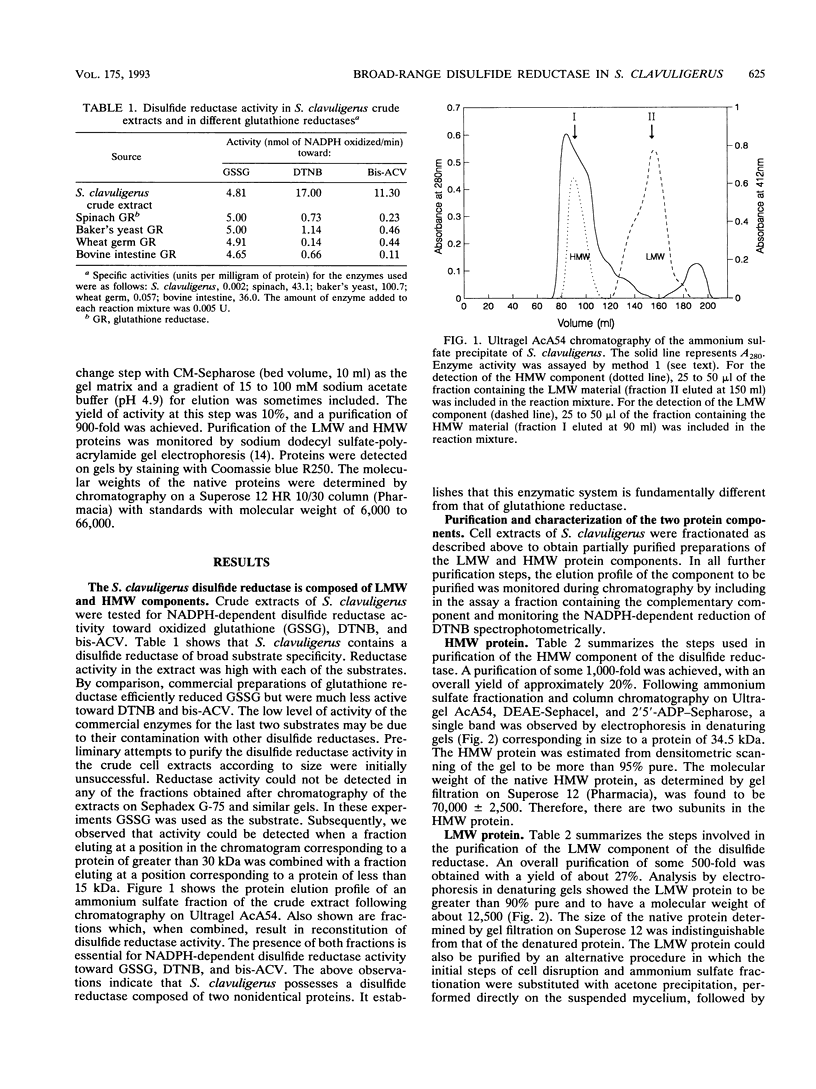
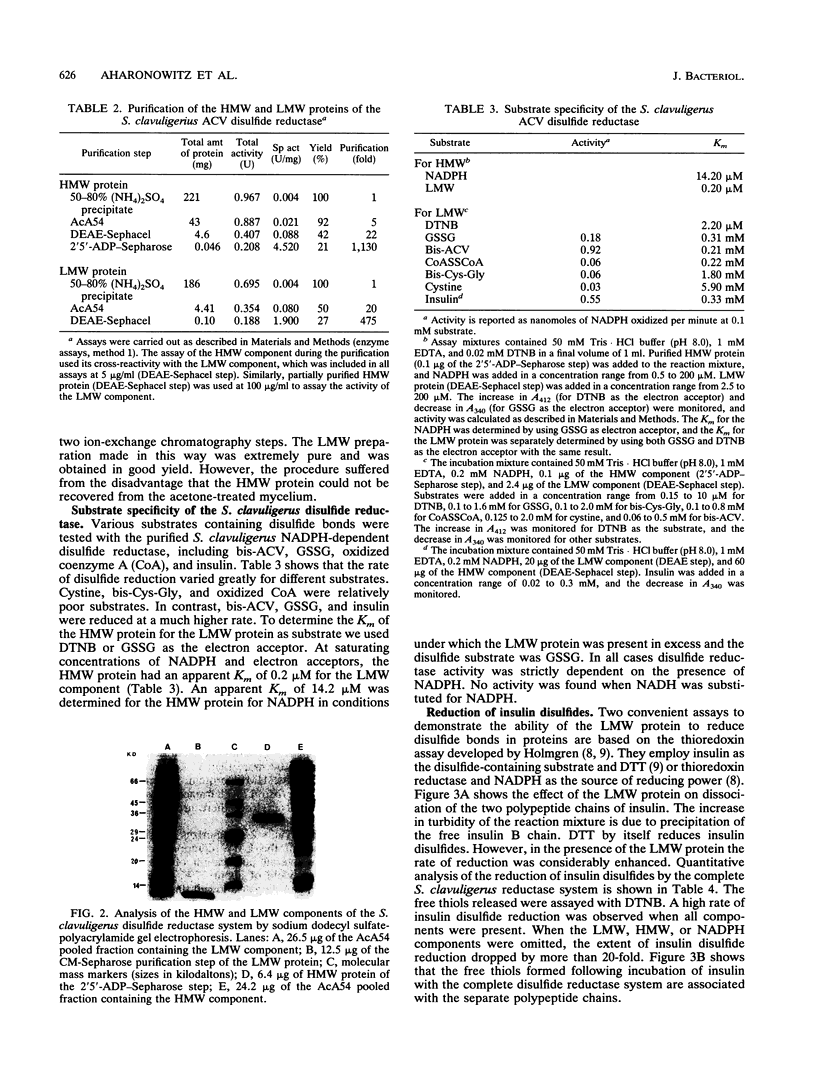
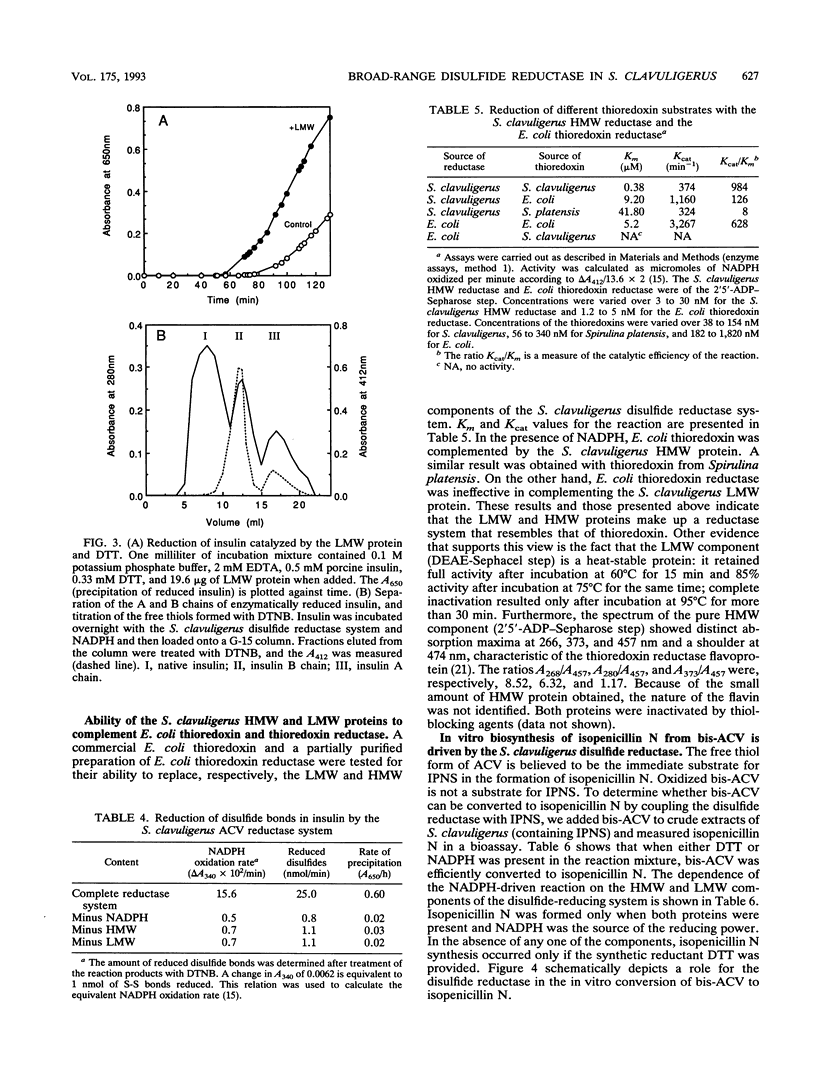
Abstract
Streptomyces clavuligerus is a potent producer of penicillin and cephalosporin antibiotics. A key step in the biosynthesis of these beta-lactam compounds is the cyclization of the thiol tripeptide delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine (ACV) to isopenicillin N by the enzyme isopenicillin N synthase (IPNS). However, bis-ACV, the oxidized disulfide form of the tripeptide, is not a substrate for IPNS. We show here that S. clavuligerus possesses an NADPH-dependent disulfide reductase of broad substrate specificity that efficiently catalyzes the reduction of disulfide bonds in bis-ACV and in other low-molecular-weight disulfide containing compounds and proteins. The disulfide reductase comprises two protein components, a 70-kDa reductase consisting of two identical subunits, and a 12-kDa heat-stable protein reductant. The structural and functional properties of the disulfide reductase resemble those of the thioredoxin class of oxidoreductases. When the disulfide reductase system is coupled with IPNS, it quantitatively converts bis-ACV to isopenicillin N. These findings suggest that the disulfide reductase may play a role in the biosynthesis of penicillins and cephalosporins in streptomycetes. We also show here that S. clavuligerus lacks glutathione reductase and have previously reported that Streptomyces species do not contain glutathione. This