Abstract
In vivo expression technology (IVET) has been used to identify >100 Salmonella typhimurium genes that are specifically expressed during infection of BALB/c mice and/or murine cultured macrophages. Induction of these genes is shown to be required for survival in the animal under conditions of the IVET selection. One class of in vivo induced (ivi) genes, iviVI-A and iviVI-B, constitute an operon that resides in a region of the Salmonella genome with low G+C content and presumably has been acquired by horizontal transfer. These ivi genes encode predicted proteins that are similar to adhesins and invasins from prokaryotic and eukaryotic pathogens (Escherichia coli [tia], Plasmodium falciparum [PfEMP1]) and have coopted the PhoPQ regulatory circuitry of Salmonella virulence genes. Examination of the in vivo induction profile indicates (i) many ivi genes encode regulatory functions (e.g., phoPQ and pmrAB) that serve to enhance the sensitivity and amplitude of virulence gene expression (e.g., spvB); (ii) the biochemical function of many metabolic genes may not represent their sole contribution to virulence; (iii) the host ecology can be inferred from the biochemical functions of ivi genes; and (iv) nutrient limitation plays a dual signaling role in pathogenesis: to induce metabolic functions that complement host nutritional deficiencies and to induce virulence functions required for immediate survival and spread to subsequent host sites.
Keywords: pathogenesis, in vivo expression technology, ivi, phoPQ, Salmonella
Microbial pathogenicity may be defined by the ability to propagate and persist at sites in the host that are inaccessible to commensal species (1). Many virulence determinants that contribute to this ability share a unique phenotype: induction in the host. Previously, we have established a genetic approach, termed in vivo expression technology (IVET), which uses the animal as a selective medium to identify bacterial genes specifically induced during infection (2, 3). These in vivo induced (ivi) genes were shown to be poorly expressed on laboratory medium but exhibit relatively elevated levels of expression in host tissues or in cultured macrophages. It is not anticipated that all ivi genes will have an essential role in virulence. However, their in vivo induction suggests that they contribute to growth in restricted host tissues and thus enhance pathogenicity.
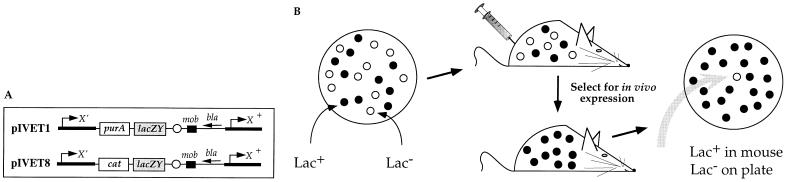
The IVET selection is a promoter trap, whereby bacterial promoters are selected that drive the expression of a gene that is required for virulence (Fig. 1). A promoterless lacZ gene is fused downstream of the promoterless purA gene to monitor the expression of the entire synthetic operon when cells are grown on laboratory medium or in animal tissues. Two variations of the IVET selection strategy have been employed, using purA and cat (chloramphenicol acetyltransferase) as the in vivo-selected markers (2, 3). A positive selection for ivi genes is provided by the need to complement a purA nutritional deficiency or to express cat in response to a host drug regimen. Here we show that induction of ivi genes is required for survival in the animal under the conditions of the IVET selection. This collection of ivi genes comprises an in vivo induction profile that reveals a broad array of regulatory, metabolic, and virulence functions that contribute to enhanced growth and persistence in host tissues.
Figure 1.
Selection for bacterial genes that are specifically induced during infection. (A) Random fragments of bacterial DNA (dark arrows) were cloned into an IVET vector, 5′ to a promoterless purA or cat gene (2, 3). (B) The recombinant pool was used as an inoculum for infection of BALB/c mice and/or RAW 264.7 cultured macrophages. Bacterial survival in the animal (or cultured macrophage) is dependent on in vivo selection of bacterial promoters that drive the expression of the promoterless purA or cat genes. After incubation in the animal (or cultured macrophage), bacterial fusion strains were recovered from host tissues and plated on lactose MacConkey indicator medium. The gray arrow indicates an ivi fusion-bearing strain which is Lac+ (ferments lactose) (•) when grown in the animal and Lac− (○) when grown on laboratory medium.
MATERIALS AND METHODS
Media.
Laboratory media used in these studies included Luria broth (LB) (4) and lactose MacConkey indicator medium prepared by adding 1% filter-sterilized lactose (Baker) to previously autoclaved MacConkey agar base (Difco). Final concentrations of antibiotics (Sigma) were as follows: ampicillin, 50 μg/ml; tetracycline, 20 μg/ml; and chloramphenicol, 20 μg/ml, unless otherwise designated. LB and MacConkey medium were supplemented with adenine (13.5 mg/ml) for the growth of purA strains.
Bacterial Strains and Phage.
All Salmonella typhimurium strains used in this study were derived from strain ATCC 14028 (CDC 6516–60). The high-frequency generalized transducing bacteriophage P22 mutant HT 105/1, int-201, was used for all transductional crosses (5), and phage-free phage-sensitive transductants were isolated as previously described (6). Strains used for PhoPQ regulation studies were constructed by transduction of the IVET-selected fusion into ATCC 14028 (wild type), and isogenic phoPQ derivatives, KK16 [phoP102::Tn10d-Cm (7)] and KK64 [phoQ24 (8)] kindly provided by Karl Klose (Harvard Medical School, Boston).
Cell Culture.
The murine macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection (ATCC TIB-71) and maintained in minimal essential medium (MEM) supplemented with Earle’s salts, 10% heat-inactivated fetal calf serum, and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes), pH 7.0. Cells were grown in a humidified atmosphere of 5% carbon dioxide and 95% air at 37°C in 75-cm2 plastic flasks (Corning). For experiments, cells were harvested by scraping with a rubber policeman and were plated at a density of 1 × 106 cells per ml in 35-mm diameter, six-well dishes (Corning) and grown 24 hr to approximately 80–90% confluence (2–5 × 106 cells per well) (9).
