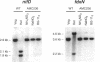Abstract
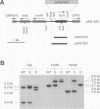
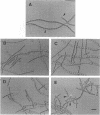
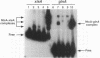
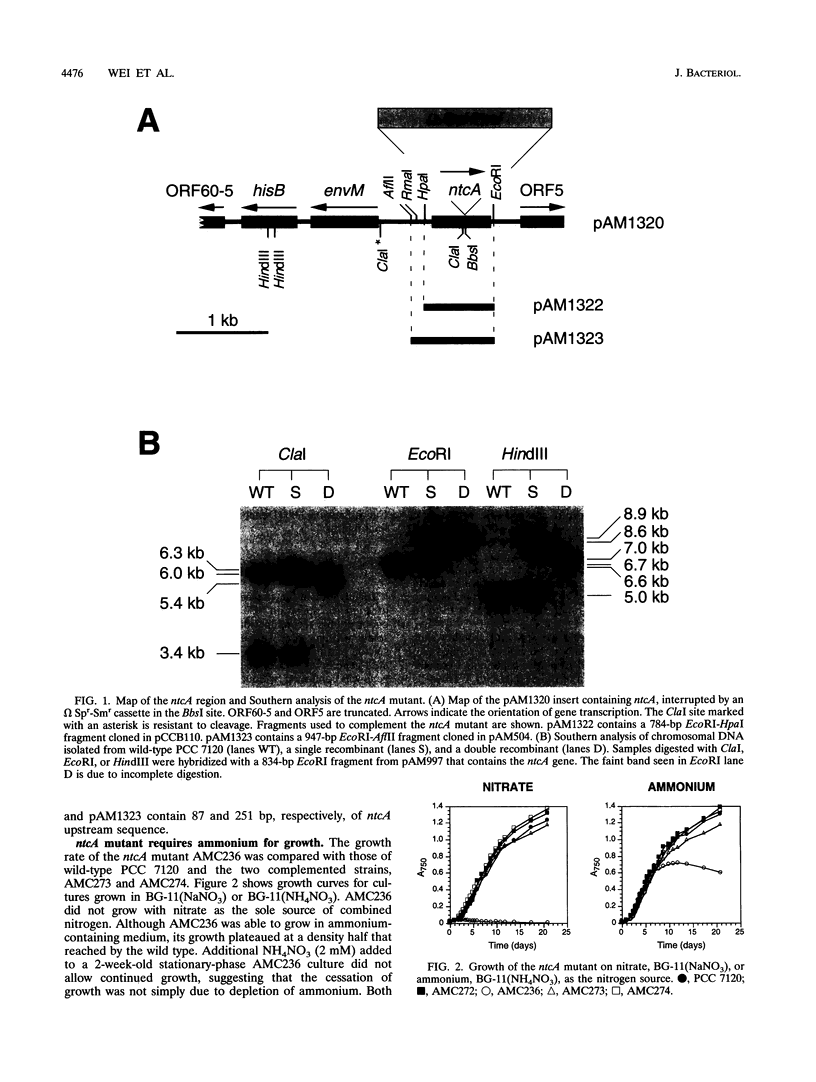
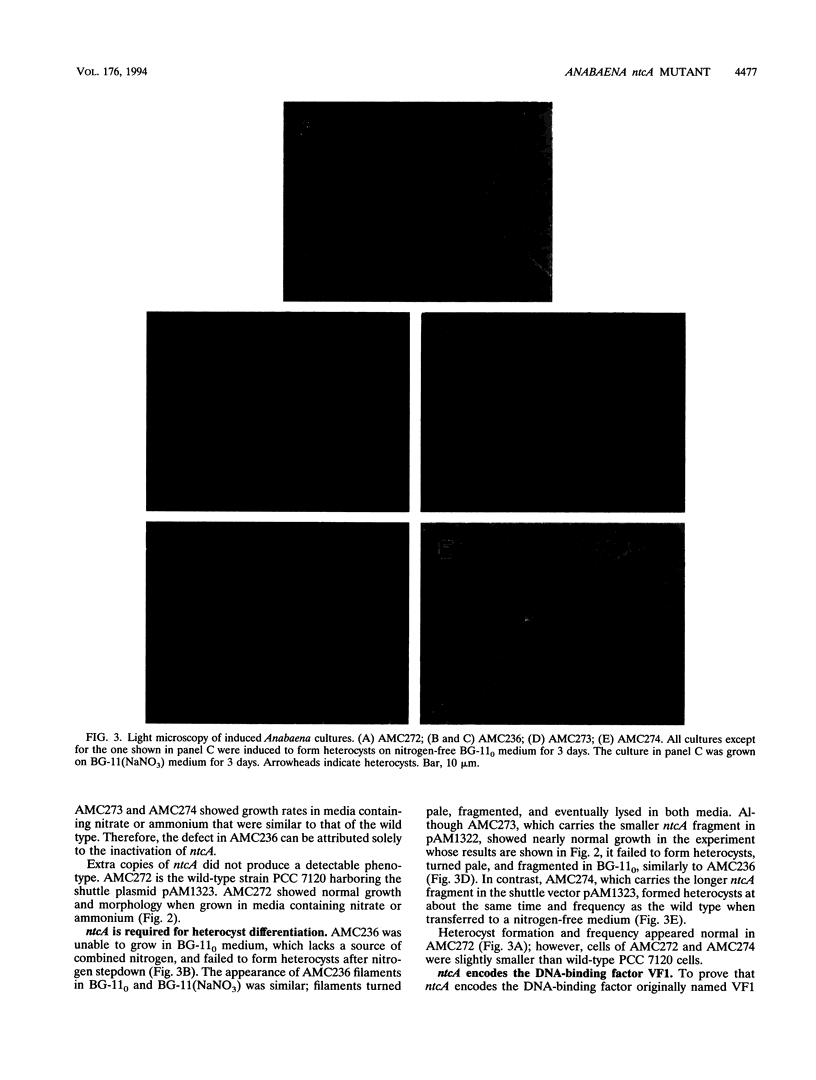
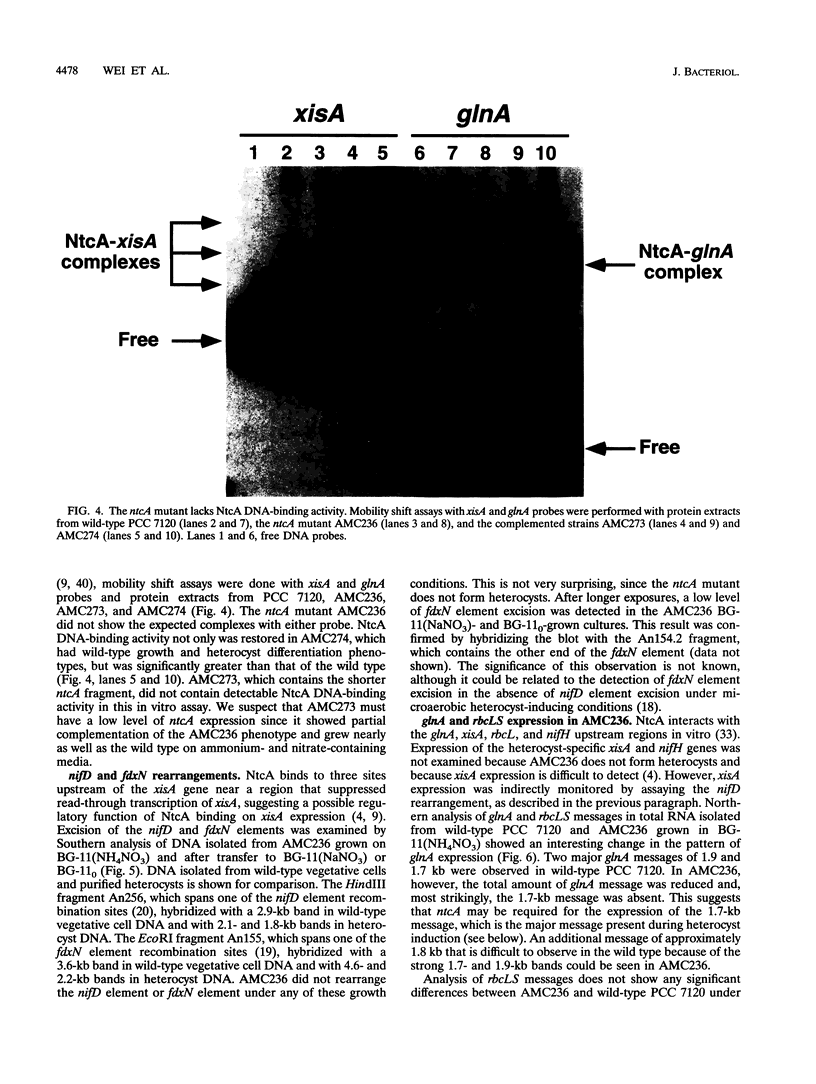
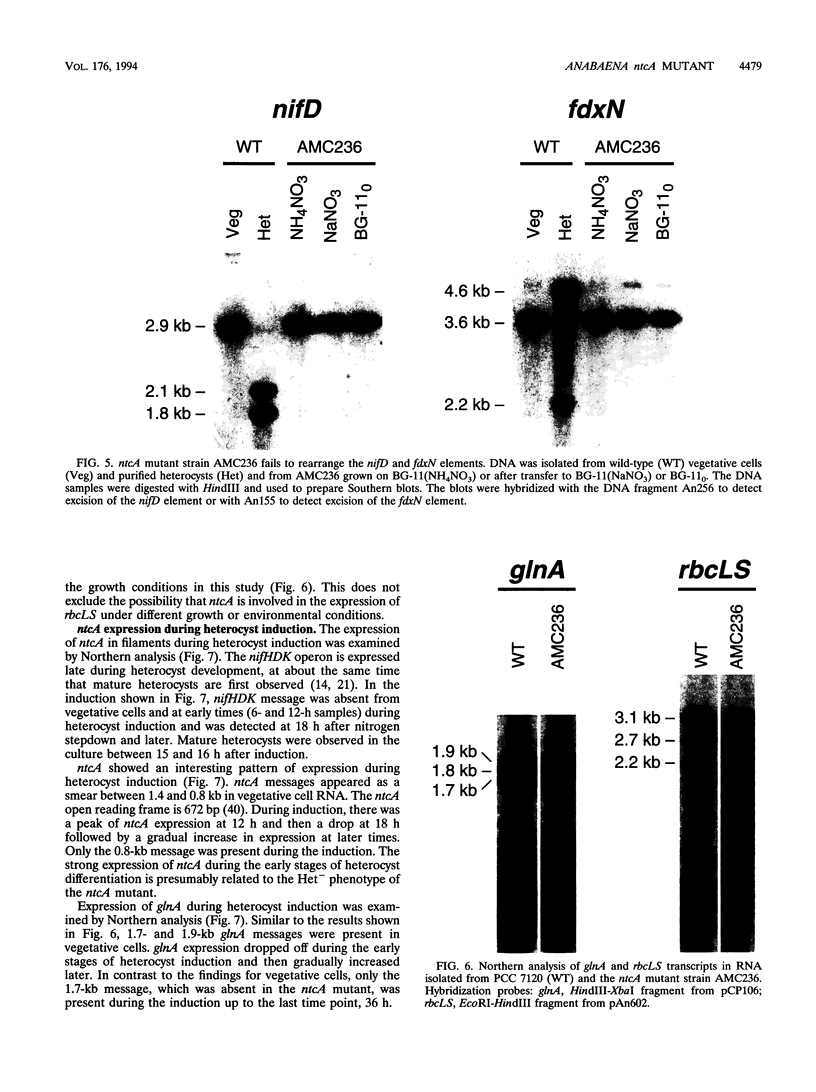
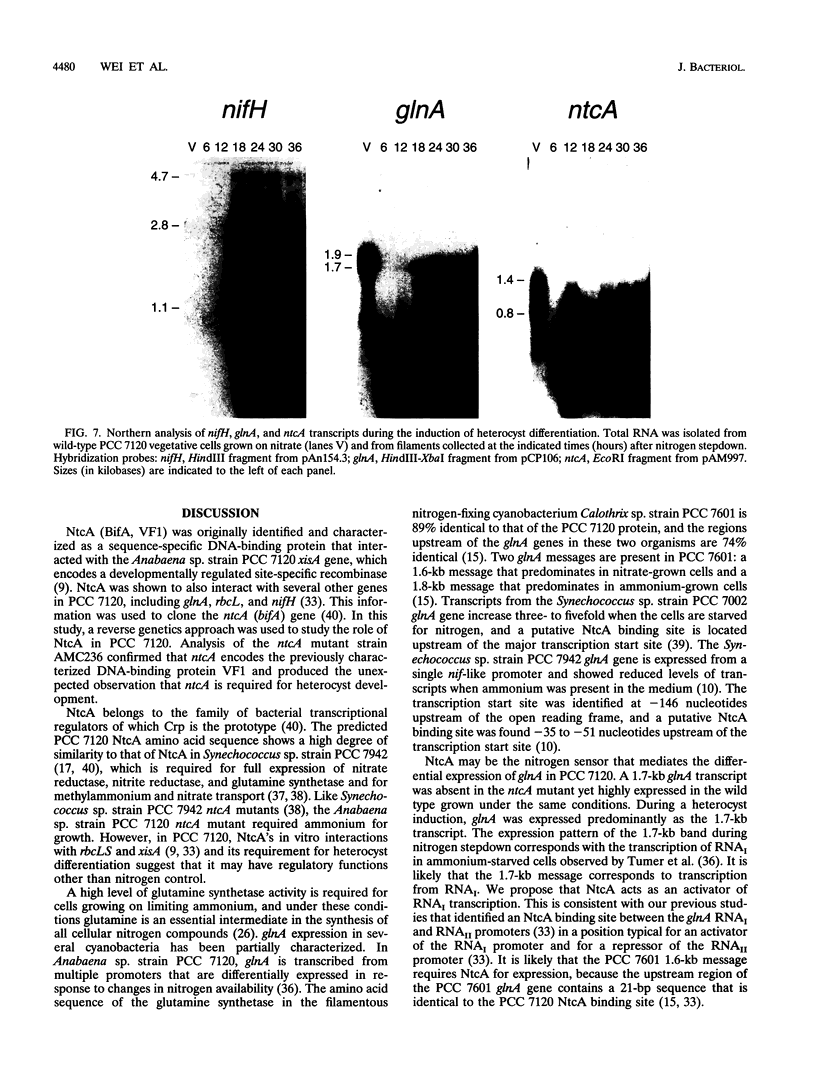
The Anabaena sp. strain PCC 7120 ntcA (bifA) gene encodes a sequence-specific DNA-binding protein, NtcA (BifA, VF1) that interacts with the upstream region of several genes, including glnA, xisA, rbcL, and nifH. We have constructed a ntcA null mutant by interrupting the gene with an omega Spr-Smr cassette. The ntcA mutant was not able to grow with nitrate or atmospheric dinitrogen as the sole nitrogen source but could be grown on medium containing ammonium. The ntcA mutant was unable to form heterocysts and did not rearrange the nifD or fdxN elements after induction on a medium lacking combined nitrogen. Northern (RNA) analysis of ntcA in the wild-type strain during nitrogen stepdown showed a peak of ntcA message at an early stage (12 h) of heterocyst induction. Complementation of the ntcA mutant with a DNA fragment containing the ntcA gene and 251 bp of upstream sequence on a shuttle vector restored a wild-type phenotype; however, a similar construction containing 87 bp of upstream sequence only partially restored the phenotype. Northern analysis of RNA samples isolated from ammonium-grown cultures of the ntcA mutant showed reduced amounts