Abstract
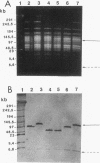
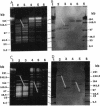
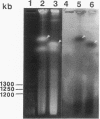
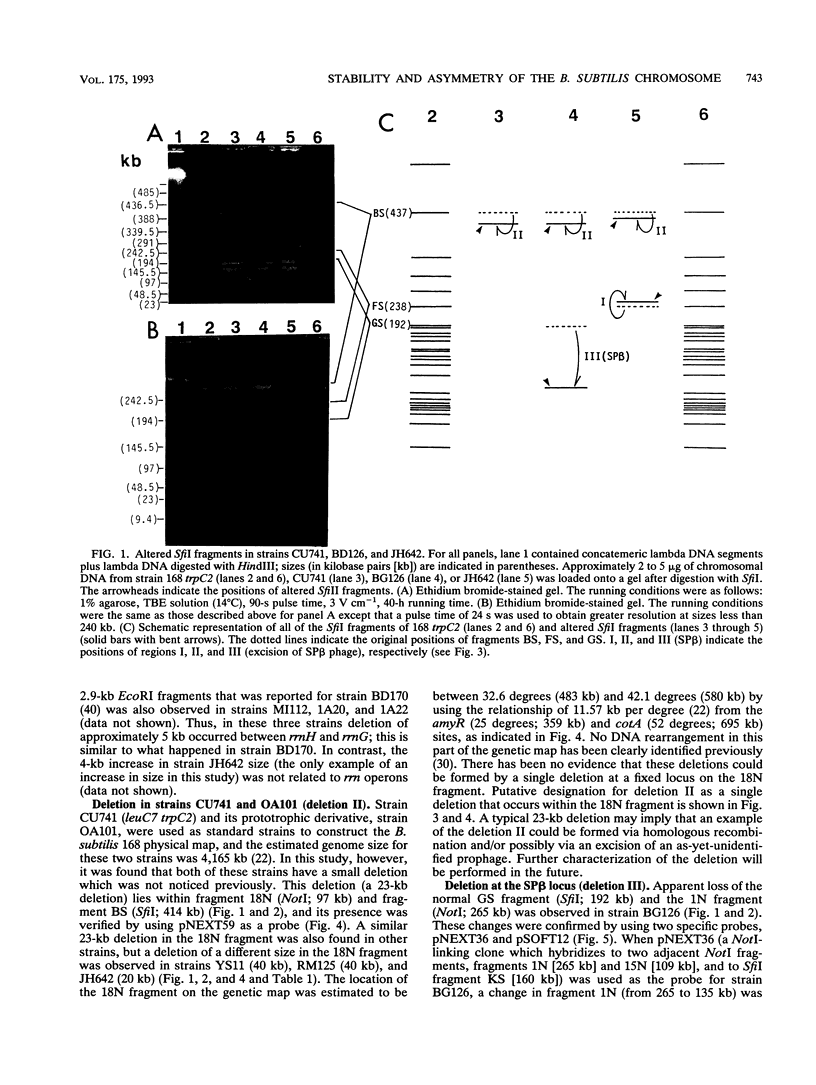
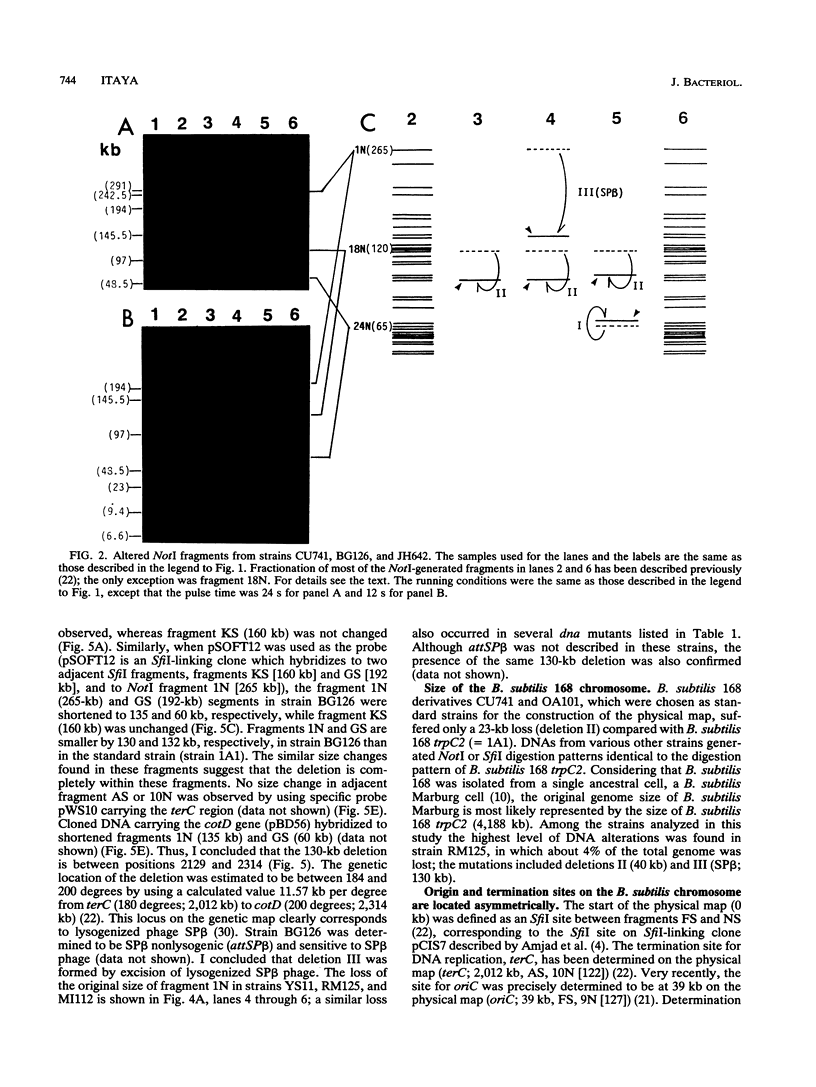
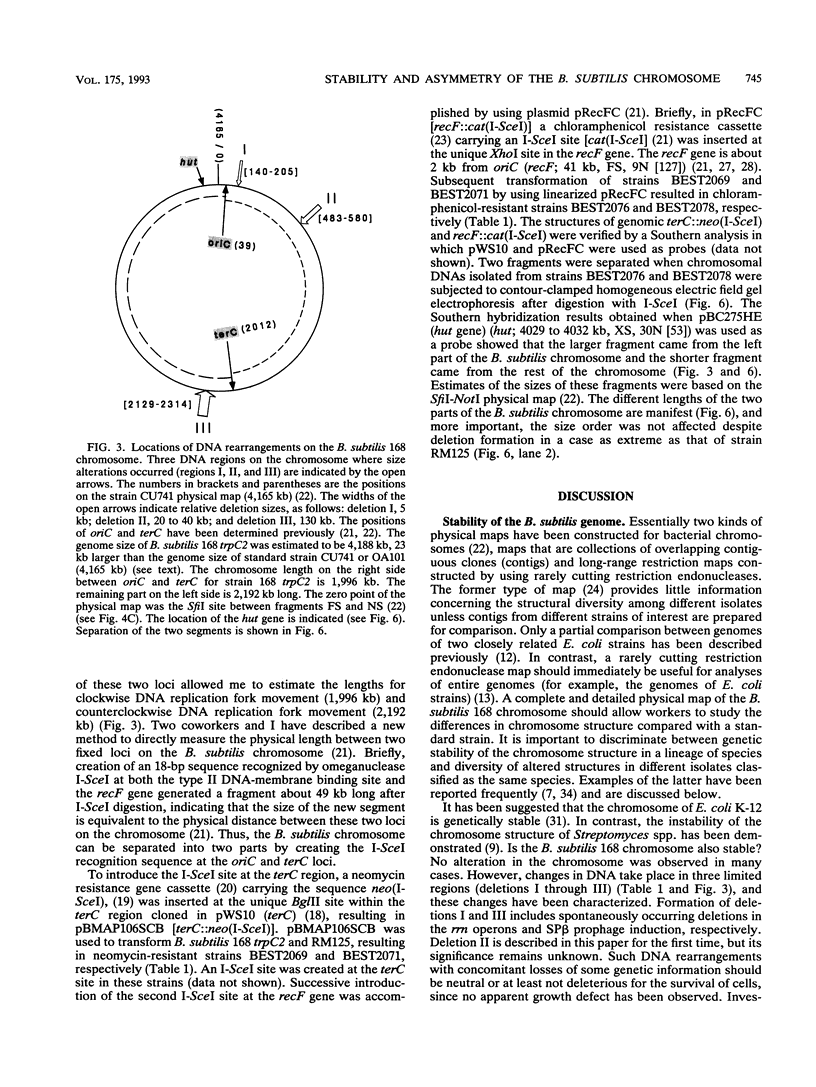
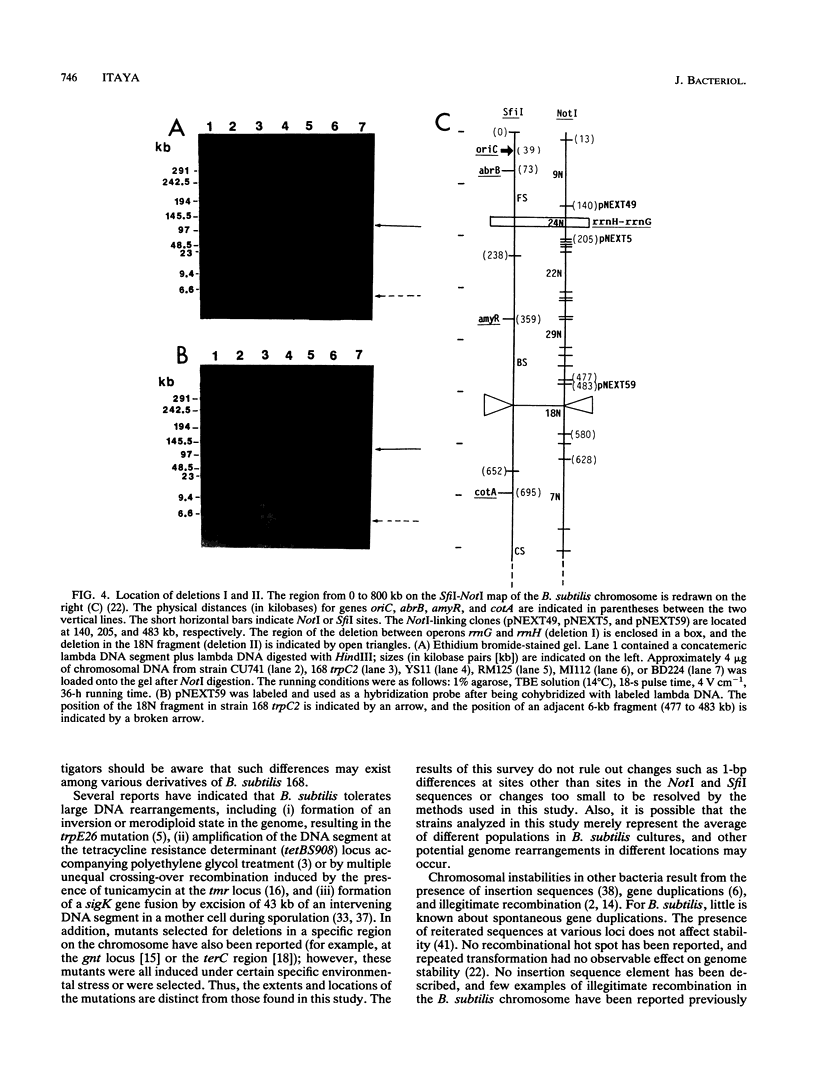
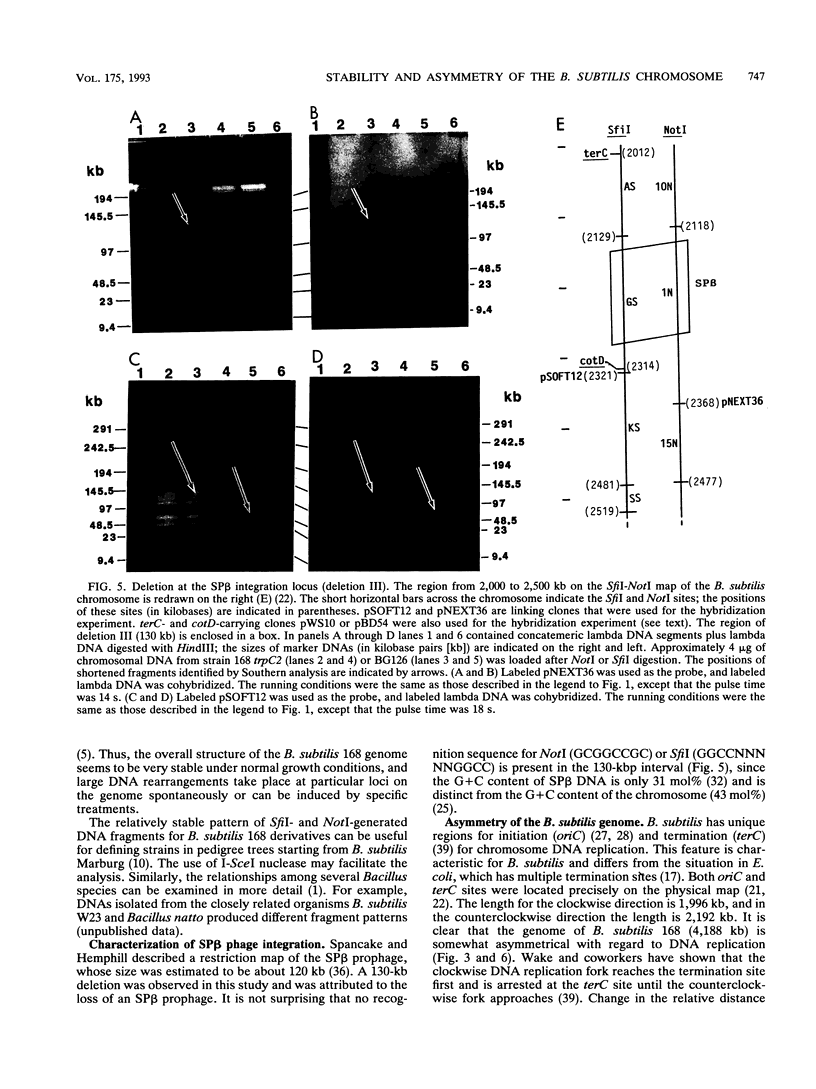
Chromosomal DNAs from a number of strains derived from Bacillus subtilis 168 were digested with restriction endonucleases NotI or SfiI, and the locations of chromosomal alterations were compared with the recently constructed standard NotI-SfiI restriction map (M. Itaya and T. Tanaka, J. Mol. Biol. 220:631-648, 1991). In general, the chromosome structure of B. subtilis 168 was found to be stable, as expected from the genetic stability of this species. DNA alterations, typically deletions, are formed in three limited loci on the chromosome. One of these alterations was characterized as a spontaneous deletion formed between rrn operons, and another occurred as a result of prophage SP beta excision. I found that oriC and terC are not located on precisely opposite sides of the chromosome. Replication in the counter clockwise direction was 196 kb longer than replication in the clockwise direction. The characteristic of length difference is not changed by deletion formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn K. S., Wake R. G. Variations and coding features of the sequence spanning the replication terminus of Bacillus subtilis 168 and W23 chromosomes. Gene. 1991 Feb 1;98(1):107–112. doi: 10.1016/0378-1119(91)90111-n. [DOI] [PubMed] [Google Scholar]