Construction of IVET Fusion Pools.
pIVET1 and pIVET8 pools were made as described previously (2, 3) with the following modifications. Sau3AI partial restriction digests of Salmonella DNA were size fractionated (1–4 kb) on a 0.8% agarose gel. Tn10d-Tc insertion mutations were used to (i) provide a counterselectable marker for the introduction of the recombinant pools from E. coli into Salmonella, and (ii) create a nutritional deficiency (purA) that will be the basis for the in vivo selection. Thus, all pIVET1-containing strains harbor a purA3131::Tn10dTc insertion mutation; all pIVET8-containing strains harbor a zjf-7501::Tn10d-Tc insertion mutation.
IVET Selection in Cultured Macrophages.
One hundred microliters of the S. typhimurium cat-lac fusion pools (5 × 108 cells) grown overnight in LB containing ampicillin and tetracycline was added to 5 × 107 RAW 264.7 macrophages that had been washed twice with cell-culture medium. After a 3-hr incubation, the coculture was washed four times with cell-culture medium and incubated for 2 hr in the presence of 100 μg/ml gentamicin to kill extracellular bacteria (10). These cells were washed four times with cell-culture medium and incubated overnight with 5 μg/ml gentamicin and 20 μg/ml chloramphenicol. The overnight coculture was washed three times with cell-culture medium, and the surviving intracellular bacteria were recovered by lysing the macrophages in 1 ml of distilled deionized H2O. The recovered bacterial cells were grown overnight in LB containing ampicillin and tetracycline and used as inoculum for a second round of macrophage selection.
IVET Selection in BALB/c Mice.
Intragastric inoculation. Pooled ivi fusions were grown overnight in LB, serially diluted to 108-109 cells, and used to i.g. (intragastrically) infect BALB/c mice. Bacteria were recovered from the small intestine after either 24- or 48-hr incubation or, in independent experiments, bacteria were recovered from the spleen or liver after mice showed visible signs of illness (6 days). The recovered bacterial cells were grown overnight in LB containing ampicillin and tetracycline and used as inoculum for a second round of selection.
Intraperitoneal infection. Pooled ivi fusions were grown overnight in LB, serially diluted to 5 × 105 cells, and used to intraperitoneally (i.p.) infect BALB/c mice. Bacteria were recovered from the spleen after mice showed visible signs of illness (3 days). The recovered bacterial cells were grown overnight in LB containing ampicillin and tetracycline and used as inoculum for a second round of selection. Mice inoculated i.p. with cat-lac fusions were administered chloramphenicol as previously described (3).
Cloning and Sequencing IVET Fusions.
ivi fusion strains were cloned by transduction as described previously (11) or by triparental mating (P. Rainey, D.M.H., and M.J.M,, unpublished work). ivi fusion junctions (200–400 bp) were sequenced by using the following primers that are homologous to the 5′ end of the selected gene reading upstream into S. typhimurium sequence: pIVET1 (purA) [5′-CATTGGGTGCCCAGTACG-3′] and pIVET8 (cat) [5′-CAACGGTGGTATATCCAG-3′].
RESULTS
Selection of in Vivo Induced Genes.
Following implementation of the pIVET1 (purA) and pIVET8 (cat) selection strategies in BALB/c mice and/or RAW 264.7 cultured macrophages, the recovered bacterial cells were plated on lactose MacConkey indicator medium. Both selections resulted in an enrichment in the fraction of Lac+ clones in the postselected pool of fusions (red or pink colonies) compared with the preselected pool, indicating that transcriptionally active promoters were selected in vivo, as has been shown previously (refs. 2 and 3; Fig. 1). For example, the preselected pIVET1 pool consists of 48% Lac+ and 52% Lac− colonies. After separate i.g. infections, the ratio of Lac+ to Lac− colonies increased to 99:1 and 97:3 among bacteria recovered from the intestine or the spleen, respectively. This indicates that there is a mucosal and systemic purine deficiency and thus IVET-selected promoters are required at both early and late stages of infection under these conditions. Each IVET selection resulted in a similar enrichment (data not shown).
From 212,000 IVET-selected colonies plated, 2647 ivi fusions (Lac−) were isolated, cloned, and restriction mapped. Siblings were identified by identical restriction patterns, and 476 fusion joint points were sequenced, resulting in the identification of >100 unique genes, of which >50% are unknown; i.e., they have no significant homology with sequences in the DNA data base or encode open reading frames (ORFs) with no assigned function. A map position on the Salmonella chromosome or virulence plasmid (pSLT) was assigned to each unique fusion by Mud-P22 mapping (12). Each unique fusion was reintroduced into the wild-type chromosome and shown to map at the expected location. In the case of ivi fusions to known genes, all were shown to integrate at their known position in the E. coli or Salmonella genomic maps. All fusions listed in Table 1 are in the known coding sequence or in the predicted ORF of the gene indicated, with the exception of cfa, where the joint point is 23 bp before the ATG start codon, cirA, where the fusion joint point is 104 bp after the translational stop codon, and iviXV, where the coding sequence has not been identified.
Table 1.
