Abstract
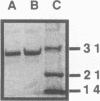
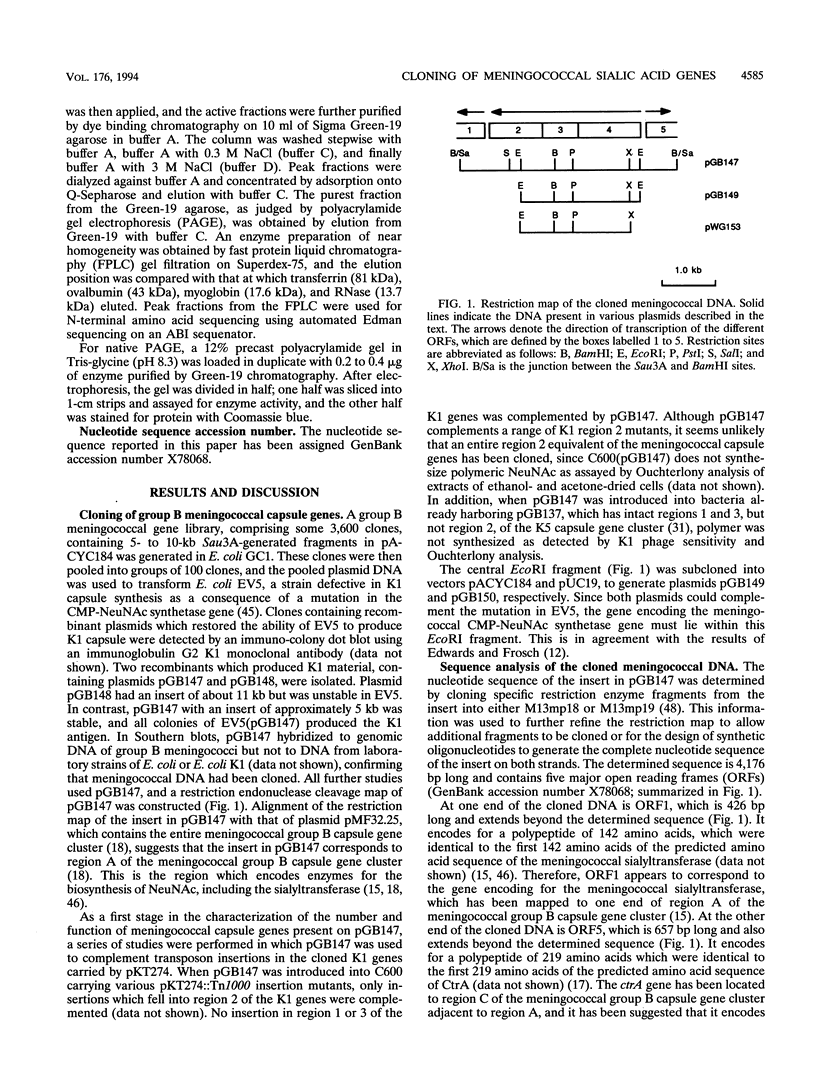
The gene encoding for the CMP-NeuNAc synthetase enzyme of Neisseria meningitidis group B was cloned by complementation of a mutant of Escherichia coli defective for this enzyme. The gene (neuA) was isolated on a 4.1-kb fragment of meningococcal chromosomal DNA. Determination of the nucleotide sequence of this fragment revealed the presence of three genes, termed neuA, neuB, and neuC, organized in a single operon. The presence of a truncated ctrA gene at one end of the cloned DNA and a truncated gene encoding for the meningococcal sialyltransferase at the other confirmed that the cloned DNA corresponded to region A and part of region C of the meningococcal capsule gene cluster. The predicted amino acid sequence of the meningococcal NeuA protein was 57% homologous to that of NeuA, the CMP-NeuNAc synthetase encoded by E. coli K1. The predicted molecular mass of meningococcal NeuA protein was 24.8 kDa, which was 6 kDa larger than that formerly predicted (U. Edwards and M. Frosch, FEMS Microbiol. Lett. 96:161-166, 1992). Purification of the recombinant meningococcal NeuA protein together with determination of the N-terminal amino acid sequence confirmed that this 24.8-kDa protein was indeed the meningococcal CMP-NeuNAc synthetase. The predicted amino acid sequences of the two other encoded proteins were homologous to those of the NeuC and NeuB proteins of E. coli K1, two proteins involved in the synthesis of NeuNAc. These results indicate that common steps exist in the biosynthesis of NeuNAc in these two microorganisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Jann K. Bacterial polysaccharide capsule synthesis, export and evolution of structural diversity. Mol Microbiol. 1989 Dec;3(12):1819–1823. doi: 10.1111/j.1365-2958.1989.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Boulnois G. J., Roberts I. S. Genetics of capsular polysaccharide production in bacteria. Curr Top Microbiol Immunol. 1990;150:1–18. doi: 10.1007/978-3-642-74694-9_1. [DOI] [PubMed] [Google Scholar]
- Boulnois G. J., Roberts I. S., Hodge R., Hardy K. R., Jann K. B., Timmis K. N. Analysis of the K1 capsule biosynthesis genes of Escherichia coli: definition of three functional regions for capsule production. Mol Gen Genet. 1987 Jun;208(1-2):242–246. doi: 10.1007/BF00330449. [DOI] [PubMed] [Google Scholar]
- Bronner D., Sieberth V., Pazzani C., Roberts I. S., Boulnois G. J., Jann B., Jann K. Expression of the capsular K5 polysaccharide of Escherichia coli: biochemical and electron microscopic analyses of mutants with defects in region 1 of the K5 gene cluster. J Bacteriol. 1993 Sep;175(18):5984–5992. doi: 10.1128/jb.175.18.5984-5992.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossmer R., Rose U., Kaspar D., Smith T. L., Grasmuk H., Unger F. M. Enzymic synthesis of 5-acetamido-9-azido-3,5,9-trideoxy-D-glycero-D-galacto-2-nonulosonic acid, a 9-azido-9-deoxy derivative of N-acetylneuraminic acid. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1282–1289. doi: 10.1016/0006-291x(80)90090-x. [DOI] [PubMed] [Google Scholar]