- Amano H., Ives C. L., Bott K. F., Shishido K. A limited number of Bacillus subtilis strains carry a tetracycline-resistance determinant at a site close to the origin of replication. Biochim Biophys Acta. 1991 Feb 16;1088(2):251–258. doi: 10.1016/0167-4781(91)90061-p. [DOI] [PubMed] [Google Scholar]
- Amjad M., Castro J. M., Sandoval H., Wu J. J., Yang M., Henner D. J., Piggot P. J. An SfiI restriction map of the Bacillus subtilis 168 genome. Gene. 1991 May 15;101(1):15–21. doi: 10.1016/0378-1119(91)90219-2. [DOI] [PubMed] [Google Scholar]
- Anderson P., Roth J. Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between rRNA (rrn) cistrons. Proc Natl Acad Sci U S A. 1981 May;78(5):3113–3117. doi: 10.1073/pnas.78.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeit R. D., Arthur M., Dunn R., Kim C., Selander R. K., Goldstein R. Resolution of recent evolutionary divergence among Escherichia coli from related lineages: the application of pulsed field electrophoresis to molecular epidemiology. J Infect Dis. 1990 Feb;161(2):230–235. doi: 10.1093/infdis/161.2.230. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch A., Häusler A., Hütter R. Genome rearrangement and genetic instability in Streptomyces spp. J Bacteriol. 