disulfide reductase may therefore be important in determining the thiol-disulfide redox balance in streptomycetes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Cohen G., Martin J. F. Penicillin and cephalosporin biosynthetic genes: structure, organization, regulation, and evolution. Annu Rev Microbiol. 1992;46:461–495. doi: 10.1146/annurev.mi.46.100192.002333. [DOI] [PubMed] [Google Scholar]
- Cohen G., Shiffman D., Mevarech M., Aharonowitz Y. Microbial isopenicillin N synthase genes: structure, function, diversity and evolution. Trends Biotechnol. 1990 Apr;8(4):105–111. doi: 10.1016/0167-7799(90)90148-q. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Sundquist A. R. Evolution of glutathione metabolism. Adv Enzymol Relat Areas Mol Biol. 1991;64:1–53. doi: 10.1002/9780470123102.ch1. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its function in disulfide reduction. J Biol Chem. 1977 Jul 10;252(13):4600–4606. [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J Biol Chem. 1979 May 10;254(9):3672–3678. [PubMed] [Google Scholar]
- Holmgren A. Reduction of disulfides by thioredoxin. Exceptional reactivity of insulin and suggested functions of thioredoxin in mechanism of hormone action. J Biol Chem. 1979 Sep 25;254(18):9113–9119. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979 Oct 10;254(19):9627–9632. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Leskiw B. K., Vining L. C., Aharonowitz Y., Westlake D. W., Wolfe S. Purification of isopenicillin N synthetase from Streptomyces clavuligerus. Can J Microbiol. 