S. typhimurium genes that are induced in vivo
| Strain | Gene* | Function | Role in pathogenesis | Parameters† |
|---|---|---|---|---|
| Regulatory genes | ||||
| MT1466 | phoP | Virulence regulator | Invasion/macrophage survival | 1BC |
| MT1731 | pmrB | Polymyxin resistance | Neutrophil survival | 8D |
| MT1396 | cadC | Cadaverine synthesis | Acid tolerance | 1A, C |
| MT1398 | iviXIII‡ | ChvD-like | Regulator induction | 1B; 99 min |
| MT1632/1633 | vacB‡/vacC | RNA processing | Post-transcriptional regulation | 1BC/1A; 95 min/9 min |
| RpoS-regulated genes | ||||
| MT1483 | spvB | Plasmid virulence | Systemic survival | 8C |
| MT1397 | cfa | Membrane modification | Stationary-phase survival | 1AC |
| MT1459 | otsA | Trehalose synthesis | Stationary-phase/osmoprotectant | 1A |
| Metabolic functions | ||||
| MT1562 | recD | Recombination/repair | Macrophage survival | 8D |
| MT1426 | hemA | Catalase cofactor | Peroxide resistance | 1C |
| MT1505 | entF | Enterobactin synthesis | Iron acquisition | 8D |
| MT1415 | fhuA | Iron transport | Iron uptake | 1A |
| MT1399 | cirA | Colicin I receptor | Catechol transport | 8D |
| MT1442/1443 | mgtA‡/mgtB‡ | Mg2+ transport | Mg2+ uptake | 8CD/8C |
| MT1498 | iviX‡ | Heavy metal transport | Cu2+ homeostasis | 8D; 11 min |
| MT1446 | ndk | Nucleotide balance | Alarmone synthesis | 1AC |
| Systemic adhesin- and invasin-like genes | ||||
| MT1461 | iviVI-A‡ | Tia/Hra1-like | Adhesion/invasion | 8C; 7 min |
| MT1461 | iviVI-B‡ | PfEMP1-like | Adhesion/invasion | 8C; 7 min |
| Mouse spleen and cultured macrophages | ||||
| MT1799 | iviXI‡ | Unknown | Macrophage survival | 8CD; 53 min |
| MT1501/1733 | iviXII | Unknown | Macrophage survival | 8D/1C; 9 min |
| MT1500 | iviXV | Unknown | Macrophage survival | 8CD; 9 min |
Listed are functions or attributes of ivi genes and their known or inferred roles in pathogenesis.
ivi fusions were cloned using a transductional method (11), and the first 200–400 base pairs were sequenced using an oligonucleotide primer that directs synthesis from the 5′ end of the selected promoterless gene (purA or cat) into the cloned fragment. Nucleotide and deduced amino acid sequences were compared with known data bases by using the fasta and blast programs of GCG (Madison, WI). All fusions listed are in the known coding sequence or in the predicted ORF of the gene indicated with the following exceptions: cfa, where the joint point is 23 bp before the ATG start codon, cirA, where the fusion joint point is 104 bp after the translational stop codon, and iviXV, where the coding sequence has not been identified although it has been recovered from three independent experiments (from the spleen after an i.p. infection and from two cultured macrophage selections). A
designation after the gene indicates that an insertion mutation in the coding sequence was isolated and assayed for a virulence defect in an LD50 study, 500-fold and >100-fold above the i.g. and i.p. LD50, respectively. None of these mutations conferred a virulence defect by this assay.
The numbers refer to the IVET vector used in the selection: 1 = pIVET1 (purA); 8 = pIVET8 (cat). The capital letters denote the route of delivery and the host tissue (BALB/c mice) from which the bacteria were recovered. A = i.g., small intestine; B = i.g., spleen; C = i.p., spleen; and D = cultured RAW 264.7 macrophages. Map positions in minutes are provided for ivi genes that have not been previously described in Salmonella. Map position on the Salmonella chromosome was determined by Mud-P22 transductional mapping (12).
Induction of ivi Fusions Is Required for Survival in the Animal under Conditions of the IVET Selection.
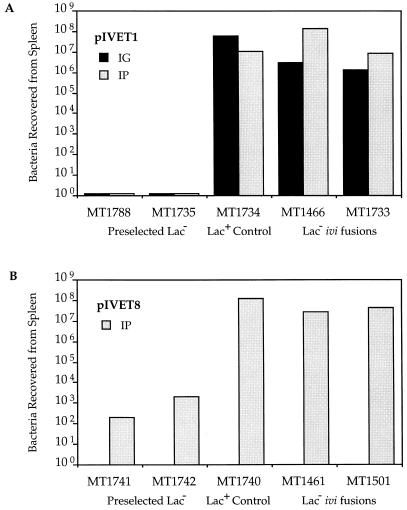
Induction of a given ivi gene in the animal was assessed by the ability to synthesize sufficient quantities of the purA or cat gene products to allow sufficient bacterial growth to cause morbidity or mortality. BALB/c mice were infected with either an ivi or a preselected Lac− or Lac+ fusion strain. After 5 days of incubation, the number of bacterial cells present in the spleen was determined. β-Galactosidase assays of the Lac− ivi fusions and the preselected Lac− fusions showed similar levels of in vitro promoter activity when grown in LB liquid culture (data not shown). Fig. 2 shows that after i.g. infection with 106 cells, pIVET1 ivi fusion strains, MT1466 (phoP) and MT1733 (iviXII), and a preselected Lac+ fusion strain, MT1734, exhibited a 106- to 108-fold growth advantage over two preselected Lac− fusion strains (MT1788 and MT1735). Moreover, i.p. infection with 5 × 102 cells of either pIVET1 (MT1466 and MT1733) or pIVET8 fusions MT1461 (iviVI-A) and MT1501 (iviXII) showed a 104- to 106-fold growth advantage over the preselected Lac− control strains. These data indicate that the induction of an individual IVET-selected fusion is required for survival in the animal under conditions of the IVET selection.
Figure 2.