of glnA message and the absence of a 1.7-kb transcript. In the wild type, the 1.7-kb transcript represents the majority of glnA transcripts after nitrogen stepdown. The ntcA mutant showed a normal pattern of rbcLS messages under these growth conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black T. A., Cai Y., Wolk C. P. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol Microbiol. 1993 Jul;9(1):77–84. doi: 10.1111/j.1365-2958.1993.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Botsford J. L., Harman J. G. Cyclic AMP in prokaryotes. Microbiol Rev. 1992 Mar;56(1):100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusca J. S., Chastain C. J., Golden J. W. Expression of the Anabaena sp. strain PCC 7120 xisA gene from a heterologous promoter results in excision of the nifD element. J Bacteriol. 1990 Jul;172(7):3925–3931. doi: 10.1128/jb.172.7.3925-3931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buikema W. J., Haselkorn R. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 1991 Feb;5(2):321–330. doi: 10.1101/gad.5.2.321. [DOI] [PubMed] [Google Scholar]
- Cai Y. P., Wolk C. P. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J Bacteriol. 1990 Jun;172(6):3138–3145. doi: 10.1128/jb.172.6.3138-3145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco C. D., Ramaswamy K. S., Ramasubramanian T. S., Golden J. W. Anabaena xisF gene encodes a developmentally regulated site-specific recombinase. Genes Dev. 1994 Jan;8(1):74–83. doi: 10.1101/gad.8.1.74. [DOI] [PubMed] [Google Scholar]
