Abstract
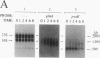
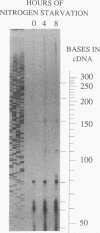
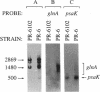
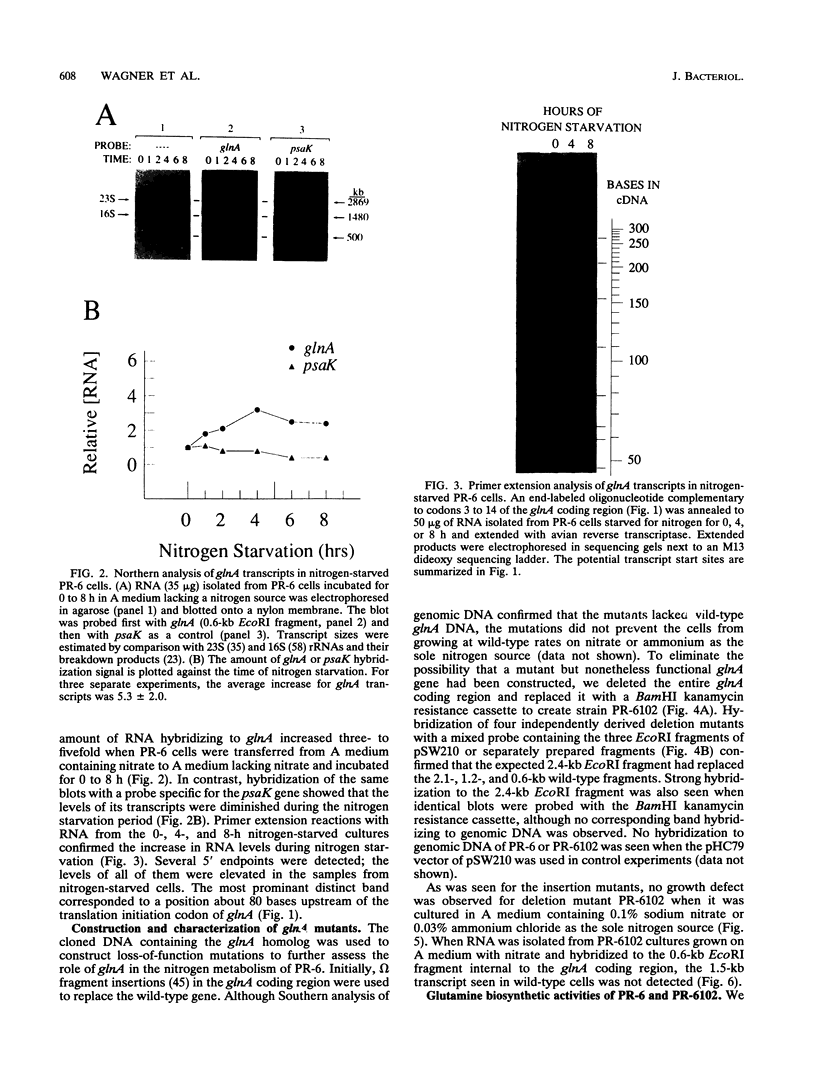
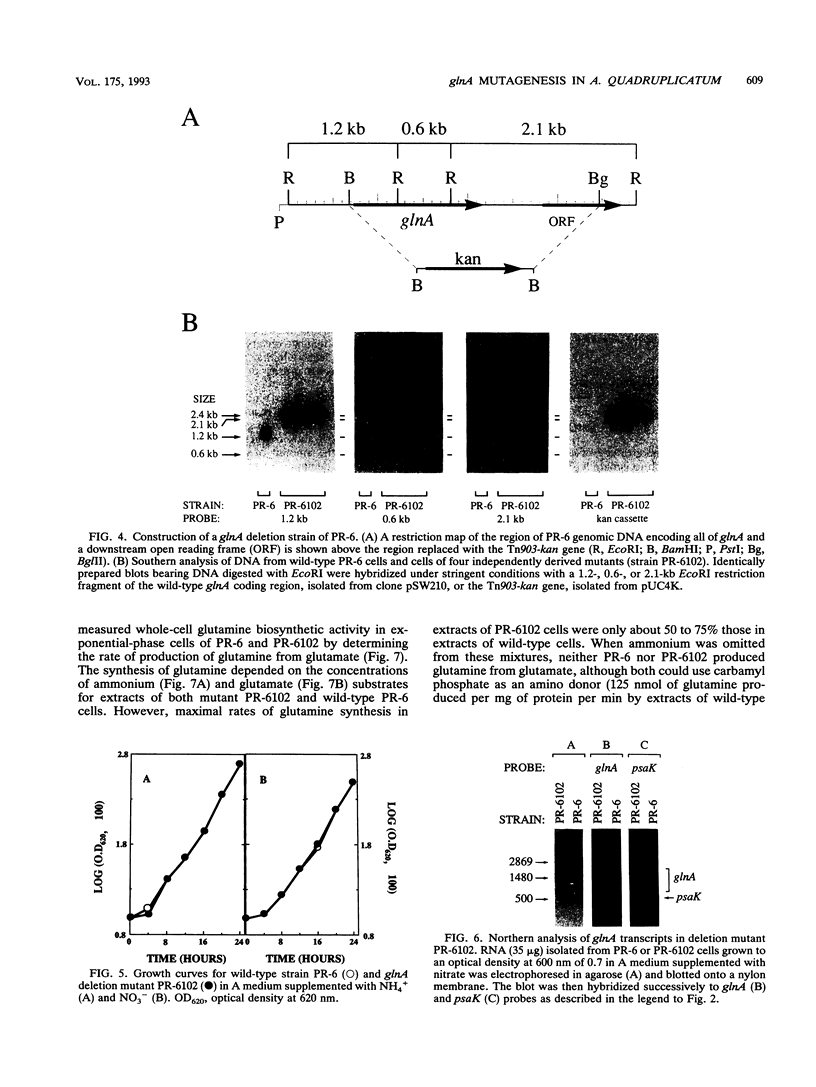
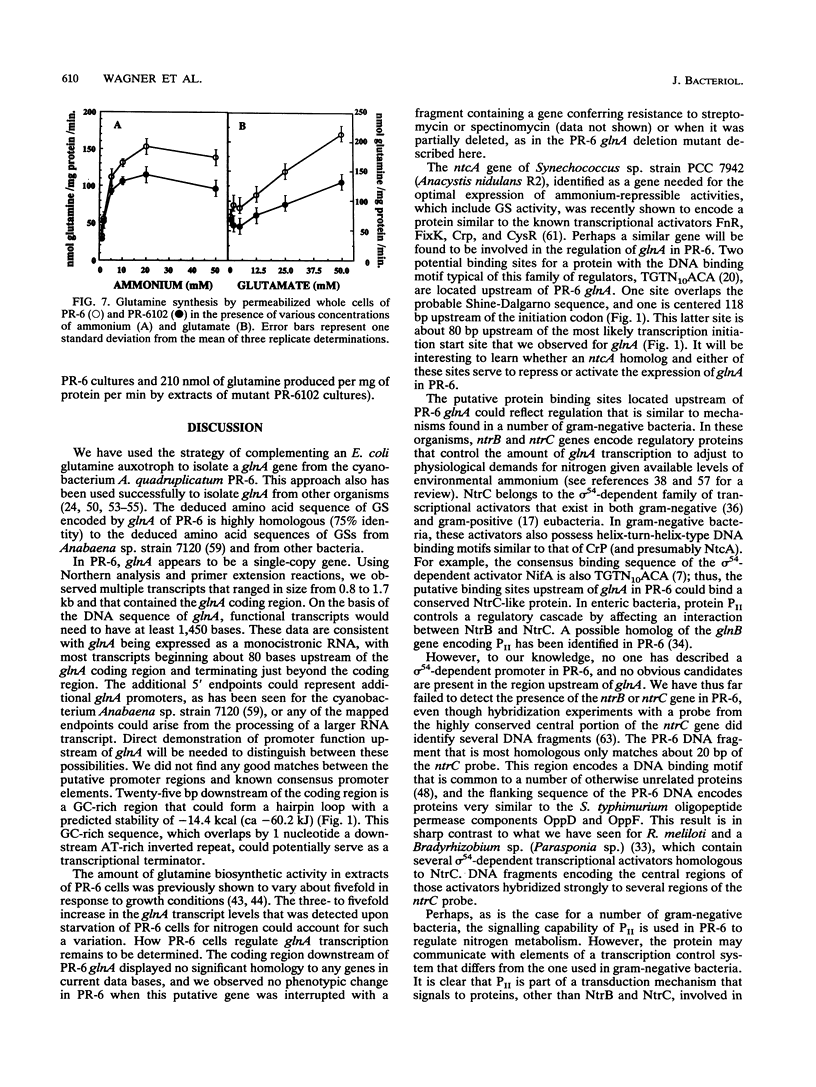
The glnA gene of the cyanobacterium Agmenellum quadruplicatum PR-6 (Synechococcus sp. strain PCC 7002) was isolated by complementing an Escherichia coli strain auxotrophic for glutamine (YMC11) with a PR-6 cosmid library. PR-6 glnA is a single-copy gene that encodes a deduced amino acid sequence that is highly homologous to the deduced glnA amino acid sequences reported for other bacteria. No homology was found between the PR-6 glnA flanking sequences and the ntrB, ntrC, or glnB genes of other bacteria. Northern (RNA) and primer extension analyses of PR-6 RNA revealed one predominant and several minor glnA transcripts of about 1.5 to 1.7 kb. The steady-state amounts of these transcripts increased three- to fivefold when the cells were starved for nitrogen. However, we found that mutant PR-6 cells lacking glnA were still able to use nitrate or ammonium as a sole nitrogen source. Although no RNA homologous to an internal fragment of the glnA gene could be detected in the mutant cells, they retained about 60% of wild-type glutamine biosynthetic activity. The mutant cells were more sensitive than the wild-type cells to methionine sulfoximine, a transition state analog of glutamate, a result that might indicate the presence of an additional glutamine synthetase; however, cell extracts of wild-type PR-6 cells and those lacking glnA were both able to use carbamyl phosphate instead of ammonium as a nitrogen donor for the synthesis of glutamine, a result that indicates the use of carbamyl phosphate synthetase to assimilate ammonium and produce glutamine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science. 1985 Nov 15;230(4727):805–807. doi: 10.1126/science.2997920. [DOI] [PubMed] [Google Scholar]
- Carlson T. A., Guerinot M. L., Chelm B. K. Characterization of the gene encoding glutamine synthetase I (glnA) from Bradyrhizobium japonicum. J Bacteriol. 1985 May;162(2):698–703. doi: 10.1128/jb.162.2.698-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T. A., Martin G. B., Chelm B. K. Differential transcription of the two glutamine synthetase genes of Bradyrhizobium japonicum. J Bacteriol. 1987 Dec;169(12):5861–5866. doi: 10.1128/jb.169.12.5861-5866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Van Baalen C., Tabita F. R. DL-7-azatryptophan and citrulline metabolism in the cyanobacterium Anabaena sp. strain 1F. J Bacteriol. 