Induction of ivi genes is required for survival in the animal under conditions of the IVET selection. BALB/c mice were infected i.g. (106 cells) or i.p. (500 cells) with either an ivi or preselected Lac− or Lac+ bacterial fusion strain. The number of bacterial cells recovered from the spleen was determined after morbidity was observed in the Lac+-infected controls (5 days). (A) pIVET1 (purA) i.g. and i.p. selections. (B) pIVET8 (cat) i.p. selection. The number of bacteria recovered from the spleen after i.g. (IG) or i.p. (IP) infection is indicated by dark or gray bars, respectively. The ivi fusion joint points are as follows: phoP (MT1466); iviXII (MT1733); iviVI-A; MT1461, and iviXII (MT1501).
LD50 Studies of ivi Genes.
ivi genes or operons already known to be essential for virulence were not tested here (e.g., phoPQ, spvB, hemA, recBCD). Insertion mutations (Tn10d-Tc and/or pGP704) in many of the ivi genes have been constructed and tested for virulence defects as assessed by 50% lethal dose (LD50) studies. Table 1 shows the ivi genes tested (indicated by ‡). None of the insertions conferred a virulence defect when BALB/c mice were challenged either i.g. or i.p. at a dose 500-fold or >100-fold, respectively, above the LD50. These studies show that although these ivi genes are not essential in these inocula to cause lethality, their induction in host tissues may reflect their contribution and perhaps requirement for growth at specific sites (see Discussion).
Regulatory Genes.
Table 1 shows that several known regulatory genes are induced during infection, including phoP, pmrB, and cadC. The phoPQ operon encodes a two-component regulator of Salmonella virulence that controls the expression of functions required for invasion of mammalian cells, survival in macrophages, and resistance to low pH and to defensins (reviewed in ref. 13). Fig. 2 shows that the induction of phoP (MT1466) after either an i.g. or an i.p. infection is sufficient to satisfy both the mucosal and systemic purA requirements in the animal, which is consistent with a role at early and late stages of infection. Table 2 shows that phoP is autoregulated as shown previously (16) and PhoPQ controls the expression of the ivi operon, pmrAB, encoding a two-component regulatory system involved in resistance to cationic antibacterial proteins (CAP) of human neutrophils (17). The expression of virulence factors by such a regulatory cascade provides a mechanism to modulate the protective response.
Table 2.
PhoPQ regulation of ivi genes
| ivi-lac fusion* | β-Galactosidase, units†
|
||
|---|---|---|---|
| phoP+Q+ | phoP102::Tn10d-Cm | phoQ24 | |
| phoP | 110 | 11 | 132 |
| spvB | 118 | 14 | 294 |
| pmrB | 122 | 31 | 187 |
| mgtB | 48 | 9 | 35 |
| iviVI-A | 236 | 5 | 563 |
| ndk | 18 | 21 | 21 |
Wild-type, PhoP− [phoP102::Tn10d-Cm (7)], and PhoQc [phoQ24, which constitutively activates PhoP-activated genes (8)] strains were grown in Mops media (15), where Mops [3-(N-morpholino)propanesulfonic acid] was replaced with Mes [2-(N-morpholino)ethanesulfonic acid] and buffered to pH 5.5 (50 μM Mg2+). The defects observed in phoP102::Tn10d-Cm may also be attributed to the lack of phoQ.
Numbers given indicate β-galactosidase activities assayed according to Slauch and Silhavy (14). Units are given as (units per OD600 unit × ml of cell suspension) × 103, where 1 unit = 1 μmol of o-nitrophenol formed per min (n = 3, SD < 10%).
The ability to respond to low-pH environments is an important aspect of Salmonella pathogenicity. cadC controls the expression of lysine decarboxylase, which may increase the pH of host cell organelles by the production of cadaverine, a primary amine (18). Moreover, CadC is topologically similar to ToxR, the global regulator of virulence in Vibrio cholerae (18, 19). Both CadC and ToxR respond to low pH and media composition, but it is unclear whether ToxR regulates polyamine synthesis in V. cholerae or whether CadC regulates other virulence genes in Salmonella spp.
Some virulence functions are utilized by both plant and animal pathogens. The chvD gene product of the plant pathogen Agrobacterium tumefaciens is involved in the expression of the VirA/VirG two- component regulatory system required for full virulence. Under conditions of low pH and phosphate starvation, ChvD is required for the induction of virG transcription (20). The ivi gene iviXIII is the Salmonella homologue of E. coli ORF orf579, encoding a predicted peptide that is ≈55% identical to the entire predicted internal fragment of ChvD. The in vivo induction of IviXIII in S. typhimurium suggests that this protein may function as a sensory element, shared by animal and plant pathogens, which modulates the expression of regulatory systems.
Two additional loci previously undescribed in Salmonella, homologues of Shigella spp. vacB and vacC, have been identified by the IVET selection. These virulence-associated chromosomal loci affect virulence plasmid gene expression in Shigella spp. and enteroinvasive E. coli (EIEC). In Shigella spp., both VacB and VacC are involved in the post-transcriptional regulation of ipa (invasion plasmid antigens) and ics (intercellular spread) gene products, which are required for invasion and lateral spread within host cells (21, 22).
RpoS-Regulated Genes.
The stationary-phase sigma factor, RpoS, which is required for full virulence (23), regulates the expression of at least three ivi fusions—e.g., spvB (24), which encodes a Salmonella plasmid virulence function that facilitates growth at systemic sites of infection (reviewed in ref. 25). Table 2 shows that spvB is also regulated by PhoPQ, demonstrating that the expression of spvB is influenced by both a sigma factor and a two-component regulator. Members of the spv operon have also been recovered by other methods to identify genes required in vivo (26).
rpoS controls the expression of two other ivi fusions, cfa (cyclopropane fatty acid synthase) (27) and otsA (osmoregulatory trehalose synthetase) (28). Cfa introduces a cyclopropane ring into bacterial membrane fatty acids during growth under conditions of amino acid limitation, preventing the loss of cellular proteins (29). Moreover, Mycobacterium tuberculosis cma1 encodes a similar modification that has been implicated in resistance to peroxides (30). OtsBA synthesizes trehalose, which is important for survival during osmotic (31) and thermal (28) stress. Induction of these RpoS-regulated genes suggests that nutrient limitation, osmolarity, and temperature are relevant components of the host ecology that signal the expression of virulence genes.