- Brown P. K., Romana L. K., Reeves P. R. Molecular analysis of the rfb gene cluster of Salmonella serovar muenchen (strain M67): the genetic basis of the polymorphism between groups C2 and B. Mol Microbiol. 1992 May;6(10):1385–1394. doi: 10.1111/j.1365-2958.1992.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echarti C., Hirschel B., Boulnois G. J., Varley J. M., Waldvogel F., Timmis K. N. Cloning and analysis of the K1 capsule biosynthesis genes of Escherichia coli: lack of homology with Neisseria meningitidis group B DNA sequences. Infect Immun. 1983 Jul;41(1):54–60. doi: 10.1128/iai.41.1.54-60.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards U., Frosch M. Sequence and functional analysis of the cloned Neisseria meningitidis CMP-NeuNAc synthetase. FEMS Microbiol Lett. 1992 Sep 15;75(2-3):161–166. doi: 10.1016/0378-1097(92)90397-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frosch M., Edwards U., Bousset K., Krausse B., Weisgerber C. Evidence for a common molecular origin of the capsule gene loci in gram-negative bacteria expressing group II capsular polysaccharides. Mol Microbiol. 1991 May;5(5):1251–1263. doi: 10.1111/j.1365-2958.1991.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Frosch M., Görgen I., Boulnois G. J., Timmis K. N., Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1194–1198. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch M., Müller D., Bousset K., Müller A. Conserved outer membrane protein of Neisseria meningitidis involved in capsule expression. Infect Immun. 1992 Mar;60(3):798–803. doi: 10.1128/iai.60.3.798-803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch M., Weisgerber C., Meyer T. F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1669–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J. Capsular polysaccharides as vaccine candidates. Curr Top Microbiol Immunol. 1990;150:97–127. doi: 10.1007/978-3-642-74694-9_6. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Zollinger W. D., Brandt B. L., Artenstein M. S. Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J Immunol. 1973 Jan;110(1):262–268. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Dunne F. T., Jonssen E. K. Studies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharide. J Biol Chem. 1971 Aug 10;246(15):4703–4712. [PubMed] [Google Scholar]
- Masson L., Holbein B. E. Physiology of sialic acid capsular polysaccharide synthesis in serogroup B Neisseria meningitidis. J Bacteriol. 1983 May;154(2):728–736. doi: 10.1128/jb.154.2.728-736.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- O'Neill M. C. Escherichia coli promoters. I. Consensus as it relates to spacing class, specificity, repeat substructure, and three-dimensional organization. J Biol Chem. 1989 Apr 5;264(10):5522–5530. [PubMed] [Google Scholar]
- Pavelka M. S., Jr, Wright L. F., Silver R. P. Identification of two genes, kpsM and kpsT, in region 3 of the polysialic acid gene cluster of Escherichia coli K1. J Bacteriol. 1991 Aug;173(15):4603–4610. doi: 10.1128/jb.173.15.4603-4610.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazzani C., Rosenow C., Boulnois G. J., Bronner D., Jann K., Roberts I. S. Molecular analysis of region 1 of the Escherichia coli K5 antigen gene cluster: a region encoding proteins involved in cell surface expression of capsular polysaccharide. J Bacteriol. 1993 Sep;175(18):5978–5983. doi: 10.1128/jb.175.18.5978-5983.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I. S., Mountford R., Hodge R., Jann K. B., Boulnois G. J. Common organization of gene clusters for production of different capsular polysaccharides (K antigens) in Escherichia coli. J Bacteriol. 