1987 Mar;169(3):1114–1119. doi: 10.1128/jb.169.3.1114-1119.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow R. A., Knotts R. R. Two forms of glutamine synthetase in free-living root-nodule bacteria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):554–559. doi: 10.1016/0006-291x(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Dixon R. A. The genetic complexity of nitrogen fixation. The ninth Fleming lecture. J Gen Microbiol. 1984 Nov;130(11):2745–2755. doi: 10.1099/00221287-130-11-2745. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F. Ribosomal ribonucleic acid synthesis and maturation in the blue-green alga Anacystis nidulans. J Bacteriol. 1972 Aug;111(2):316–324. doi: 10.1128/jb.111.2.316-324.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Débarbouillé M., Martin-Verstraete I., Klier A., Rapoport G. The transcriptional regulator LevR of Bacillus subtilis has domains homologous to both sigma 54- and phosphotransferase system-dependent regulators. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2212–2216. doi: 10.1073/pnas.88.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filser D. M., Moscatelli C., Lamberti A., Vincze E., Guida M., Salzano G., Iaccarino M. Characterization and cloning of two Rhizobium leguminosarum genes coding for glutamine synthetase activities. J Gen Microbiol. 1986 Sep;132(9):2561–2569. doi: 10.1099/00221287-132-9-2561. [DOI] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Comparative properties of glutamine synthetases I and II in Rhizobium and Agrobacterium spp. J Bacteriol. 1980 Nov;144(2):641–648. doi: 10.1128/jb.144.2.641-648.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Identification of two glutamine synthetases in Agrobacterium. J Bacteriol. 1980 Feb;141(2):996–998. doi: 10.1128/jb.141.2.996-998.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Hirschman J., Wong P. K., Sei K., Keener J., Kustu S. Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro: evidence that the ntrA product is a sigma factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7525–7529. doi: 10.1073/pnas.82.22.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Hunt T. P., Magasanik B. Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG, and glnL. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8453–8457. doi: 10.1073/pnas.82.24.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Gu B. H., Albright L. M., Nixon B. T. Conservation between coding and regulatory elements of Rhizobium meliloti and Rhizobium leguminosarum dct genes. J Bacteriol. 1989 Oct;171(10):5244–5253. doi: 10.1128/jb.171.10.5244-5253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumano M., Tomioka N., Sugiura M. The complete nucleotide sequence of a 23S rRNA gene from a blue-green alga, Anacystis nidulans. Gene. 1983 Oct;24(2-3):219–225. doi: 10.1016/0378-1119(83)90082-3. [DOI] [PubMed] [Google Scholar]