Metabolic Functions.
The multisubunit complex RecBCD, the major recombination and repair system in bacteria, has also been implicated in superoxide resistance (32). HemA, involved in the synthesis of heme, an iron-containing compound that serves a role in electron transport processes (33), may, in part, mediate protection from oxidative damage by the synthesis of the heme component of catalase (34), although catalase mutants do not show a reduction in virulence (35). recBD and hemA are in ivi operons required for full virulence (32, 33), and their in vivo induction in the macrophage or spleen provides an added benefit to the pathogen: a protective response against protein and DNA damage resulting from the macrophage oxidative burst.
The availability of metal ions, including Fe2+, Mg2+, and Cu2+, affects the expression of genes required for their transport as well as other virulence functions. For example, Fe2+ is limited in the host by the action of host iron-binding proteins. Induction of entF, involved in the synthesis of enterobactin, as well as the siderophore transport system, fhuA (ferric hydroxamate uptake), and the catechol transporter, cirA (colicin I receptor) (reviewed in ref. 36), reflects the pathogen’s attempt to overcome this well characterized barrier to infection (reviewed in ref. 37). As expected, entF, fhuA, and cirA fusions are induced under iron-limiting conditions in vitro (U.H., C.P.C., D.M.H., F. Govantes, and M.J.M., unpublished results).
Mg2+ levels are estimated to be low (<50 μM) in the phagosome (38) and have been shown to induce the expression not only of two high-affinity Mg2+ transport systems, but also phoPQ and genes under its control (refs. 38 and 39; U.H., C.P.C., D.M.H., F. Govantes, and M.J.M., unpublished results). Table 1 shows that ivi fusions to these Mg2+ transport genes have been recovered from BALB/c mice (mgtA and mgtB) and cultured RAW 264.7 macrophages (mgtA). Moreover, Table 2 shows that mgtB is regulated by PhoPQ as indicated previously (39). While the induction of mgtA and mgtB may reflect the pathogen’s attempt to counter the inhibitory effects of low Mg2+ in the phagosome, it also demonstrates the role of Mg2+ as an environmental signal that contributes to the expression of genes required for macrophage survival (39). As expected, mgtA and mgtB fusions are induced under Mg2+-limiting conditions in vitro (ref. 39; U.H., C.P.C., D.M.H., F. Govantes, and M.J.M., unpublished results).
iviX has been recovered from two independent selections performed in cultured macrophages infected in vitro. The predicted protein sequence of IviX shows significant homology (33% identity) to heavy metal transporters of many bacteria and is regulated in response to the level of Cu2+ in the medium (data not shown).
In addition to metals, nucleotide availability may also serve as a signal for virulence gene expression. For example, vacB (discussed above) is an RNase II homologue which may serve to reconstitute the depleted nucleotide pools in vivo by means of increased RNA turnover. Moreover, mutations in vacB affect the expression of ipa and ics virulence genes of Shigella spp. (21, 22). ndk is an ivi gene encoding nucleoside diphosphate kinase that maintains proper nucleotide (NTP) balance in both prokaryotes and eukaryotes. Ndk is involved in the synthesis of GTP, a precursor of the alarmone ppGpp, which is known to signal nutrient-limiting conditions (40). Moreover, Ndk-dependent alterations in metabolism during stationary phase affect Pseudomonas aeruginosa virulence (see Discussion). ndk is closely linked to two other independently isolated ivi genes, iviVIII-A and iviVIII-B, Salmonella homologues of the previously reported E. coli ORFs, orf384 and orf337 (41). However, their biochemical functions or roles in virulence are unknown.
Systemic Induction of Adhesin- and Invasin-Like Genes.
Two ivi genes encoding adhesin- or invasin-like proteins have been identified from a fusion recovered from the spleen after i.p. infection, suggesting a continued systemic role for adhesion and/or invasion factors. iviVI-A and iviVI-B reside in a region of exceptionally low G+C content and thus are presumed to have been acquired by horizontal transfer (42). iviVI-A encodes a predicted product that shows significant sequence similarity (>35% identity) over its entire length to both enterotoxigenic E. coli Tia, an outer membrane protein required for toxigenic to and invasion of cultured gut epithelial cells (43), and to E. coli Hra1, an enteric pathogen afimbrial adhesin (44). Fig. 2 shows that induction of iviVI-A is required for survival in the animal under the conditions of the IVET selection.
A Tn10d-Tc insertion in the upstream, closely linked gene iviVI-B shows polarity on the transcriptional activity of iviVI-A, indicating these two genes are in the same operon. IviVI-B shows regions of similarity (33% to 47%) to several Duffy binding-like (DBL) domains of the malarial virulence factor PfEMP1 (Plasmodium falciparum-infected erythrocyte membrane protein 1) (45). PfEMP1 belongs to the plasmodial family of DBL proteins involved in red blood cell (RBC) invasion and surface modifications (reviewed in ref. 46). Table 2 shows that iviVI-A (and presumably iviVI-B) is PhoPQ regulated, indicating that genes acquired by horizontal transfer have adopted the regulatory circuitry of Salmonella virulence genes.
Mouse Spleen and Cultured Macrophages.
iviXI, iviXII, iviXV, and mgtA represent four examples of specific ivi fusions recovered from spleens and from cultured macrophages. iviXI resides in a previously reported ORF of Salmonella (orf179), iviXII encodes a predicted peptide with similarity (52%) to an ORF of Haemophilus influenzae HI1305. iviXV has been recovered from three independent experiments, although the coding sequence has not been identified. Fig. 2 shows that induction of iviXII is sufficient for survival in BALB/c mice under both the pIVET1 (purA) and pIVET8 (cat) selections. Taken together, these data indicate that fusions recovered from cultured macrophages are expressed at levels sufficient to answer the IVET selection in the animal.
