycin macrolactone and a rational approach for producing hybrid macrolides. Gene. 1992 Jun 15;115(1-2):97–103. doi: 10.1016/0378-1119(92)90546-2. [DOI] [PubMed] [Google Scholar]
- Donadio S., Staver M. J., McAlpine J. B., Swanson S. J., Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991 May 3;252(5006):675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- Haydock S. F., Dowson J. A., Dhillon N., Roberts G. A., Cortes J., Leadlay P. F. Cloning and sequence analysis of genes involved in erythromycin biosynthesis in Saccharopolyspora erythraea: sequence similarities between EryG and a family of S-adenosylmethionine-dependent methyltransferases. Mol Gen Genet. 1991 Nov;230(1-2):120–128. doi: 10.1007/BF00290659. [DOI] [PubMed] [Google Scholar]
- He J. S., Ruettinger R. T., Liu H. M., Fulco A. J. Molecular cloning, coding nucleotides and the deduced amino acid sequence of P-450BM-1 from Bacillus megaterium. Biochim Biophys Acta. 1989 Dec 22;1009(3):301–303. doi: 10.1016/0167-4781(89)90120-6. [DOI] [PubMed] [Google Scholar]
- Horii M., Ishizaki T., Paik S. Y., Manome T., Murooka Y. An operon containing the genes for cholesterol oxidase and a cytochrome P-450-like protein from a Streptomyces sp. J Bacteriol. 1990 Jul;172(7):3644–3653. doi: 10.1128/jb.172.7.3644-3653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto R. H., Powell A. T., Akiyoshi D. E., Regier D. A., Kerstetter R. A., Nester E. W., Hawes M. C., Gordon M. P. Nucleotide sequence and analysis of the plant-inducible locus pinF from Agrobacterium tumefaciens. J Bacteriol. 1989 May;171(5):2506–2512. doi: 10.1128/jb.171.5.2506-2512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A. Genetic manipulation of Streptomyces: integrating vectors and gene replacement. Methods Enzymol. 1991;204:430–458. doi: 10.1016/0076-6879(91)04023-h. [DOI] [PubMed] [Google Scholar]
- Kizawa H., Tomura D., Oda M., Fukamizu A., Hoshino T., Gotoh O., Yasui T., Shoun H. Nucleotide sequence of the unique nitrate/nitrite-inducible cytochrome P-450 cDNA from Fusarium oxysporum. J Biol Chem. 1991 Jun 5;266(16):10632–10637. [PubMed] [Google Scholar]
- Leskiw B. K., Mevarech M., Barritt L. S., Jensen S. E., Henderson D. J., Hopwood D. A., Bruton C. J., Chater K. F. Discovery of an insertion sequence, IS116, from Streptomyces clavuligerus and its relatedness to other transposable elements from actinomycetes. J Gen Microbiol. 1990 Jul;136(7):1251–1258. doi: 10.1099/00221287-136-7-1251. [DOI] [PubMed] [Google Scholar]
- Majer J., Martin J. R., Egan R. S., Corcoran J. W. Antibiotic glycosides. 8. Erythromycin D, a new macrolide antibiotic. J Am Chem Soc. 1977 Mar 2;99(5):1620–1622. doi: 10.1021/ja00447a055. [DOI] [PubMed] [Google Scholar]
- Martín M. F., Liras P. Organization and expression of genes involved in the biosynthesis of antibiotics and other secondary metabolites. Annu Rev Microbiol. 1989;43:173–206. doi: 10.1146/annurev.mi.43.100189.001133. [DOI] [PubMed] [Google Scholar]
- McAlpine J. B., Tuan J. S., Brown D. P., Grebner K. D., Whittern D. N., Buko A., Katz L. New antibiotics from genetically engineered actinomycetes. I. 2-Norerythromycins, isolation and structural determinations. J Antibiot (Tokyo) 1987 Aug;40(8):1115–1122. doi: 10.7164/antibiotics.40.1115. [DOI] [PubMed] [Google Scholar]
- O'Keefe D. P., Harder P. A. Occurrence and biological function of cytochrome P450 monooxygenases in the actinomycetes. Mol Microbiol. 1991 Sep;5(9):2099–2105. doi: 10.1111/j.1365-2958.1991.tb02139.x. [DOI] [PubMed] [Google Scholar]