- Chastain C. J., Brusca J. S., Ramasubramanian T. S., Wei T. F., Golden J. W. A sequence-specific DNA-binding factor (VF1) from Anabaena sp. strain PCC 7120 vegetative cells binds to three adjacent sites in the xisA upstream region. J Bacteriol. 1990 Sep;172(9):5044–5051. doi: 10.1128/jb.172.9.5044-5051.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kupiec R., Gurevitz M., Zilberstein A. Expression of glnA in the cyanobacterium Synechococcus sp. strain PCC 7942 is initiated from a single nif-like promoter under various nitrogen conditions. J Bacteriol. 1993 Dec;175(23):7727–7731. doi: 10.1128/jb.175.23.7727-7731.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Vides J., Magasanik B., Gralla J. D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991 Sep;55(3):371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhai J. Strong and regulated promoters in the cyanobacterium Anabaena PCC 7120. FEMS Microbiol Lett. 1993 Dec 1;114(2):179–184. doi: 10.1111/j.1574-6968.1993.tb06570.x. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990 Oct;9(10):3379–3388. doi: 10.1002/j.1460-2075.1990.tb07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmorjani K., Liotenberg S., Houmard J., de Marsac N. T. Molecular characterization of the gene encoding glutamine synthetase in the cyanobacterium Calothrix sp. PCC 7601. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1296–1302. doi: 10.1016/0006-291x(92)90214-6. [DOI] [PubMed] [Google Scholar]