DISCUSSION
We have employed IVET, a genetic system that uses the animal as a selective medium, to enrich for bacterial genes induced in vivo. During infection of its host, the pathogen elaborates a broad array of regulatory, metabolic, and virulence functions that contribute to pathogenicity. Inspection of the in vivo induction profile reveals (i) many ivi genes encode regulatory functions that serve to enhance the sensitivity and amplitude of the in vivo response; (ii) many metabolic genes have a direct role in virulence; (iii) some ivi genes encode functions that are similar to those of pathogens that span vast evolutionary distances ranging from bacterial pathogens of plants and animals to eukaryotic human parasites; and (iv) the host ecology provides an environmental address to which the pathogen responds with the coordinate expression of metabolic and virulence functions.
The multifactorial nature of virulence indicates that pathogenesis is not restricted to a single linear pathway from infection to mortality. Thus, since many alternative routes of spread are available, individual mutations in genes that affect the growth of a pathogen at a specific host site may not affect lethality. The contribution of these genes will be overlooked in a standard LD50 assay which lacks the resolution to elaborate a specific function that is available in more defined in vitro or in vivo infection models (e.g., invasion of epithelial cells, Peyer’s patches, or phagocytes). A classic example is demonstrated by the fact that mutants in the well characterized Yersinia enterocolitica invasion gene, inv, do not confer a virulence defect as measured by LD50 studies, but they do show a dramatic defect in the ability to invade cultured epithelial cells (80-fold) and in the ability to colonize Peyer’s patches (up to 107-fold) early after infection (47). IVET provides a means to identify essential virulence determinants as well as those elusive functions whose role may be characterized in more defined systems.
The ability to sense and respond to environmental signals is a key component to pathogenicity. Thus, several ivi genes encode regulatory functions, including PhoPQ, the global regulator of Salmonella virulence, which is shown to be induced after either an i.g. or an i.p. infection, suggesting a role at both early and late stages of infection. PhoPQ regulates its own expression as well as that of several other ivi genes of known and unknown function, including the regulatory gene pmrB. The control of one regulatory gene by another provides a means to fine-tune and/or amplify the pathogen’s response to host signals encountered during infection. Signals that induce regulatory genes may be distinct from those sensed by the proteins they encode, providing a mechanism to satisfy immediate requirements and anticipate future needs. Further, the ivi gene spvB is controlled by both the sigma factor RpoS and PhoPQ, demonstrating that virulence genes respond to independent and/or overlapping regulatory signals.
The in vivo induction of two genes previously described in Shigella spp. indicates that post-transcriptional processes make a significant contribution to virulence. vacB is a member of the RNase II family and may exert its effect by regulating the message stability of components required for translation of ipa and ics mRNAs. vacC encodes a tRNA guanine transglycosylase that exerts its effect through a specific tRNA modification that leads to increased translation of the positive regulatory element virF (22). The involvement of tRNAs in the expression of virulence genes has been demonstrated in uropathogenic E. coli (48).
The induction of four independent metal transport systems reflects an aspect of host ecology wherein metal ions and other small molecules such as nucleotides serve as cofactors for enzymatic activity and also as signals that direct bacterial gene expression. For example, the limited amount of Mg2+ in the phagosome may signal not only the induction of Mg2+ transport systems but also the expression of phoPQ and the genes under its control (refs. 38 and 39; U.H., C.P.C., D.M.H., F. Govantes, and M.J.M., unpublished work). Moreover, the limited availability of iron is a well characterized signal for the expression of genes involved in iron acquisition and other virulence genes in several pathogens. Despite the recognized role of iron in infection, the loss of a single gene involved in iron acquisition is not always associated with a virulence defect (37). This does not, however, diminish the contribution of each individual iron-acquisition system to pathogenicity.
The importance of Cu2+ transport systems that maintain Cu2+ balance, sufficient for enzyme activity while avoiding toxic concentrations, is demonstrated by the fact that mutations which disrupt Cu2+ transporters lead to defects in bacterial growth (49) or to human genetic disease (50).
De novo synthesis of pyrimidines and purines has been shown to be a strict requirement for bacterial growth and persistence under the nucleotide-limiting conditions of the host (2, 26, 51, 52). Additionally, several genes involved in the synthesis and recycling of nucleotides have answered the IVET selection, including vacB and ndk. vacB (RNaseII homologue)-directed alterations in RNA metabolism may provide a source of nucleotides to reconstitute the depleted pools in vivo. Under these conditions, vacB may affect the stability of a specific set of messages whose functions contribute to virulence (e.g., ipa and ics of Shigella spp. discussed above). ndk plays a direct role in the virulence of P. aeruginosa. In stationary phase, the activity of Ndk is modified by protease cleavage, followed by insertion into the membrane, where it produces GTP exclusively (53), possibly for the use in the synthesis of alginate (54). In Salmonella, the production of GTP, a precursor to the alarmone ppGpp, may be involved in signaling nutrient limitation and directing the induction of other virulence genes. Indeed, ppGpp affects the expression of rpoS, a stationary-phase sigma factor required for full virulence (40).
Thus components of the host ecology such as the availability of metal ions and nucleotides serve a dual role in pathogenesis: to induce metabolic functions that overcome nutritional deficiencies and to induce virulence functions required for immediate survival and spread to subsequent anatomical sites of infection.