- Kustu S., Santero E., Keener J., Popham D., Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989 Sep;53(3):367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Physiological roles of glutamine synthetases I and II in ammonium assimilation in Rhizobium sp. 32H1. J Bacteriol. 1980 Mar;141(3):1209–1216. doi: 10.1128/jb.141.3.1209-1216.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- Martin F., Suzuki A., Hirel B. A new high-performance liquid chromatography assay for glutamine synthetase and glutamate synthase in plant tissues. Anal Biochem. 1982 Sep 1;125(1):24–29. doi: 10.1016/0003-2697(82)90378-5. [DOI] [PubMed] [Google Scholar]
- Martin G. B., Chapman K. A., Chelm B. K. Role of the Bradyrhizobium japonicum ntrC gene product in differential regulation of the glutamine synthetase II gene (glnII). J Bacteriol. 1988 Dec;170(12):5452–5459. doi: 10.1128/jb.170.12.5452-5459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Guild G. M., Prestidge L. S., Hogness D. S. A new high-capacity cosmid vector and its use. Gene. 1980 Nov;11(3-4):271–282. doi: 10.1016/0378-1119(80)90067-0. [DOI] [PubMed] [Google Scholar]
- Paone D. A., Stevens S. E. Nitrogen Starvation and the Regulation of Glutamine Synthetase in Agmenellum quadruplicatum. Plant Physiol. 1981 Jun;67(6):1097–1100. doi: 10.1104/pp.67.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Expression of glnA in Escherichia coli is regulated at tandem promoters. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1979–1983. doi: 10.1073/pnas.82.7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Astwood P. M., Nixon B. T., Ausubel F. M. Deduced products of C4-dicarboxylate transport regulatory genes of Rhizobium leguminosarum are homologous to nitrogen regulatory gene products. Nucleic Acids Res. 1987 Oct 12;15(19):7921–7934. doi: 10.1093/nar/15.19.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossbach S, Schell J, de Bruijn F J. The ntrC gene of Agrobacterium tumefaciens C58 controls glutamine synthetase (GSII) activity, growth on nitrate and chromosomal but not Ti-encoded arginine catabolism pathways. Mol Gen Genet. 1987 Oct;209(3):419–426. doi: 10.1007/BF00331144. [DOI] [PubMed] [Google Scholar]