1990 Aug;172(8):4138–4142. doi: 10.1128/jb.172.8.4138-4142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan C. M., Pack R. A., Smith M. T., Wake R. G. Normal terC-region of the Bacillus subtilis chromosome acts in a polar manner to arrest the clockwise replication fork. J Mol Biol. 1991 Nov 20;222(2):197–207. doi: 10.1016/0022-2836(91)90206-l. [DOI] [PubMed] [Google Scholar]
- Daniels D. L. The complete AvrII restriction map of the Escherichia coli genome and comparisons of several laboratory strains. Nucleic Acids Res. 1990 May 11;18(9):2649–2651. doi: 10.1093/nar/18.9.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Fujita T. Genetic analysis of a pleiotropic deletion mutation (delta igf) in Bacillus subtilis. J Bacteriol. 1983 May;154(2):864–869. doi: 10.1128/jb.154.2.864-869.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusato T., Takano J., Yamane K., Hashiguchi K., Tanimoto A., Mori M., Yoda K., Yamasaki M., Tamura G. Amplification and deletion of the amyE+-tmrB+ gene region in a Bacillus subtilis recombinant-phage genome by the tmrA7 mutation. J Bacteriol. 1986 Feb;165(2):549–556. doi: 10.1128/jb.165.2.549-556.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka M., Kobayashi T., Horiuchi T. A newly identified DNA replication terminus site, TerE, on the Escherichia coli chromosome. J Bacteriol. 1991 Jan;173(1):391–393. doi: 10.1128/jb.173.1.391-393.