The need for adhesins and invasins is most often discussed in the context of early infection stages (e.g., mucosal epithelium). However, the recovery from the spleen after an i.p. infection of iviVI-A and iviVI-B, encoding predicted products that resemble adherence and/or invasion factors of other pathogens, suggests a continued need for these factors at late stages of infection. IviVI-A shows homology to Tia and Hra1, adherence and invasion factors of enteric pathogenic E. coli. Upstream in the same operon lies iviVI-B, which shows regions of similarity to the conserved DBL domains of PfEMP1, an adherence factor of the eukaryotic systemic pathogen Plasmodium falciparum. PfEMP1 is produced by the parasite within an infected RBC and is localized to the RBC plasma membrane, where it directs adherence to vascular epithelia, preventing elimination of the infected RBC in the spleen. The function of IviVI-B in Salmonella is not necessarily limited to adherence, as the DBL family of proteins are also involved in invasion, antigenic variation, and possibly chemokine signaling: a member of the DBL family (DABP) binds to the RBC Duffy blood group antigen, a receptor of a family of chemokines including interleukin 8 (46).
The low G+C content of the chromosomal region in which iviVI-AB resides suggests that these genes have been acquired by horizontal transfer (42). The PhoPQ regulation of iviVI-A (and presumably iviVI-B) reveals that selection has favored the coordinate expression of these acquired genes with other presumed systemic ivi virulence and metabolic functions of Salmonella, including phoP, spvB, pmrB, and mgtB (Table 2). The requirement of type III secretion systems involved in systemic survival has recently been described in studies using differential hybridization methods (55) and directed mutagenesis of a region unique to salmonellae (56). Both of these type III secretion systems map to the same region of the Salmonella chromosome (distinct from iviVI-AB) and are characterized by low G+C content. These “pathogenicity islands” are believed to have been acquired by horizontal transfer. Mutations in this region confer defects in virulence after i.p. infection and in macrophage survival, suggesting they are required at systemic sites of infection. Likewise, iviVI-AB were recovered from the spleen after an i.p. infection, and their PhoPQ-dependent expression (Table 2) is consistent with their systemic induction (Table 1), possibly within the macrophage as in the case of other PhoPQ-activated genes [e.g., pagC (57)].
The independent recovery of specific ivi fusions from infected mice and from cultured macrophages provides complementary information regarding both infection models: it (i) validates both selections, (ii) defines the relevant host tissue, and (iii) provides clues to the function of ivi genes. The recovery of iviXI, iviXII, iviXV, and mgtA from spleens and cultured macrophages may define the relevant mammalian cell type for the expression of these four fusions and suggests that they play a role in bacterial survival within splenic phagocytes.
The ability to sense and respond to complex and overlapping signals encountered at each anatomical site is a key component to pathogenicity. This ability results in the elaboration of ivi regulatory, metabolic, and virulence functions that contribute to growth and persistence in host tissues. Virulence is the sum of these contributions.
Acknowledgments
We thank Matthew K. Ritter for assistance in mapping. We also thank Laszlo Csonka for critically reading the manuscript. This work was supported by National Institutes of Health Grant AI36373, American Cancer Society Junior Faculty Research Award 554, and a Beckman Young Investigator Award (M.J.M.), and by American Cancer Society Grant IRG-158 (P.C.H.).
Footnotes
Abbreviations: IVET, in vivo expression technology; i.g., intragastric(ally); DBL, Duffy binding-like; ORF, open reading frame.
References
- 1.Falkow S. In: Escherichia coli and Salmonella Cellular and Molecular Biology. 2nd Ed. Neidhardt F C, Curtiss R III, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 2723–2729. [Google Scholar]
- 2.Mahan M J, Slauch J M, Mekalanos J J. Science. 1993;259:686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- 3.Mahan M J, Tobias J W, Slauch J M, Hanna P C, Collier R J, Mekalanos J J. Proc Natl Acad Sci USA. 1995;92:669–673. doi: 10.1073/pnas.92.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis R W, Botstein D, Roth J R. Advanced Bacterial Genetics. Plainview, New York: Cold Spring Harbor Lab. Press; 1980. [Google Scholar]
- 5.Schmieger H. Mol Gen Genet. 1972;119:75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- 6.Chan R K, Botstein D, Watanabe T, Ogata Y. Virology. 1972;50:883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- 7.Miller S I, Kukral A M, Mekalanos J J. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller S I, Mekalanos J J. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanna P C, Acosta D, Collier R J. Proc Natl Acad Sci USA. 1993;90:10198–10201. doi: 10.1073/pnas.90.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isberg R R, Falkow S. Nature (London) 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 11.Mahan M J, Slauch J M, Mekalanos J J. J Bacteriol. 1993;175:7086–7091. doi: 10.1128/jb.175.21.7086-7091.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson N, Goldman B S. J Bacteriol. 1992;174:1673–1681. doi: 10.1128/jb.174.5.1673-1681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groisman E A, Heffron F. In: Two-Component Signal Transduction. Hoch J A, Silhavy T J, editors. Washington, DC: Am. Soc. Microbiol.; 1995. pp. 319–332. [Google Scholar]