- Schön A., Kannangara C. G., Gough S., Söll D. Protein biosynthesis in organelles requires misaminoacylation of tRNA. Nature. 1988 Jan 14;331(6152):187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Virosco J., Haselkorn R. The wild-type gene for glutamine synthetase restores ammonia control of nitrogen fixation to Gln- (glnA) mutants of Rhodopseudomonas capsulata. J Bacteriol. 1983 Jul;155(1):180–185. doi: 10.1128/jb.155.1.180-185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P., Hunsperger J. P., Chereskin B. M., Messing J. Maize glutamine synthetase cDNAs: isolation by direct genetic selection in Escherichia coli. Genetics. 1988 Dec;120(4):1111–1123. doi: 10.1093/genetics/120.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville J. E., Kahn M. L. Cloning of the glutamine synthetase I gene from Rhizobium meliloti. J Bacteriol. 1983 Oct;156(1):168–176. doi: 10.1128/jb.156.1.168-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S. E., Porter R. D. Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6052–6056. doi: 10.1073/pnas.77.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka N., Sugiura M. The complete nucleotide sequence of a 16S ribosomal RNA gene from a blue-green alga, Anacystis nidulans. Mol Gen Genet. 1983;191(1):46–50. doi: 10.1007/BF00330888. [DOI] [PubMed] [Google Scholar]
- Vega-Palas M. A., Flores E., Herrero A. NtcA, a global nitrogen regulator from the cyanobacterium Synechococcus that belongs to the Crp family of bacterial regulators. Mol Microbiol. 1992 Jul;6(13):1853–1859. doi: 10.1111/j.1365-2958.1992.tb01357.x. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- White B. N., Bayley S. T. Further codon assignments in an extremely halophilic bacterium using a cell-free protein-synthesizing system and a ribosomal binding assay. Can J Biochem. 1972 Jun;50(6):600–609. doi: 10.1139/o72-082. [DOI] [PubMed] [Google Scholar]
- Wilcox M. Gamma-glutamyl phosphate attached to glutamine-specific tRNA. A precursor of glutaminyl-tRNA in Bacillus subtilis. Eur J Biochem. 1969 Dec;11(3):405–412. doi: 10.1111/j.1432-1033.1969.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Nirenberg M. Transfer RNA as a cofactor coupling amino acid synthesis with that of protein. Proc Natl Acad Sci U S A. 1968 Sep;61(1):229–236. doi: 10.1073/pnas.61.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Rossbach S., Schneider M., Ratet P., Messmer S., Szeto W. W., Ausubel F. M., Schell J. Rhizobium meliloti 1021 has three differentially regulated loci involved in glutamine biosynthesis, none of which is essential for symbiotic nitrogen fixation. J Bacteriol. 1989 Mar;171(3):1673–1682. doi: 10.1128/jb.171.3.1673-1682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Porter R. D., Liu W. Y., Jay E., Stevens S. E., Jr Genes for the alpha and beta subunits of phycocyanin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]