1986 Dec;32(12):953–958. doi: 10.1139/m86-176. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Westlake D. W., Wolfe S. Cyclization of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine to penicillins by cell-free extracts of Streptomyces clavuligerus. J Antibiot (Tokyo) 1982 Apr;35(4):483–490. doi: 10.7164/antibiotics.35.483. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luthman M., Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982 Dec 21;21(26):6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- Perry D., Abraham E. P., Baldwin J. E. Factors affecting the isopenicillin N synthetase reaction. Biochem J. 1988 Oct 1;255(1):345–351. [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Sequence of thioredoxin reductase from Escherichia coli. Relationship to other flavoprotein disulfide oxidoreductases. J Biol Chem. 1988 Jun 25;263(18):9015–9019. [PubMed] [Google Scholar]
- Swerdlow R. D., Setlow P. Purification and characterization of a Bacillus megaterium disulfide reductase specific for disulfides containing pantethine 4',4"-diphosphate. J Bacteriol. 1983 Jan;153(1):475–484. doi: 10.1128/jb.153.1.475-484.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing A., Follmann H., Auling G. Nucleotide and thioredoxin specificity of the manganese ribonucleotide reductase from Brevibacterium ammoniagenes. Eur J Biochem. 1988 Jul 15;175(1):167–173. doi: 10.1111/j.1432-1033.1988.tb14179.x. [DOI] [PubMed] [Google Scholar]