Abstract
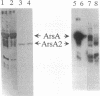
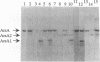
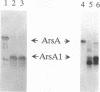
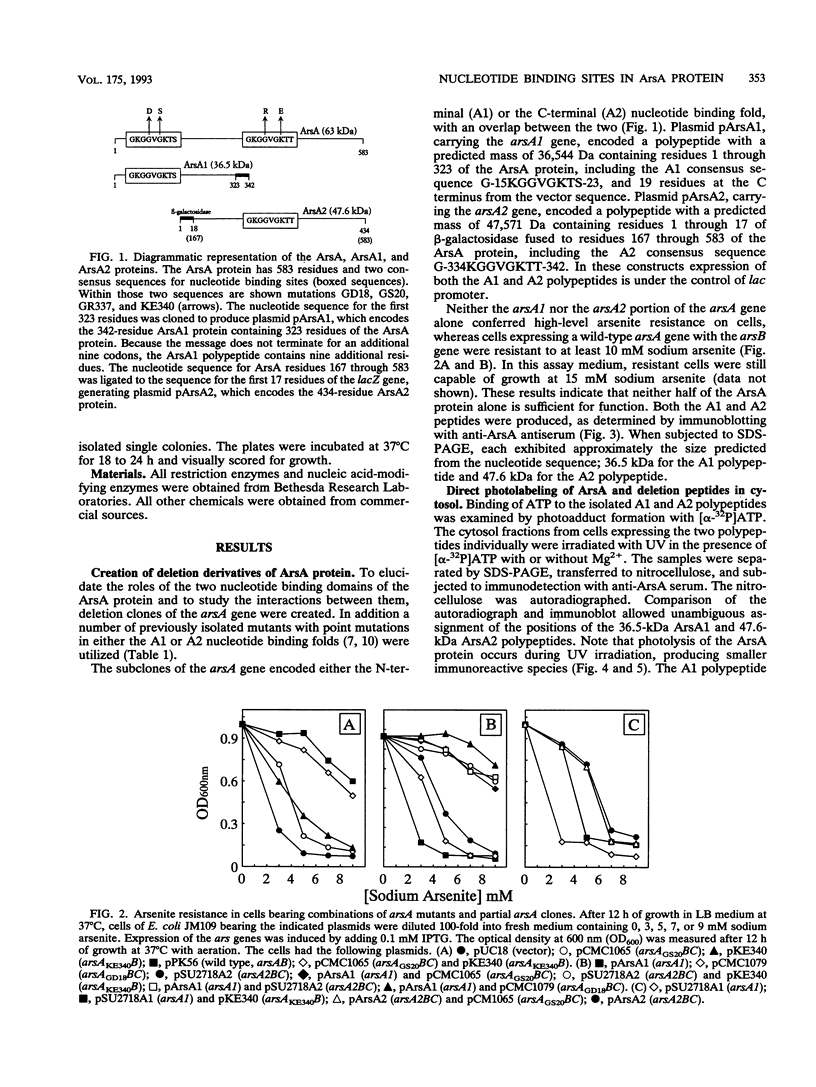
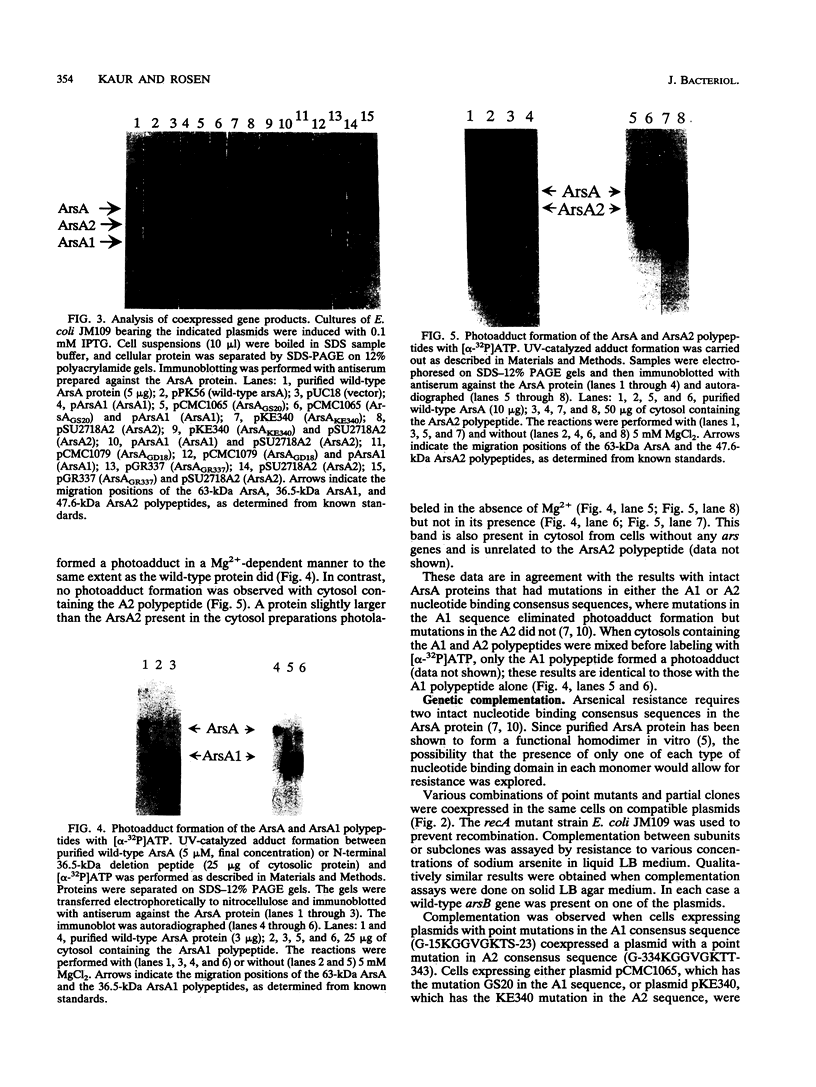
The catalytic component of the oxyanion-translocating ATPase of the plasmid-encoded ars operon of Escherichia coli is a homodimer of the ArsA protein. This enzyme is an oxyanion-stimulated ATPase with two consensus nucleotide binding sequences in each subunit, one in the N-terminal (A1) half and one in the C-terminal (A2) half of the ArsA protein. The two halves of both the arsA gene and the ArsA protein exhibit similar nucleotide and amino acid sequences, respectively. The two halves of the arsA gene were subcloned into compatible plasmids. Neither alone was sufficient to confer resistance, but cells in which the arsA1 and arsA2 half genes were coexpressed were resistant to arsenicals. Genetic complementation was also observed in cells bearing plasmids with point mutations in the two halves of the arsA gene and between cells with plasmids carrying combinations of the arsA1 or arsA2 subclones and point mutations. In every case, complementation was observed only when one plasmid contained a wild-type arsA1 sequence and the other contained a wild-type arsA2 sequence. These results demonstrate that both sites are required for resistance but that the two nucleotide binding domains need not reside in a single polypeptide. We propose a model in which the ArsA dimer has two catalytic units, each composed of an A1 domain from one monomer and an A2 domain from the other monomer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibi E., Kaback H. R. Functional complementation of internal deletion mutants in the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1524–1528. doi: 10.1073/pnas.89.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Misra T. K., Silver S., Rosen B. P. Nucleotide sequence of the structural genes for an anion pump. The plasmid-encoded arsenical resistance operon. J Biol Chem. 1986 Nov 15;261(32):15030–15038. [PubMed] [Google Scholar]