- Omer C. A., Lenstra R., Litle P. J., Dean C., Tepperman J. M., Leto K. J., Romesser J. A., O'Keefe D. P. Genes for two herbicide-inducible cytochromes P-450 from Streptomyces griseolus. J Bacteriol. 1990 Jun;172(6):3335–3345. doi: 10.1128/jb.172.6.3335-3345.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus T. J., Tuan J. S., Luebke V. E., Maine G. T., DeWitt J. P., Katz L. Mutation and cloning of eryG, the structural gene for erythromycin O-methyltransferase from Saccharopolyspora erythraea, and expression of eryG in Escherichia coli. J Bacteriol. 1990 May;172(5):2541–2546. doi: 10.1128/jb.172.5.2541-2546.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissowotzki K., Mansouri K., Piepersberg W. Genetics of streptomycin production in Streptomyces griseus: molecular structure and putative function of genes strELMB2N. Mol Gen Genet. 1991 Dec;231(1):113–123. doi: 10.1007/BF00293829. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Finzel B. C., Gunsalus I. C., Wagner G. C., Kraut J. The 2.6-A crystal structure of Pseudomonas putida cytochrome P-450. J Biol Chem. 1985 Dec 25;260(30):16122–16130. [PubMed] [Google Scholar]
- Schoner B., Geistlich M., Rosteck P., Jr, Rao R. N., Seno E., Reynolds P., Cox K., Burgett S., Hershberger C. Sequence similarity between macrolide-resistance determinants and ATP-binding transport proteins. Gene. 1992 Jun 15;115(1-2):93–96. doi: 10.1016/0378-1119(92)90545-z. [DOI] [PubMed] [Google Scholar]
- Shafiee A., Hutchinson C. R. Macrolide antibiotic biosynthesis: isolation and properties of two forms of 6-deoxyerythronolide B hydroxylase from Saccharopolyspora erythraea (Streptomyces erythreus). Biochemistry. 1987 Sep 22;26(19):6204–6210. doi: 10.1021/bi00393a037. [DOI] [PubMed] [Google Scholar]
- Tuan J. S., Weber J. M., Staver M. J., Leung J. O., Donadio S., Katz L. Cloning of genes involved in erythromycin biosynthesis from Saccharopolyspora erythraea using a novel actinomycete-Escherichia coli cosmid. Gene. 1990 May 31;90(1):21–29. doi: 10.1016/0378-1119(90)90435-t. [DOI] [PubMed] [Google Scholar]
- Uchiyama H., Weisblum B. N-Methyl transferase of Streptomyces erythraeus that confers resistance to the macrolide-lincosamide-streptogramin B antibiotics: amino acid sequence and its homology to cognate R-factor enzymes from pathogenic bacilli and cocci. Gene. 1985;38(1-3):103–110. doi: 10.1016/0378-1119(85)90208-2. [DOI] [PubMed] [Google Scholar]
- Unger B. P., Gunsalus I. C., Sligar S. G. Nucleotide sequence of the Pseudomonas putida cytochrome P-450cam gene and its expression in Escherichia coli. J Biol Chem. 1986 Jan 25;261(3):1158–1163. [PubMed] [Google Scholar]
- Vara J., Lewandowska-Skarbek M., Wang Y. G., Donadio S., Hutchinson C. R. Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus). J Bacteriol. 1989 Nov;171(11):5872–5881. doi: 10.1128/jb.171.11.5872-5881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. M., Leung J. O., Maine G. T., Potenz R. H., Paulus T. J., DeWitt J. P. Organization of a cluster of erythromycin genes in Saccharopolyspora erythraea. J Bacteriol. 1990 May;172(5):2372–2383. doi: 10.1128/jb.172.5.2372-2383.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. M., Leung J. O., Swanson S. J., Idler K. B., McAlpine J. B. An erythromycin derivative produced by targeted gene disruption in Saccharopolyspora erythraea. Science. 1991 Apr 5;252(5002):114–117. doi: 10.1126/science.2011746. [DOI] [PubMed] [Google Scholar]
- Weber J. M., Losick R. The use of a chromosome integration vector to map erythromycin resistance and production genes in Saccharopolyspora erythraea (Streptomyces erythraeus). Gene. 1988 Sep 7;68(2):173–180. doi: 10.1016/0378-1119(88)90019-4. [DOI] [PubMed] [Google Scholar]
- Weber J. M., Schoner B., Losick R. Identification of a gene required for the terminal step in erythromycin A biosynthesis in Saccharopolyspora erythraea (Streptomyces erythreus). Gene. 1989 Feb 20;75(2):235–241. doi: 10.1016/0378-1119(89)90269-2. [DOI] [PubMed] [Google Scholar]
- Wright F., Bibb M. J. Codon usage in the G+C-rich Streptomyces genome. Gene. 1992 Apr 1;113(1):55–65. doi: 10.1016/0378-1119(92)90669-g. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Maurer K. H., Hutchinson C. R. Transformation of Streptomyces erythraeus. J Antibiot (Tokyo) 1986 Sep;39(9):1304–1313. doi: 10.7164/antibiotics.39.1304. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]