- 14.Slauch J M, Silhavy T J. J Bacteriol. 1991;173:4039–4048. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neidhardt F C, Bloch P L, Smith D F. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soncini F C, Vescovi E G, Groisman E A. J Bacteriol. 1995;177:4364–4371. doi: 10.1128/jb.177.15.4364-4371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roland K L, Martin L E, Esther C R, Spitznagel J. J Bacteriol. 1993;75:4154–4164. doi: 10.1128/jb.175.13.4154-4164.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson E R. Mol Microbiol. 1993;8:5–14. doi: 10.1111/j.1365-2958.1993.tb01198.x. [DOI] [PubMed] [Google Scholar]
- 19.Watson N, Dunyak D S, Rosey E L, Slonczewski J L, Olson E R. J Bacteriol. 1992;174:530–540. doi: 10.1128/jb.174.2.530-540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winans S C, Kerstetter R A, Nester E W. J Bacteriol. 1988;170:4047–4054. doi: 10.1128/jb.170.9.4047-4054.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobe T, Sasakawa C, Okada N, Honma Y, Yoshikawa M. J Bacteriol. 1992;174:6359–6367. doi: 10.1128/jb.174.20.6359-6367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durand J M, Okada N, Tobe T, Watarai M, Fukuda I, Suzuki T, Nakata N, Kamatsu K, Yoshikawa M, Sasakawa C. J Bacteriol. 1994;176:4627–4634. doi: 10.1128/jb.176.15.4627-4634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang F C, Krause M, Roudier M, Fierer J, Guiney D G. J Bacteriol. 1991;173:6783–6789. doi: 10.1128/jb.173.21.6783-6789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulig P A, Danbara H, Guiney D G, Lax A J, Norel F, Rhen M. Mol Microbiol. 1993;7:825–830. doi: 10.1111/j.1365-2958.1993.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 26.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 27.Wang A Y, Cronan J E., Jr Mol Microbiol. 1994;11:1009–1017. doi: 10.1111/j.1365-2958.1994.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 28.Hengge-Aronis R, Kleine W, Lange R, Rimmele M, Boos W. J Bacteriol. 1991;173:7918–7924. doi: 10.1128/jb.173.24.7918-7924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gitter B, Diefenbach R, Keweloh H, Riesenberg D. Appl Microbiol Biotechnol. 1995;43:89–92. doi: 10.1007/BF00170628. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Y, Lee R E, Besra G S, Belisle J T, Barry C E., III Proc Natl Acad Sci USA. 1995;92:6630–6634. doi: 10.1073/pnas.92.14.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strom A R, Kaasen I. Mol Microbiol. 1993;8:205–210. doi: 10.1111/j.1365-2958.1993.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 32.Buchmeier N A, Lipps C J, So M Y, Heffron F. Mol Microbiol. 1993;7:933–936. doi: 10.1111/j.1365-2958.1993.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 33.Benjamin W H, Jr, Hall P, Briles D E. Microbial Pathogen. 1991;1:289–295. doi: 10.1016/0882-4010(91)90033-7. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg J T, Demple B. EMBO J. 1988;7:2611–2617. doi: 10.1002/j.1460-2075.1988.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchmeier N A, Libby S J, Xu Y, Loewen P C, Switala J, Guiney D G, Fang F C. J Clin Invest. 1995;95:1047–1053. doi: 10.1172/JCI117750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Earhart C F. In: Escherichia coli and Salmonella Cellular and Molecular Biology. 2nd Ed. Neidhardt F C, et al., editors. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1075–1090. [Google Scholar]
- 37.Bullen J J, Griffiths E. Iron and Infection. New York: Wiley; 1987. [Google Scholar]
- 38.Garcia-del Portillo F, Foster J W, Maguire M E, Finlay B B. Mol Microbiol. 1992;6:3289–3297. doi: 10.1111/j.1365-2958.1992.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Vescovi E G, Soncini F C, Groisman E A. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 40.Cashel M, Gentry D R, Hernandez V J, Vinella D. In: Escherichia coli and Salmonella Cellular and Molecular Biology. 2nd Ed. Neidhardt F C, et al., editors. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1458–1496. [Google Scholar]
- 41.Baker J, Parker J. FEMS Microbiol Lett. 1994;121:293–296. doi: 10.1111/j.1574-6968.1994.tb07115.x. [DOI] [PubMed] [Google Scholar]
- 42.Groisman E A, Sturmoski M A, Solomon F R, Lin R, Ochman H. Proc Natl Acad Sci USA. 1993;90:1033–1037. doi: 10.1073/pnas.90.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleckenstein J M, Kopecko D J, Warren R L, Elsinghorst E A. Infect Immun. 1996;64:2256–2265. doi: 10.1128/iai.64.6.2256-2265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lutwyche P, Rupps R, Cavanagh J, Warren R A, Brooks D. Infect Immun. 1994;62:5020–5026. doi: 10.1128/iai.62.11.5020-5026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 46.Borst P, Bitter W, McCulloch R, Van Leeuwen F, Rudenko G. Cell. 1995;82:1–4. doi: 10.1016/0092-8674(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 47.Pepe J C, Miller V L. Proc Natl Acad Sci USA. 1993;90:6473–6477. doi: 10.1073/pnas.90.14.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritter A, Blum G, Emody L, Kerenyi M, Bock A, Neuhierl B, Rabsch W, Scheutz F, Hacker J. Mol Microbiol. 1995;17:109–121. doi: 10.1111/j.1365-2958.1995.mmi_17010109.x. [DOI] [PubMed] [Google Scholar]
- 49.Odermatt A, Suter H, Krapf R, Solioz M. J Biol Chem. 1993;268:12775–12779. [PubMed] [Google Scholar]
- 50.Bull P C, Cox D W. Trends Genet. 1994;10:246–252. doi: 10.1016/0168-9525(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 51.Fields P I, Swanson R V, Haidaris C J, Heffron F. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McFarland W C, Stocker B A D. J Microb Pathol. 1987;3:129–141. doi: 10.1016/0882-4010(87)90071-4. [DOI] [PubMed] [Google Scholar]
- 53.Shankar S, Kamath S, Chakrabarty A M. J Bacteriol. 1996;178:1777–1781. doi: 10.1128/jb.178.7.1777-1781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.May T B, Shinabarger D, Boyd A, Chakrabarty A M. J Biol Chem. 1994;269:4872–4877. [PubMed] [Google Scholar]
- 55.Shea J E, Hensel M, Gleeson C, Holden D W. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ochman H, Soncini F C, Solomon F, Groisman E A. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alpuche-Aranda C M, Swanson J A, Loomis W P, Miller S I. Proc Natl Acad Sci USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]