- Ching M. H., Kaur P., Karkaria C. E., Steiner R. F., Rosen B. P. Substrate-induced dimerization of the ArsA protein, the catalytic component of an anion-translocating ATPase. J Biol Chem. 1991 Feb 5;266(4):2327–2332. [PubMed] [Google Scholar]
- Distefano M. D., Moore M. J., Walsh C. T. Active site of mercuric reductase resides at the subunit interface and requires Cys135 and Cys140 from one subunit and Cys558 and Cys559 from the adjacent subunit: evidence from in vivo and in vitro heterodimer formation. Biochemistry. 1990 Mar 20;29(11):2703–2713. doi: 10.1021/bi00463a013. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Baumberg S. Resistance to arsenic compounds conferred by a plasmid transmissible between strains of Escherichia coli. J Bacteriol. 1973 Jul;115(1):459–460. doi: 10.1128/jb.115.1.459-460.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. M., Rosen B. P. Characterization of the catalytic subunit of an anion pump. J Biol Chem. 1989 Oct 15;264(29):17349–17354. [PubMed] [Google Scholar]
- Karkaria C. E., Chen C. M., Rosen B. P. Mutagenesis of a nucleotide-binding site of an anion-translocating ATPase. J Biol Chem. 1990 May 15;265(14):7832–7836. [PubMed] [Google Scholar]
- Karkaria C. E., Rosen B. P. Trinitrophenyl-ATP binding to the ArsA protein: the catalytic subunit of an anion pump. Arch Biochem Biophys. 1991 Jul;288(1):107–111. doi: 10.1016/0003-9861(91)90170-n. [DOI] [PubMed] [Google Scholar]
- Kaur P., Rosen B. P. Mutagenesis of the C-terminal nucleotide-binding site of an anion-translocating ATPase. J Biol Chem. 1992 Sep 25;267(27):19272–19277. [PubMed] [Google Scholar]
- Kaur P., Rosen B. P. Plasmid-encoded resistance to arsenic and antimony. Plasmid. 1992 Jan;27(1):29–40. doi: 10.1016/0147-619x(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martinez E., Bartolomé B., de la Cruz F. pACYC184-derived cloning vectors containing the multiple cloning site and lacZ alpha reporter gene of pUC8/9 and pUC18/19 plasmids. Gene. 1988 Aug 15;68(1):159–162. doi: 10.1016/0378-1119(88)90608-7. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Kimura E., Mizushima S. Complementation of two overlapping fragments of SecA, a protein translocation ATPase of Escherichia coli, allows ATP binding to its amino-terminal region. J Biol Chem. 1990 May 25;265(15):8760–8765. [PubMed] [Google Scholar]
- Mobley H. L., Chen C. M., Silver S., Rosen B. P. Cloning and expression of R-factor mediated arsenate resistance in Escherichia coli. Mol Gen Genet. 1983;191(3):421–426. doi: 10.1007/BF00425757. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Rosen B. P. Energetics of plasmid-mediated arsenate resistance in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6119–6122. doi: 10.1073/pnas.79.20.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., Borbolla M. G. A plasmid-encoded arsenite pump produces arsenite resistance in Escherichia coli. Biochem Biophys Res Commun. 1984 Nov 14;124(3):760–765. doi: 10.1016/0006-291x(84)91023-4. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Weigel U., Karkaria C., Gangola P. Molecular characterization of an anion pump. The arsA gene product is an arsenite(antimonate)-stimulated ATPase. J Biol Chem. 1988 Mar 5;263(7):3067–3070. [PubMed] [Google Scholar]
- Senior A. E. The proton-translocating ATPase of Escherichia coli. Annu Rev Biophys Biophys Chem. 1990;19:7–41. doi: 10.1146/annurev.bb.19.060190.000255. [DOI] [PubMed] [Google Scholar]
- Tsai M. D., Yan H. G. Mechanism of adenylate kinase: site-directed mutagenesis versus X-ray and NMR. Biochemistry. 1991 Jul 16;30(28):6806–6818. doi: 10.1021/bi00242a002. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrubel W., Stochaj U., Sonnewald U., Theres C., Ehring R. Reconstitution of an active lactose carrier in vivo by simultaneous synthesis of two complementary protein fragments. J Bacteriol. 1990 Sep;172(9):5374–5381. doi: 10.1128/jb.172.9.5374-5381.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]