- Finkel S. E., Johnson R. C. The Fis protein: it's not just for DNA inversion anymore. Mol Microbiol. 1992 Nov;6(22):3257–3265. doi: 10.1111/j.1365-2958.1992.tb02193.x. [DOI] [PubMed] [Google Scholar]
- Frías J. E., Mérida A., Herrero A., Martín-Nieto J., Flores E. General distribution of the nitrogen control gene ntcA in cyanobacteria. J Bacteriol. 1993 Sep;175(17):5710–5713. doi: 10.1128/jb.175.17.5710-5713.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Carrasco C. D., Mulligan M. E., Schneider G. J., Haselkorn R. Deletion of a 55-kilobase-pair DNA element from the chromosome during heterocyst differentiation of Anabaena sp. strain PCC 7120. J Bacteriol. 1988 Nov;170(11):5034–5041. doi: 10.1128/jb.170.11.5034-5041.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Mulligan M. E., Haselkorn R. Different recombination site specificity of two developmentally regulated genome rearrangements. Nature. 1987 Jun 11;327(6122):526–529. doi: 10.1038/327526a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Whorff L. L., Wiest D. R. Independent regulation of nifHDK operon transcription and DNA rearrangement during heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991 Nov;173(22):7098–7105. doi: 10.1128/jb.173.22.7098-7105.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Wiest D. R. Genome rearrangement and nitrogen fixation in Anabaena blocked by inactivation of xisA gene. Science. 1988 Dec 9;242(4884):1421–1423. doi: 10.1126/science.3144039. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Lammers P. J., Golden J. W., Haselkorn R. Identification and sequence of a gene required for a developmentally regulated DNA excision in Anabaena. Cell. 1986 Mar 28;44(6):905–911. doi: 10.1016/0092-8674(86)90013-9. [DOI] [PubMed] [Google Scholar]