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iismaa T. P., Carrigan C. M., Wake R. G. Relocation of the replication terminus, terC, of Bacillus subtilis to a new chromosomal site. Gene. 1988 Jul 30;67(2):183–191. doi: 10.1016/0378-1119(88)90395-2. [DOI] [PubMed] [Google Scholar]
- Itaya M. Construction of a novel tetracycline resistance gene cassette useful as a marker on the Bacillus subtilis chromosome. Biosci Biotechnol Biochem. 1992 Apr;56(4):685–686. doi: 10.1271/bbb.56.685. [DOI] [PubMed] [Google Scholar]
- Itaya M., Kondo K., Tanaka T. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res. 1989 Jun 12;17(11):4410–4410. doi: 10.1093/nar/17.11.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya M., Laffan J. J., Sueoka N. Physical distance between the site of type II DNA binding to the membrane and oriC on the Bacillus subtilis 168 chromosome. J Bacteriol. 1992 Aug;174(16):5466–5470. doi: 10.1128/jb.174.16.5466-5470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya M., Tanaka T. Complete physical map of the Bacillus subtilis 168 chromosome constructed by a gene-directed mutagenesis method. J Mol Biol. 1991 Aug 5;220(3):631–648. doi: 10.1016/0022-2836(91)90106-g. [DOI] [PubMed] [Google Scholar]