1988 Mar;170(3):1305–1310. doi: 10.1128/jb.170.3.1305-1310.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I., Mountford R., High N., Bitter-Suermann D., Jann K., Timmis K., Boulnois G. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Vann W. F., Aaronson W. Genetic and molecular analyses of Escherichia coli K1 antigen genes. J Bacteriol. 1984 Feb;157(2):568–575. doi: 10.1128/jb.157.2.568-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. N., Boulnois G. J., Roberts I. S. Molecular analysis of the Escherichia coli K5 kps locus: identification and characterization of an inner-membrane capsular polysaccharide transport system. Mol Microbiol. 1990 Nov;4(11):1863–1869. doi: 10.1111/j.1365-2958.1990.tb02035.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steenbergen S. M., Vimr E. R. Mechanism of polysialic acid chain elongation in Escherichia coli K1. Mol Microbiol. 1990 Apr;4(4):603–611. doi: 10.1111/j.1365-2958.1990.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Steenbergen S. M., Wrona T. J., Vimr E. R. Functional analysis of the sialyltransferase complexes in Escherichia coli K1 and K92. J Bacteriol. 1992 Feb;174(4):1099–1108. doi: 10.1128/jb.174.4.1099-1108.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Boulnois G. J., Bitter-Suermann D., Cabello F. C. Surface components of Escherichia coli that mediate resistance to the bactericidal activities of serum and phagocytes. Curr Top Microbiol Immunol. 1985;118:197–218. doi: 10.1007/978-3-642-70586-1_11. [DOI] [PubMed] [Google Scholar]
- Troy F. A., 2nd The chemistry and biosynthesis of selected bacterial capsular polymers. Annu Rev Microbiol. 1979;33:519–560. doi: 10.1146/annurev.mi.33.100179.002511. [DOI] [PubMed] [Google Scholar]
- Vann W. F., Silver R. P., Abeijon C., Chang K., Aaronson W., Sutton A., Finn C. W., Lindner W., Kotsatos M. Purification, properties, and genetic location of Escherichia coli cytidine 5'-monophosphate N-acetylneuraminic acid synthetase. J Biol Chem. 1987 Dec 25;262(36):17556–17562. [PubMed] [Google Scholar]
- Vimr E. R., Aaronson W., Silver R. P. Genetic analysis of chromosomal mutations in the polysialic acid gene cluster of Escherichia coli K1. J Bacteriol. 1989 Feb;171(2):1106–1117. doi: 10.1128/jb.171.2.1106-1117.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgerber C., Hansen A., Frosch M. Complete nucleotide and deduced protein sequence of CMP-NeuAc: poly-alpha-2,8 sialosyl sialyltransferase of Escherichia coli K1. Glycobiology. 1991 Sep;1(4):357–365. doi: 10.1093/glycob/1.4.357. [DOI] [PubMed] [Google Scholar]
- Wilkins B. M., Boulnois G. J., Lanka E. A plasmid DNA primase active in discontinuous bacterial DNA replication. Nature. 1981 Mar 19;290(5803):217–221. doi: 10.1038/290217a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zapata G., Crowley J. M., Vann W. F. Sequence and expression of the Escherichia coli K1 neuC gene product. J Bacteriol. 1992 Jan;174(1):315–319. doi: 10.1128/jb.174.1.315-319.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata G., Vann W. F., Aaronson W., Lewis M. S., Moos M. Sequence of the cloned Escherichia coli K1 CMP-N-acetylneuraminic acid synthetase gene. J Biol Chem. 1989 Sep 5;264(25):14769–14774. [PubMed] [Google Scholar]