- Landy A. Dynamic, structural, and regulatory aspects of lambda site-specific recombination. Annu Rev Biochem. 1989;58:913–949. doi: 10.1146/annurev.bi.58.070189.004405. [DOI] [PubMed] [Google Scholar]
- Magasanik B. The regulation of nitrogen utilization in enteric bacteria. J Cell Biochem. 1993 Jan;51(1):34–40. doi: 10.1002/jcb.240510108. [DOI] [PubMed] [Google Scholar]
- Mohamed A., Jansson C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol. 1989 Dec;13(6):693–700. doi: 10.1007/BF00016024. [DOI] [PubMed] [Google Scholar]
- Morett E., Buck M. In vivo studies on the interaction of RNA polymerase-sigma 54 with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters. The role of NifA in the formation of an open promoter complex. J Mol Biol. 1989 Nov 5;210(1):65–77. doi: 10.1016/0022-2836(89)90291-x. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Buikema W. J., Haselkorn R. Bacterial-type ferredoxin genes in the nitrogen fixation regions of the cyanobacterium Anabaena sp. strain PCC 7120 and Rhizobium meliloti. J Bacteriol. 1988 Sep;170(9):4406–4410. doi: 10.1128/jb.170.9.4406-4410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Haselkorn R. Nitrogen fixation (nif) genes of the cyanobacterium Anabaena species strain PCC 7120. The nifB-fdxN-nifS-nifU operon. J Biol Chem. 1989 Nov 15;264(32):19200–19207. [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubramanian T. S., Wei T. F., Golden J. W. Two Anabaena sp. strain PCC 7120 DNA-binding factors interact with vegetative cell- and heterocyst-specific genes. J Bacteriol. 