- Itaya M., Yamaguchi I., Kobayashi K., Endo T., Tanaka T. The blasticidin S resistance gene (bsr) selectable in a single copy state in the Bacillus subtilis chromosome. J Biochem. 1990 Jun;107(6):799–801. doi: 10.1093/oxfordjournals.jbchem.a123128. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Moriya S., Atlung T., Hansen F. G., Yoshikawa H., Ogasawara N. Cloning of an autonomously replicating sequence (ars) from the Bacillus subtilis chromosome. Mol Microbiol. 1992 Feb;6(3):309–315. doi: 10.1111/j.1365-2958.1992.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Moriya S., Ogasawara N., Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. III. Nucleotide sequence of some 10,000 base pairs in the origin region. Nucleic Acids Res. 1985 Apr 11;13(7):2251–2265. doi: 10.1093/nar/13.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M., Sugishita A., Furukawa K. Cloning and nucleotide sequences of histidase and regulatory genes in the Bacillus subtilis hut operon and positive regulation of the operon. J Bacteriol. 1988 Jul;170(7):3199–3205. doi: 10.1128/jb.170.7.3199-3205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Hoch J. A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985 Jun;49(2):158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Samori Y., Kobayashi Y. The cisA cistron of Bacillus subtilis sporulation gene spoIVC encodes a protein homologous to a site-specific recombinase. J Bacteriol. 1990 Feb;172(2):1092–1098. doi: 10.1128/jb.172.2.1092-1098.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobral B. W., Honeycutt R. J., Atherly A. G. The genomes of the family Rhizobiaceae: size, stability, and rarely cutting restriction endonucleases. J Bacteriol. 