1994 Mar;176(5):1214–1223. doi: 10.1128/jb.176.5.1214-1223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Palas M. A., Flores E., Herrero A. NtcA, a global nitrogen regulator from the cyanobacterium Synechococcus that belongs to the Crp family of bacterial regulators. Mol Microbiol. 1992 Jul;6(13):1853–1859. doi: 10.1111/j.1365-2958.1992.tb01357.x. [DOI] [PubMed] [Google Scholar]
- Vega-Palas M. A., Madueño F., Herrero A., Flores E. Identification and cloning of a regulatory gene for nitrogen assimilation in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 1990 Feb;172(2):643–647. doi: 10.1128/jb.172.2.643-647.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S. J., Thomas S. P., Kaufman R. I., Nixon B. T., Stevens S. E., Jr The glnA gene of the cyanobacterium Agmenellum quadruplicatum PR-6 is nonessential for ammonium assimilation. J Bacteriol. 1993 Feb;175(3):604–612. doi: 10.1128/jb.175.3.604-612.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
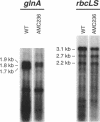
- Wei T. F., Ramasubramanian T. S., Pu F., Golden J. W. Anabaena sp. strain PCC 7120 bifA gene encoding a sequence-specific DNA-binding protein cloned by in vivo transcriptional interference selection. J Bacteriol. 1993 Jul;175(13):4025–4035. doi: 10.1128/jb.175.13.4025-4035.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]