1991 Jan;173(2):704–709. doi: 10.1128/jb.173.2.704-709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spancake G. A., Hemphill H. E. Deletion mutants of Bacillus subtilis bacteriophage SP beta. J Virol. 1985 Jul;55(1):39–44. doi: 10.1128/jvi.55.1.39-44.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier P., Kunkel B., Kroos L., Losick R. Chromosomal rearrangement generating a composite gene for a developmental transcription factor. Science. 1989 Jan 27;243(4890):507–512. doi: 10.1126/science.2536191. [DOI] [PubMed] [Google Scholar]
- Umeda M., Ohtsubo E. Mapping of insertion elements IS1, IS2 and IS3 on the Escherichia coli K-12 chromosome. Role of the insertion elements in formation of Hfrs and F' factors and in rearrangement of bacterial chromosomes. J Mol Biol. 1989 Aug 20;208(4):601–614. doi: 10.1016/0022-2836(89)90151-4. [DOI] [PubMed] [Google Scholar]
- Widom R. L., Jarvis E. D., LaFauci G., Rudner R. Instability of rRNA operons in Bacillus subtilis. J Bacteriol. 1988 Feb;170(2):605–610. doi: 10.1128/jb.170.2.605-610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M., Ehrlich S. D. Stability of reiterated sequences in the Bacillus subtilis chromosome. J Bacteriol. 1989 May;171(5):2653–2656. doi: 10.1128/jb.171.5.2653-2656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]